Abstract
The contribution of the adaptive and innate immune systems to the pathogenesis and outcome of sepsis remains a fundamental yet controversial question. Here, we use mice lacking the recombination activating gene-1 (Rag-1) to study the role of T and B cells in sepsis after cecal ligation and puncture (CLP). Spleens of Rag-1−/− mice were atrophic and completely devoid of CD3+ T cells and CD19+ B cells. Wildtype mice and Rag-1−/− mice (both on a C57BL/6J background) underwent CLP or sham surgery. Both wildtype and Rag-1−/− mice developed clinical signs of sepsis within the first day after CLP. This included severe hypothermia as measured by a decrease in body surface temperature and organ dysfunction as detected by plasma increases in BUN and LDH levels. Survival curves of wildtype and Rag-1−/− mice after CLP were superimposable, with 35% survival in the wildtype group and 27% survival in the Rag-1−/− group, respectively (not significant, P=0.875). Using multiplex bead-based assays, the mediator concentrations for 23 cytokines and chemokines were measured in plasma of wildtype and Rag-1−/− mice 8 h after CLP or sham surgery. Compared to sham surgery mice, the highest mediator levels were observed for G-CSF, KC, IL-6, MCP-1 and IL-10. Levels for most mediators were unaffected by the absence of T and B lymphocytes. Only the concentrations of IL-6 and IL-17 were found to be significantly lower in Rag-1−/− mice compared to wildtype mice. In conclusion, the absence of T and B cells in the CLP model employed does not appear to affect the acute outcome of severe sepsis.
Keywords: cecal ligation and puncture, Rag-1, septic peritonitis, lymphocytes, Luminex, bead-based assay
INTRODUCTION
The annual incidence of severe sepsis has dramatically increased over the past decades. In the United States there are estimated 600,000–750,000 cases of sepsis per year, resulting in ~250,000 annual deaths. (1,2). Despite tremendous scientific efforts, the pathophysiology of sepsis and subsequent multi-organ failure remains poorly understood (3). The roles of innate and adaptive immune responses are controversial in the setting of sepsis. It has been widely speculated that engagement of the immune system contains invading pathogens, but may also contribute to tissue damage. Since both hyperactivation but also anergy of immune cells seem to occur during the course of sepsis (4,5), it remains unclear what the most beneficial nature of an immune response during sepsis should look like. Many humoral and cellular components of the innate immune system, including cytokines, the complement system, neutrophils and antigen presenting cells, have been implicated in the pathogenesis of sepsis (6). The role of adaptive immune cells, namely T and B lymphocytes, is even more complex. It is undisputed, that the events of clonal selection and expansion of antigen specific T and B cells require at least 2–5 days and that in experimental sepsis the majority of animals usually succumb within this time frame. As the influence of antigen specific T and B cells may be minor for the acute events during sepsis, lymphocytes are also capable of responding to bacterial products such as endotoxin via Toll-like-receptors and express different classes of cytokine receptors to respond to the ‘cytokine storm’ (7,8). An import chain of observations has established the importance of lymphocyte apoptosis during sepsis (9,10). Interestingly, apoptosis occurring in lymphoid and non-lymphoid organs after cecal ligation and puncture (CLP) is largely independent of endotoxin and TNFα (11). The genetic engineering of mice to knockout the recombination activation genes (Rag-1, Rag-2) has produced strains that do not possess mature T and B cells (12). During experimental sepsis in Rag-1−/− mice, these mice have similar degrees of apoptosis of parenchymal cells, suggesting that T and B cells are not necessary for apoptosis to occur and that apoptotic cell death is not restricted to lymphocytes after CLP (13). This finding might not apply to the intestinal tract, where apoptosis of the gut epithelium was found 5-fold augmented in septic Rag-1−/− mice (14). Furthermore, in a model of colon ascendens stent peritonitis, Rag-1−/− mice have been described as having a survival disadvantage (15). Studies on subsets of lymphocytes imply that CD4+ T cells may confer anti-apoptotic effects that are protective for sepsis survival (14). Additionally, we have recently shown that γδ T cells are an important source of IL-17A during sepsis and that neutralization of IL-17A improves survival in this setting (16). To further elucidate the role of lymphocytes in sepsis, we have investigated survival and mediator release in Rag-1−/− mice using the CLP model.
MATERIALS AND METHODS
Animals
All procedures were performed in accordance with the National Institutes of Health guidelines and the University Committee on Use and Care of Animals (UCUCA), University of Michigan. Male mice of the strains C57BL/6J (wildtype) and B6.129S7-Rag1tm1Mom/J (Rag-1−/−, on a C57BL/6J background) were purchased from the Jackson Laboratories (Bar Harbor, USA) at 8–10 weeks of age. All animals were housed under specific pathogen free conditions.
