Abstract
Background
Excessive complement activation is an integral part of ischemia and reperfusion (IR) injury (IRI) of organs. In kidney transplantation the pathological consequence of IRI and complement activation can lead to delayed graft function which in turn is associated with acute rejection. Previous strategies to reduce complement induced IRI required systemic administration of agents, which can lead to increased susceptibility to infections/immune diseases. The objective of this study was to determine whether an increase in complement control defenses of rat kidney endothelium reduces IRI. We hypothesized that increased complement control on the endothelial barrier reduces IR-mediated complement activation and reduces kidney dysfunction.
Materials and methods
Fisher 344 rats underwent left kidney ischemia for 45 min. and treatment with a novel fusogenic lipid vesicle (FLVs) delivery system to decorate endothelial cells with Vaccinia virus complement control protein (VCP), followed by reperfusion for 24h. Assessment included renal function by serum creatinine and urea, myeloperoxidase assay for neutrophil infiltration, histopathology, and quantification of C3 production in kidneys.
Results
Animals in which the kidney endothelium was bolstered by FLVs+VCP treatment had better renal function with a significant reduction in serum creatinine as compared to vehicle controls. C3 production was significantly reduced (p<0.05) in treated animals compared to vehicle controls.
Conclusion
Increasing complement control at the endothelial barrier with FLVs+VCP modulates complement activation/production during the first 24h, reducing renal dysfunction following IRI.
Keywords: Ischemia-reperfusion injury, complement, transplantation, fusogenic lipid vesicles, vaccinia virus complement control protein
Introduction
Ischemia and reperfusion (IR) injury (IRI) is an unavoidable problem that accompanies several clinical situations and is often associated with devastating consequences and increased morbidity. Excessive complement activation following reperfusion of ischemic tissues is well documented in a variety of organs and is increasingly being recognized as a central mediator of reperfusion injury. Complement mediation has been shown to play a significant role in acute kidney injury, myocardial infarction, strokes and is also associated with primary dysfunction and delayed function of transplanted organs. [1–7]
The vascular endothelium is an important barrier, which offers protection from harmful stimuli and maintains normal homeostasis throughout the body. A key feature of IRI is the disruption of this vital barrier during reperfusion resulting in the activation of endothelium and other pro-inflammatory cells.[8–10] This triggers a set of events which involves production of inflammatory mediators, adhesion molecules, cytokines, and enhanced complement activation and deposition on the cell membranes.[11]
The majority of the complement components in the body are synthesized by the liver and are found circulating in the blood; however, specialized cells in different tissues are also capable of synthesis of complement components.[12–16] Recent evidence in animal models of kidney IRI suggests that local production of complement is a main contributor of tissue injury.[1] Nevertheless, during early reperfusion of ischemic kidneys, spontaneous activation of the alternate pathway in blood leads to cleavage of C3 into C3a and C3b, with the latter being deposited on the cell membranes and serving as a marker for macrophage cleanup.[3;17] Further propagation of the complement cascade leads to formation of C5a (a potent anaphylatoxin), and C5b which begins the assembly of the C5b-9 pore, also known as the membrane attack complex (MAC), capable of lysing cells.[18] More recently, others have shown in mice and swine models of kidney IR involvement of the lectin pathway and classical pathway of complement activation, respectively.[19–21] Irrespective of the pathway complement activation leads to amplification of the inflammatory response ultimately resulting in organ damage.
Several complement inhibitors have been employed to reduce IRI in animal models by blocking upstream to C3 production or further along the complement cascade. Such agents include therapy with C1-INH which has been shown to be beneficial in intestinal IRI, myocardial infarction, and ischemic brain injury. [22–24] Soluble complement receptor 1 (sCR1), a complement inhibitor which accelerates the decay of C3 and C5 convertase has been shown to be cardio-protective, effective in reducing hepatic damage following ischemia and reperfusion, and also in reducing vascular injury in renal allografts.[25–27] C5 was targeted in another study using a monoclonal antibody BB5.1 which protected the kidney from IRI. [28] In this study, the antibody prevented the activation of C5 and subsequent generation of MAC. More recently, newer agents have been developed to target the receptors with antagonists such as C5a receptor antagonist (A8 Δ71–773), which was shown to be beneficial in early kidney graft survival.[7]
The majority of the agents described above require systemic administration, which in certain situations such as in immunosuppressed kidney recipients further suppresses the innate immune system leaving the patients susceptible to infection. In order to avoid this potential complication, targeted therapies using second generation anti complement inhibitors are being developed.
In this study, we used a novel therapeutic approach that targets the endothelial barrier, which is the primary site involved in the inflammatory response following IR. This therapy is applied in a two-step process as previously reported,[29] first a solution containing fusogenic lipid vesicles (FLVs) is perfused via the renal artery to incorporate bivalent metal tethers on the surface of endothelial cells throughout the renal vasculature. We then used a second perfusion solution containing a modified vaccinia virus complement control protein (VCP) with a hexahistidine tag to couple with the metal tether. VCP is a highly potent complement inhibiting protein produced by vaccinia virus, which binds to C3b and C4b in the presence of factor I and prevents the formation of C3 convertase of both the alternate and classical pathways.
The purpose of this study was to determine whether the enhancement of endogenous complement control on the endothelial barrier using our targeted FLVs+VCP therapy was capable of reducing excessive complement activation and the inflammatory response during reperfusion in a rat kidney model of IRI. Here, we report that enhancement of complement control at the level of the endothelium partially mitigated the inflammatory response and reduced the degree of renal dysfunction in the first 24 h of reperfusion.