Cecal ligation and puncture
Mice were anaesthetized by intra-peritoneal injection of a mixture of Ketamine (100 mg/kg bodyweight, Fort Dodge Animal Health, USA) and Xylazine (8 mg/kg bodyweight, Akorn Inc., USA) in PBS. After disinfection of the skin with 70% ethanol, a small midline abdominal incision (1 cm) was performed with a sterile surgical blade. The peritoneum was opened with surgical scissors and the cecum located and exteriorized. Ligation of 5–7 mm cecum resulted in survival rates of 20–40%. For the ligation of the cecum we used black braided silk 4-0 (Henry Schein Inc., USA) and a through-and-through puncture was performed with a 21 Gauge needle (Becton Dickinson, USA). Patency of the puncture site was achieved by extrusion of a small amount of intestinal content (1 mm). Next, the cecum was carefully repositioned into the peritoneal cavity. The peritoneal and skin layers of the wound were closed with 3–4 simple interrupted stitches using coated braided silk 6-0 (Tyco Healthcare, USA). Sham-OP animals underwent anesthesia, laparotomy and exteriorization of the cecum as described above, but without ligation and puncture. For fluid resuscitation, all mice received 1 ml sterile NaCl 0.9% subcutaneously directly at the end of the surgery and again on the first postoperative day. Survival was monitored at least every 12 h for 7 days after surgery. The body surface temperature was measured over the lower left quadrant of the abdomen with an YSI 4600 scientific thermometer and YSI 427 small surface probe. For collection of plasma, the mice were deeply anaesthetized by isoflurane inhalation (Minrad Inc., USA). Blood was collected from the retro-orbital sinus using EDTA (5–10 mM) as an anticoagulant and plasma separated by centrifugation (2000g, 10 min, 4°C). Samples were stored at −80°C until further analysis. Spleen organ weight was measured on a Mettler AE 100 digital gram scale.
Clinical Chemistry
Plasma samples were analyzed on an Idexx VetTest 8008 Chemistry Analyzer (Diamond Diagnostics, USA). Normal reference ranges were: creatinine (0.2–0.8 mg/dL), BUN (18–29 mg/dL), ALT (28–132 U/L), AST (59–247 U/L) and LDH (1105–3993 U/L). All plasma samples were obtained 8 h after onset of CLP or after sham surgery.
Photography
Digital images of spleens were acquired with a Cyber-shot DSC-W350 digital camera (Sony Inc., USA) and edited in Adobe Photoshop CS2, Version 9.0.
Measurement of mediator concentrations by Luminex and ELISA
For simultaneous detection of 23 mouse cytokines and chemokines, we used the BioPlex Pro™ mouse cytokine assay, 96-well, 23-Plex Group I (BioRad, USA). The analytes were: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-17, Eotaxin (CCL11), G-CSF, GM-CSF, IFN-γ, KC (CXCL1), MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5) and TNF-α. The EDTA-plasma samples were diluted 10-fold in sample diluent (BioRad). The assay was performed according to the instructions of the manufacturer. For the washing steps, we used an Aurum™ vacuum manifold (BioRad). Samples were analyzed on the Luminex xMAP™/Bioplex-200 System with BioPlex Manager™ Software 5.0. Results for IL-10, G-CSF and KC were confirmed using ELISA kits from R&D systems according to the instructions of the manufacturer.
Flow Cytometry of Splenocytes
Mice were euthanized by CO2 inhalation and spleens removed in sterile working technique. The spleens were placed into cell strainers (100 μm, BD Falcon, USA) and mashed into a 50 ml tube using the plunger end of a sterile 10 ml syringe. The cell strainer was rinsed with 10 ml medium (RPMI 1640 containing 0.1% BSA, 100 U/ml Penicillin-Streptomycin, 25 mM HEPES). After filtering the cell suspension through a 40μm cell strainer, cells were counted in a hemocytometer (Hausser Scientific, USA). After centrifugation (500g, 5 min, 4°C) the cells were resuspended in blocking solution (PBS, 1% normal mouse serum, 0.1% sodium azide) supplemented with Fc Block™ (BD Biosciences). The cells were distributed in 96-well v-bottom plates (Corning, USA) at a concentration of 1 million cells in 100μl per well. Fluorescent antibodies were added at the concentration recommended by the manufacturers followed by incubation for 30 min. All antibodies used were anti-mouse together with matched fluorochrome labeled isotype controls. PerCP-Cy5.5 CD3e (clone 145-2C11), efluor450 CD45 (clone 30-F11), PE CD19 (clone eBio1D3) and isotype controls were purchased from eBioscience, USA. After 2 washing steps the cells were fixated with 2% formaldehyde solution. A minimum of 50,000 events were acquired on a BD LSR II flow cytometer (BD Biosciences, USA) and data were analyzed with the WinList for Win32 3.0 Software (Verity Software Inc., USA).
Statistical analysis
The GraphPad Prism Version 5.01 software was used for figure preparation and statistical analysis. All values are expressed as mean and error bars represent s.e.m. Data sets were analyzed by one-way ANOVA or Student’s t-test. Survival curves were analyzed by the log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests. We considered differences significant when P < 0.05.
RESULTS
Deficiency of CD19+ B cells and CD3+ T cells in Rag-1−/− mice
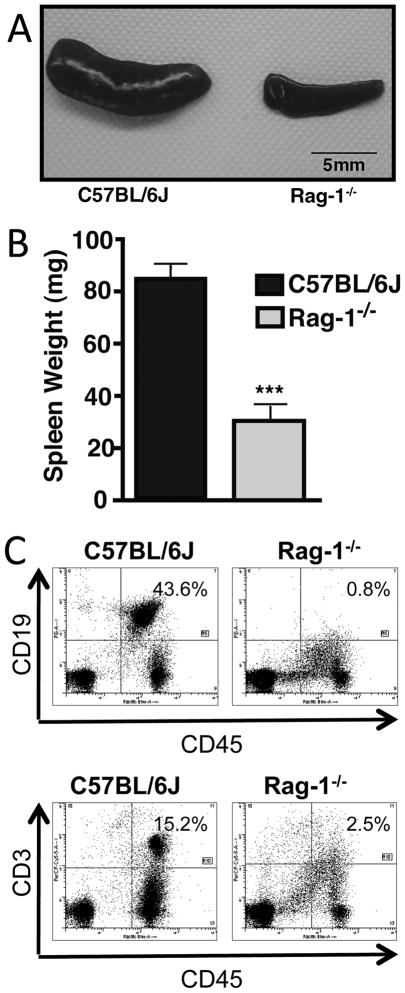
Rag-1−/− mice displayed distinct phenotypic abnormalities when compared to C57BL/6J (wildtype) mice. Specifically, the Rag-1−/− strain showed a reduction in spleen size (Fig. 1A). In wildtype mice the average spleen weight was 84 mg, which was reduced 2.7-fold in Rag-1−/− mice, with an average spleen weight of only 31 mg (Fig. 1B). On a cellular level the abnormalities of the spleen were reflected by the absence of mature B and T cells (Fig. 1C). CD19 was used as a surface marker that is present on all cells of the B cell lineage except mature plasma cells. In populations of splenocytes from untreated normal wildtype mice there were 43.6% CD19+ cells and in spleens from Rag-1−/− mice 0.8%, respectively. As a surrogate marker for T cells, we used CD3 expression. The frequency of CD3+ cells was 15.2% in wildtype and 2.5% in populations of splenocytes from Rag-1−/− mice (Fig. 1C, lower panel).
FIGURE 1.
Phenotypic characteristics of Rag-1−/− mice. (A) Photograph of spleens from a normal untreated C57BL/6J (wildtype) mouse (left) and from a Rag-1−/− mouse (right). (B) Spleen total organ weight from Rag-1−/− mice (n=6) compared to wildtype mice (n=8). (C) Flowcytometric analysis of splenocytes from untreated Rag-1−/− and wildtype mice. Cells were stained for the common leucocyte antigen CD45 and either CD19 for B cells (upper panel) or CD3 for T cells (lower panel). Error bars represent ±SEM and ***P < 0.001, student’s t-test.
Clinical outcome of Rag-1−/− mice and C57BL/6J mice after CLP
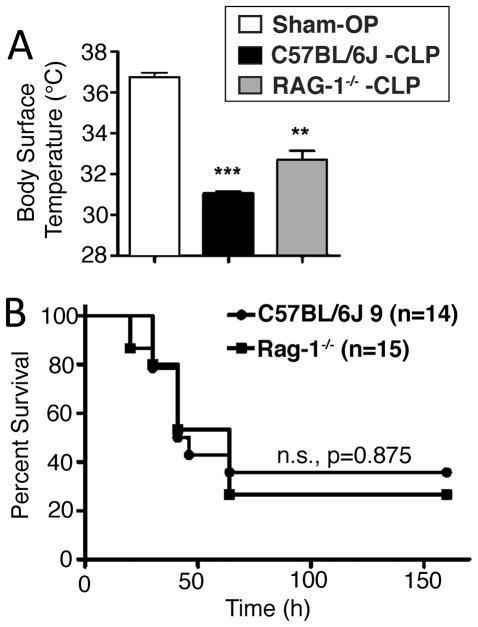
Wildtype mice (C57BL/6J, n=14) and Rag-1−/− mice (n=15) underwent cecal ligation and puncture (CLP) for the induction of polymicrobial sepsis/septic peritonitis. A third group of mice (wildtype, n=3) was subjected to sham surgery, defined as anesthesia with laparotomy without cecal ligation and puncture. All animals were monitored for hypothermia and survival. Hypothermia has been evaluated before by others as an endpoint for CLP and possibly early predictor of morbidity and subsequent death (17). Body surface temperature of all animals was recorded at 8 h after CLP (Fig. 2A). In the sham surgery group the average body surface temperature was 36.7°C (reference range: 36.0–37.0°C), failing to show signs of postoperative hypothermia after the surgical procedure. However, both wildtype mice (31.0°C) and Rag-1−/− mice (32.5°C) displayed a significant decrease in average body surface temperature after CLP compared to the sham surgery group. There was also statistical significance comparing the temperatures of wildtype to Rag-1−/− mice after CLP. The CLP Rag-1−/− mice were slightly less hypothermic than the wildtype mice.
FIGURE 2.
Survival of Rag-1−/− mice after cecal ligation and puncture. (A) Body surface temperature of wildtype mice after Sham-OP (n=3), CLP (n=6) and Rag-1−/− mice (n=6) after CLP, 8h. (B) Survival of wildtype mice (n=14) and Rag1−/− mice (n=15) was monitored for 7 days after CLP surgery. Error bars represent ±SEM and **P < 0.01, ***P < 0.001, student’s t-test.
In addition to hypothermia, mice in both groups after CLP developed progressive clinical signs of sepsis, such as tachypnea, periorbital exudates, lethargy and huddling. Survival rates are shown in Fig. 2B. The death of most animals occurred between day 2 and day 4 after surgery. In total, 5 out of 14 mice of the wildtype strain survived and fully recovered from clinical signs of disease until 8 days after surgery. In the group of the Rag-1−/− strain, 4 out of 15 mice survived. Essentially, survival curves of Rag-1−/− and wildtype mice were superimposable (P=0.875).
Evaluation of organ dysfunction after CLP
To characterize the pathophysiologic changes in organ function, we analyzed several clinical chemistry parameters after CLP or sham surgery (Table 1). Plasma samples were obtained 8 h after CLP or sham surgery. After CLP, there were moderate but not yet significant increases in the levels for the transaminases, ALT and AST. Mice in both the wildtype and Rag-1−/− group displayed signs of azotemia with a 2.5–3-fold increase in BUN levels. In the wildtype group this correlated with a declining glomerular filtration rate (GFR) as shown by a rise of creatinine levels. In the Rag-1−/− mice, creatinine levels remained in the normal range at the time point studied. LDH as a surrogate marker for cellular disintegrity and organ damage rose more than 6-fold in wildtype as well as Rag-1−/− mice. Noteworthy, LDH levels remained within the normal range after sham surgery.
TABLE 1.
Serum Markers
| Sham-OP
|
CLP
|
||
|---|---|---|---|
| C57BL/6J | C57BL/6J | RAG-1−/− | |
| ALT (U/L) | 92.5 ± 16.5 | 158.0 ± 31.4 | 130.7 ± 20.0 |
| AST (U/L) | 197.5 ± 37.5 | 420.3 ± 92.9 | 328.0 ± 69.5 |
| BUN (mg/dL) | 18.0 ± 1.0 | 62.7 ± 5.0** | 50.3 ± 2.9** |
| Crea (mg/dL) | 0.25 ± 0.05 | 0.46 ± 0.03* | 0.23 ± 0.03†† |
| LDH (U/L) | 951 ± 526 | 6410 ± 1252* | 6604 ± 1247* |
Samples were obtained 8 h after onset of CLP or after sham surgery. Data are shown as mean ± SEM.
P < 0.05 vs. Sham-OP
P < 0.01 vs. Sham-OP
P <0.01 C57BL/6J (CLP) vs. RAG-1−/− (CLP)
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase;
BUN, blood urea nitrogen; Crea, creatinine; LDH, lactate dehydrogenase
Levels of chemokines in wildtype and Rag-1−/− mice after CLP
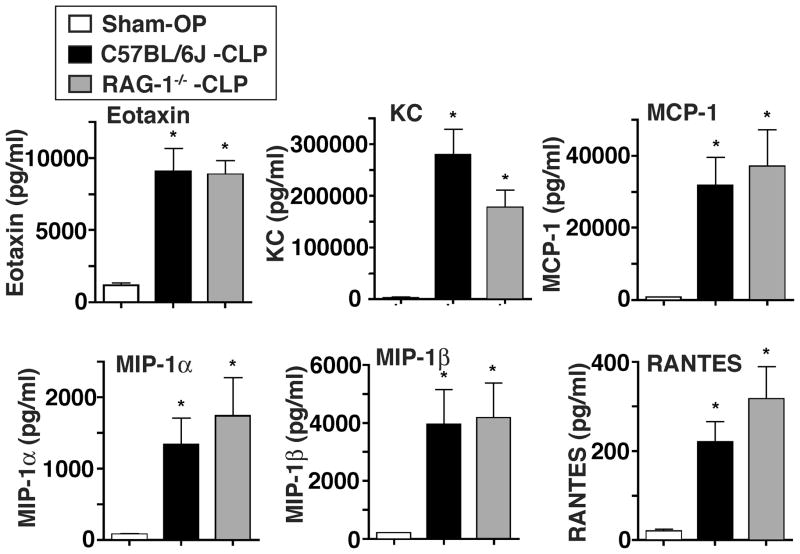
We simultaneously measured the levels of different chemokines after sham surgery or CLP using a bead-based multiplex assay. As shown in Fig. 3 levels of all mediators (eotaxin, KC, MCP-1, MIP-1α, MIP-1β and RANTES) were detectable in low concentrations in mice after sham surgery. Not surprisingly, levels for all chemokines dramatically increased in mice following CLP. These findings were similar in wildtype as well as Rag-1−/− mice after CLP. By far, the highest absolute levels of a chemokine were detected for KC, which averaged 2.2 ng/ml after sham surgery and 279 ng/ml in wildtype mice after CLP and 177 ng/ml in Rag-1−/− mice after CLP. In wildtype mice, this represented a 125-fold increase compared to sham surgery. At the 8 h time point studied, the lowest mediator levels were detected for RANTES, with an average of 220 pg/ml in wildtype mice and 316 pg/ml in Rag-1−/− mice after CLP. The absolute concentrations of RANTES were about 1000-fold lower than levels for KC. Most notably, the elevated levels for eotaxin, KC, MCP-1, MIP-1α, MIP-1β and RANTES after CLP were all not significantly different between wildtype and Rag-1−/− mice.
FIGURE 3.
Characterization of chemokine levels after cecal ligation and puncture. C57BL/6J (wildtype) mice underwent either sham surgery (Sham-OP, n=4) or CLP (n=6). Rag-1−/− mice (n=6) underwent CLP and plasma from all 3 groups was collected after 8 h. Chemokines were simultaneously detected with a bead-based assay. *P < 0.05 vs. Sham-OP. P-values of C57BL/6J-CLP vs. Rag-1−/−-CLP were not significantly different for all analytes shown.
Levels of cytokines in wildtype and Rag-1−/− mice after CLP
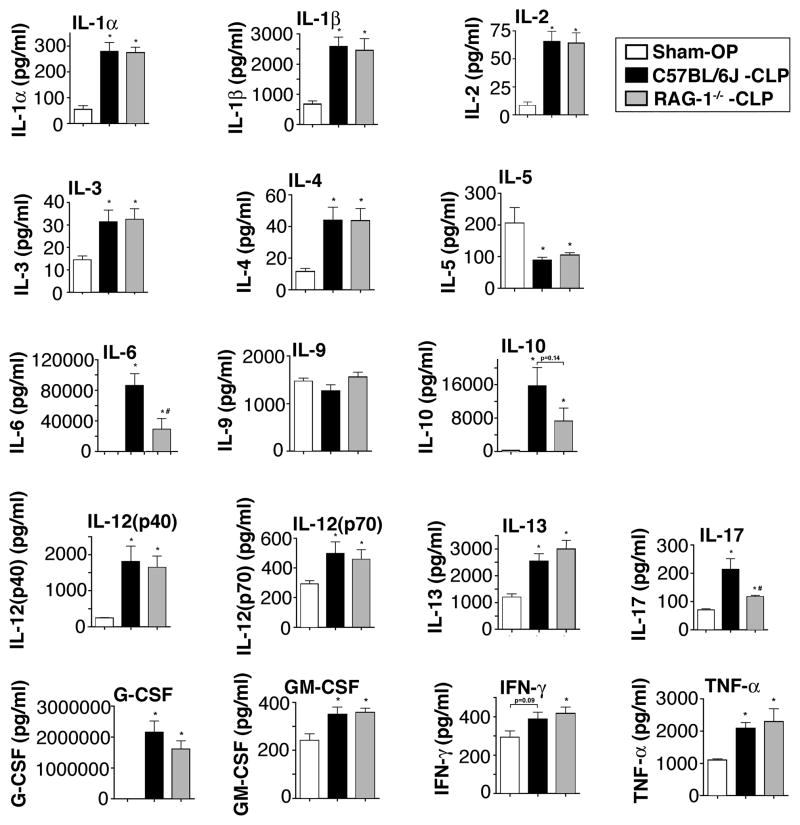
In addition to the aforementioned data, we characterized the levels of 17 cytokines in wildtype mice after sham surgery, CLP and Rag-1−/− mice 8 h after CLP (Fig. 4). We selected the 8 h time point based on our studies that show that most mediators reach a peak at 8 h when high grade CLP is employed (data not shown). Most mediators were found to be more abundant in plasma in the setting of CLP, when compared to sham-operated mice. Surprisingly, plasma levels of IL-5 were diminished about 50% from levels of 200 pg/ml after sham surgery, falling to approximately 100 pg/ml in wildtype and Rag-1−/− after CLP. For IL-9, plasma levels did not change after CLP. The levels of GM-CSF and Interferon-γ levels were only moderately elevated in septic mice. The cytokines IL-2, IL-3 and IL-4, although induced after CLP, were present in low quantities (<100 pg/ml), with virtually no differences in plasma samples from wildtype and Rag-1−/− mice. The most abundant mediator to be found was G-CSF. After sham surgery mean G-CSF levels were elevated from 2.3 ng/ml to 2150 ng/ml after CLP in wildtype mice. This meant a more than 900-fold increase in the G-CSF levels for the septic wildtype mice. Very high mediator levels were also detected for IL-6 (86 ng/ml) and IL-10 (16 ng/ml) after CLP. Interestingly, IL-6 levels in Rag-1−/− mice were only 34% of levels in wildtype mice after CLP. A trend of lower IL-10 levels in the Rag-1−/− group did not quite reach statistical significance. However, Rag-1−/− mice displayed a significant 50% reduction in the values for IL-17. Overall, 14 out of the 17 cytokines were present in virtually indistinguishable levels in the wildtype and Rag-1−/− group after CLP.
FIGURE 4.
Characterization of cytokine levels after cecal ligation and puncture. C57BL/6J (wildtype) mice underwent either sham surgery (Sham-OP, n=4) or CLP (n=6). Rag-1−/− mice (n=6) underwent CLP and plasma from all 3 groups was collected after 8 h. Mediators were simultaneously detected with a bead-based assay. *P < 0.05 vs. Sham-OP, #P < 0.05 C57BL/6J-CLP vs. Rag-1−/−-CLP.
DISCUSSION
T and B cells are the essential cellular components of adaptive immunity. The aim of the current study was to investigate the role of these cells in polymicrobial sepsis after CLP. The phenotyping of the Rag-1−/− mice used in our experiments confirmed atrophy of the spleen due to the virtual absence of CD3+ T cells and CD19+ B cells. These and similar findings have been established by other investigators before (12,18,19). After CLP clinical chemistry analytes, namely transaminases, BUN, creatinine and LDH levels increased in comparison to the animals with sham surgery. Although the rise in transaminases was not significant at the 8 h time point studied, it can be assumed that progressive liver failure develops in later stages of polymicrobial sepsis. Rag-1−/− mice maintained lower creatinine levels, which may suggest that decline of glomerular filtration rate (GFR) and loss of renal function is delayed in the absence of T and B cells. It should be noted, that creatinine levels closely correlate with GFR in a steady state situation, which most likely does not exist during the early phases of sepsis. Mice undergoing CLP rapidly developed clinical signs of septic shock, including severe hypothermia, with only moderate differences between wildtype and Rag-1−/− mice. Most importantly, survival curves were strikingly similar in the genetic absence of T and B cells, employing a severe technique in the CLP model, typically resulting in 60–80% mortality rates (20). This finding is consistent with another report, that Rag-1−/− mice after CLP did not die prematurely and displayed no apparent observable differences in severity of illness during sepsis, although in this study mice were only monitored for 24h after CLP (13). The comparable survival patterns of C57BL/6J and Rag-1−/− mice are consistent with our results obtained for the characterization of the ‘cytokine storm’ in both strains. The levels for most mediators were unaffected in the case of T and B cell deficiency, when compared to CLP wildtype mice. Only the concentrations of IL-6 and IL-17 were markedly reduced in the Rag-1−/− strain. This is consistent with the idea that T cells are a major source for IL-17, and we have recently shown that γδ T cells appear to be the source of IL-17A in the setting of CLP (16). However, cellular sources other than T cells exist for IL-17. For example, in the endotoxemia model, macrophages might be more important as early producers of IL-17A (Bosmann and Ward, unpublished findings). Reduced levels of the proinflammatory mediator IL-6 and IL-17 in Rag-1−/− mice may be counterbalanced by a concomitant trend towards reduced levels of the anti-inflammatory cytokine IL-10. In both wildtype and Rag-1−/− mice the levels for G-CSF were extraordinary high, suggesting that this mediator may be involved in the physiological responses to CLP. G-CSF has long been known to activate neutrophils and promote granulopoiesis in the bone marrow as well as neutrophilia in the peripheral blood. This effect is routinely employed using G-CSF administration to hemato-oncologic patients with chemotherapy induced neutropenia, who are very prone to develop infectious complications and subsequent sepsis. During sepsis, the influx of innate immune cells to the site of infection is orchestrated by chemokines such as KC (IL-8 in humans), MIP-1α and MIP-1β to direct the migration of neutrophils and MCP-1 for chemoattraction of macrophages/dendritic cells. We found all these mediators to be released in high concentrations after CLP, this being independent of the presence of T and B cells. On the other hand, RANTES, which is a major chemokine to attract T cells, was present in 1000-fold lower quantities after CLP.
In contrast to our findings, other studies have reported a higher mortality of Rag-1−/− mice in experimental sepsis (15,21). One report used a model of colon ascendens stent peritonitis (CASP) instead of CLP. Notably, the mortality after the CASP procedure occurred within 1–2 days, which is much more rapid than in the CLP model. The authors of that study performed intra-peritoneal fluid resuscitation during the CASP procedure, which may accelerate spreading of fecal bacteria. Rag-1−/− mice may also be more susceptible to polymicrobial sepsis than wildtype mice, when a two-step model of burn injury followed 10 days later by CLP is used (22). In an earlier report, Rag-1−/− mice after CLP had a survival of only 5% compared to 45% in the control group (C57B46 mice). In comparison to our experimental design, that group had used antibiotics (metronidazole, ceftriaxone), leading to mortality occurring at later time points (3–4 days) after surgery and likely involving a more prolonged hyperinflammatory phase (21). Apparently contradictory results in the CLP model may be related to the wide variability of this model with survival outcome dependent on severity of the surgery and the regimen for post-surgical use of antibiotics and fluid resuscitation (23,24). In addition, age and sex of mice must be carefully matched for comparing the survival after CLP and inbred strain background effects may further complicate the interpretation of results (25,26).
It is undisputed that lymphocytes participate in the complex immune response during the course of sepsis. We speculate that, with the complete absence of T and B cells, the potential favorable and detrimental properties of these cells in polymicrobial sepsis are being counterbalanced. T and B cells are very heterogeneous cell populations. It may well be that approaches specifically addressing the role of lymphocyte subsets such as CD4+, CD8+, αβ T, γδ T, Treg cells and B cells come to diverse conclusions (14, 27). For example, CD4 cell deficient mice displayed reduced survival rates after CLP when compared to CD8 cell deficient mice, although differences between CD4 deficient mice and wildtype mice were only significant 30 h after CLP and not after 192 h (27). The extensive apoptosis of lymphocytes and non-lymphocytic cells during sepsis has been intensively investigated (28–31). Furthermore, lymphocytes possibly come into play for the later phases of sepsis, especially the stages of recovery from acute disease. Indeed, it will be interesting to further elucidate to what extent the apoptotic loss of lymphocytes, including memory T and B cells, has long term effects with respect to immune suppression occurring after survival of sepsis (32,33). Recent studies also indicate that immune suppression following sepsis appears to involve Treg cells (34,35). Furthermore, experimental evidence supports the idea that individuals after sepsis are at a higher risk not only for a recurrent infectious episode but also possibly neoplasms (36). It remains to be seen if these and future findings on the role of lymphocytes during sepsis will lead to new treatment options for this disease and the means to prevent secondary complications such as recurrent infections or tumor growth.
Acknowledgments
The authors thank Rachel Voight and Mike Haggadone for technical assistance. We cordially thank Beverly Schumann and Sue Scott for assistance in the preparation of the manuscript and Robin Kunkel for assistance in the preparation of the figures.
Footnotes
Funding Disclosure: This study was supported by grants from the National Institutes of Health (GM-29507, GM-61656, to P.A.W.), the Deutsche Forschungsgemeinschaft (project 571701, BO 3482/1-1, to M.B.) and the Boehringer Ingelheim Fond (to N.F.R).
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.[see comment] Critical Care Medicine. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nature Reviews Immunology. 2008;8:776–87. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. New England Journal of Medicine. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 5.de Jong HK, van der Poll T, Wiersinga WJ. The systemic pro-inflammatory response in sepsis. Journal of Innate Immunity. 2010;2:422–30. doi: 10.1159/000316286. [DOI] [PubMed] [Google Scholar]
- 6.Ward PA. The dark side of C5a in sepsis. Nature Reviews Immunology. 2004;4:133–42. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 7.Nagai Y, Shimazu R, Ogata H, Akashi S, Sudo K, Yamasaki H, Hayashi S, Iwakura Y, Kimoto M, Miyake K. Requirement for MD-1 in cell surface expression of RP105/CD180 and B-cell responsiveness to lipopolysaccharide. Blood. 2002;99:1699–705. doi: 10.1182/blood.v99.5.1699. [DOI] [PubMed] [Google Scholar]
- 8.Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, Cohen-Sfady M, Pevsner-Fischer M, Lider O, Cohen IR. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. Journal of Immunology. 2007;179:41–4. doi: 10.4049/jimmunol.179.1.41. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nature Reviews Immunology. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Critical Care Medicine. 1999;27:1230–51. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–53. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: Findings in normal and T- and B-cell-deficient mice. Critical care medicine. 1997;25:1298–307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Stromberg PE, Woolsey CA, Clark AT, Clark JA, Turnbull IR, McConnell KW, Chang KC, Chung CS, Ayala A, Buchman TG, Hotchkiss RS, Coopersmith CM. CD4+ lymphocytes control gut epithelial apoptosis and mediate survival in sepsis. FASEB Journal. 2009;23:1817–25. doi: 10.1096/fj.08-119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reim D, Westenfelder K, Kaiser-Moore S, Schlautkotter S, Holzmann B, Weighardt H. Role of T cells for cytokine production and outcome in a model of acute septic peritonitis. Shock. 2009;31:245–50. doi: 10.1097/SHK.0b013e31817fd02c. [DOI] [PubMed] [Google Scholar]
- 16.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL, Ward PA. Adverse functions of IL-17A in experimental sepsis. FASEB Journal. 2008;22:2198–205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 17.Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG. Humane endpoints in shock research. Shock. 2004;21:17–25. doi: 10.1097/01.shk.0000101667.49265.fd. [DOI] [PubMed] [Google Scholar]
- 18.Mombaerts P. Lymphocyte development and function in T-cell receptor and RAG-1 mutant mice. International Reviews of Immunology. 1995;13:43–63. doi: 10.3109/08830189509061737. [DOI] [PubMed] [Google Scholar]
- 19.Lebleu VS, Sugimoto H, Miller CA, Gattone VH, 2nd, Kalluri R. Lymphocytes are dispensable for glomerulonephritis but required for renal interstitial fibrosis in matrix defect-induced Alport renal disease. Laboratory Investigation. 2008;88:284–92. doi: 10.1038/labinvest.3700715. [DOI] [PubMed] [Google Scholar]
- 20.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature Protocols. 2009;4:31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–56. [PubMed] [Google Scholar]
- 22.Shelley O, Murphy T, Paterson H, Mannick JA, Lederer JA. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20:123–9. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 23.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24 (Suppl 1):52–7. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 25.Schneider CP, Nickel EA, Samy TS, Schwacha MG, Cioffi WG, Bland KI, Chaudry IH. The aromatase inhibitor, 4-hydroxyandrostenedione, restores immune responses following trauma-hemorrhage in males and decreases mortality from subsequent sepsis. Shock. 2000;14:347–53. doi: 10.1097/00024382-200014030-00019. [DOI] [PubMed] [Google Scholar]
- 26.Angele MK, Wichmann MW, Ayala A, Cioffi WG, Chaudry IH. Testosterone receptor blockade after hemorrhage in males. Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg. 1997;132:1207–14. doi: 10.1001/archsurg.1997.01430350057010. [DOI] [PubMed] [Google Scholar]
- 27.Martignoni A, Tschop J, Goetzman HS, Choi LG, Reid MD, Johannigman JA, Lentsch AB, Caldwell CC. CD4-expressing cells are early mediators of the innate immune system during sepsis. Shock. 2008;29:591–7. doi: 10.1097/SHK.0b013e318157f427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis--a potential treatment of sepsis? Clinical Infectious Diseases. 2005;41 (Suppl 7):S465–9. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scandinavian Journal of Infectious Diseases. 2003;35:585–92. doi: 10.1080/00365540310015692. [DOI] [PubMed] [Google Scholar]
- 30.Hoogerwerf JJ, van Zoelen MA, Wiersinga WJ, van ’t Veer C, de Vos AF, de Boer A, Schultz MJ, Hooibrink B, de Jonge E, van der Poll T. Gene expression profiling of apoptosis regulators in patients with sepsis. Journal of Innate Immunity. 2010;2:461–8. doi: 10.1159/000317035. [DOI] [PubMed] [Google Scholar]
- 31.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 32.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infection & Immunity. 2010;78:1582–92. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. Journal of Experimental Medicine. 2007;204:1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Critical Care Medicine. 2003;31:2068–71. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 35.Wisnoski N, Chung CS, Chen Y, Huang X, Ayala A. The contribution of CD4+ CD25+ T-regulatory-cells to immune suppression in sepsis. Shock. 2007;27:251–7. doi: 10.1097/01.shk.0000239780.33398.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavassani KA, Carson WFt, Moreira AP, Wen H, Schaller MA, Ishii M, Lindell DM, Dou Y, Lukacs NW, Keshamouni VG, Hogaboam CM, Kunkel SL. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115:4403–11. doi: 10.1182/blood-2009-09-241083. [DOI] [PMC free article] [PubMed] [Google Scholar]