Materials and methods
Animals and groups: Male Fisher 344 rats (RT1lv) 8–12 weeks old were purchased from Harlan (Indianapolis, IN). All the animals were housed in the animal care facility and the procedures were in accordance to NIH guidelines for animal research and approved by the Institutional Animal Care and Use Committee of University of Louisville. The animals were assigned into the following treatment groups (n=6/group): Sham, vehicle control (lactated Ringer’s solution), FLVs only, VCP only and FLVs + VCP.
Renal IRI
Under isofluorane anesthesia and midline abdominal incision right nephrectomy was first performed. The left kidney underwent 45 min of ischemia by clamping the left renal artery. The artery was then cannulated and the kidney flushed with lactated Ringer’s solution. The effluent was collected through an adjacent venotomy in the renal vein. Sham animals underwent laparotomy only with 45 min of observation. Animals received treatment according to the groups described above with an incubation period of 15 min each for lactated Ringer’s solution, FLVs and VCP. Following repair of vessels the kidney was allowed to reperfuse for a period of 24 h. The animals were euthanized the next day with an overdose of intra-peritoneal pentobarbital and kidneys were harvested.
Renal function tests
Blood samples were collected before surgery and at 24 h after reperfusion of the treated kidney. Samples were centrifuged and serum creatinine and urea were measured according to the manufacturer’s instructions with Quantichrome™ Creatinine and Urea Assay Kits, DICT-500 and DIUR-500 (BioAssay Systems, Hayward, CA).
Histology
Treated kidneys were harvested at 24 h and three samples i.e. two coronal and one transverse were fixed with Safefix II (10.0% v/v Ethanol SDA-3A), 18.0% v/v Potassium Biphthalate, 1.42% w/v deionized water) Fisher Scientific Company L.L.C (Kalamazoo MI). Paraffin embedded specimens were cut at 6μ thickness and stained with haematoxylin and eosin. Histopathology was evaluated by a pathologist blinded to the treatment groups. An IRI scoring system developed by Jablonski was used to grade the kidney injury from Grades 1–4.[30] Briefly, Grade 1 = Mitosis and necrosis of individual cells; Grade 2 = Necrosis of all cells in adjacent proximal tubules; Grade 3 = Necrosis confined to distal third of proximal tubules with a band of necrosis extending through inner cortex; Grade 4 = Necrosis involving all three segments of the proximal tubules.
Myeloperoxidase (MPO) assay
MPO activity was measured as described before.[31] Briefly, 100mg of tissue was homogenized and diluted in 50 mM potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide, pH 6.0. After sonication and two freeze thaw cycles, samples were centrifuged at 400×g for 30 min. The supernatants were reacted with H2O2 (0.3 mM) in the presence of tetramethylbenzidine (1.6 mM). MPO activity was assessed using SpectraMax M2e (Molecular Devices, Sunnyvale, CA) plate reader by measuring the change in absorbance at 655 nm with human MPO as standard.
Western blotting
C3 deposition in the treated kidneys was quantified by western blot analysis. Briefly, kidney tissue homogenates were prepared using protein extraction buffer (0.01M cacodylic acid pH 5.0, 0.15 M Nacl, 1μM ZnCl2, 0.02 M CaCl2, 0.0015 M NaN3, and 0.01% vol/vol Triton X-100) over a period of 48 hours.[32] The extracted protein was collected, sonicated at 8,000 rpm for 5 min and 0.1 M Tris was added to raise the pH to 7.5. Protein content was determined using BCA protein assay kit (Thermo Scientific, Rockford, IL). Equal amounts of protein were separated on 12% Tris-HCl gels (Bio-rad) by electrophoresis, transferred onto polyvinylidene fluoride (PVDF) membrane. For C3 deposition the membrane was blocked for 1 hour with 2.5% nonfat dry milk and incubated with C3 monoclonal antibody (C3 (B-9) SC-28294 Santa Cruz Biotechnology, Inc, CA) at 1/100 dilution. The membrane was washed and incubated for 1 h at room temperature with a 1/1200 dilution of goat anti-mouse HRP-labeled IgG ((W4021), Promega Corp, WI). The membranes were developed using chemiluminescence (Western Lighting™ Plus-ECL (PerkinElmer Inc, MA)) and visualized by autoradiography.
Statistical analysis
Values are given as means ± SEM for each group. Two-way ANOVA was performed to identify difference between the groups followed by post-hoc pairwise comparisons using the Tukey method. A p value of <0.05 was considered significant.
Results
Renal function protection following IRI by FLVs+VCP treatment
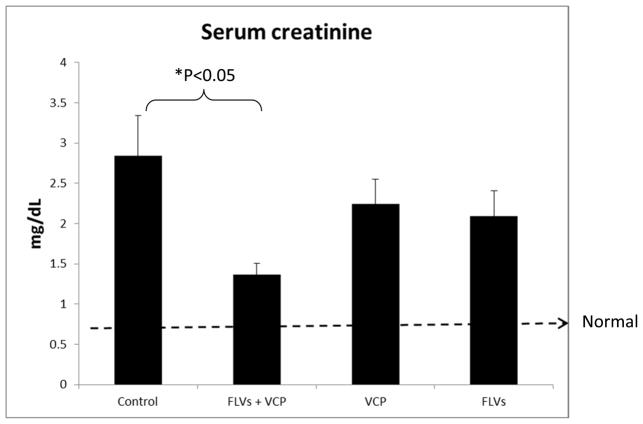
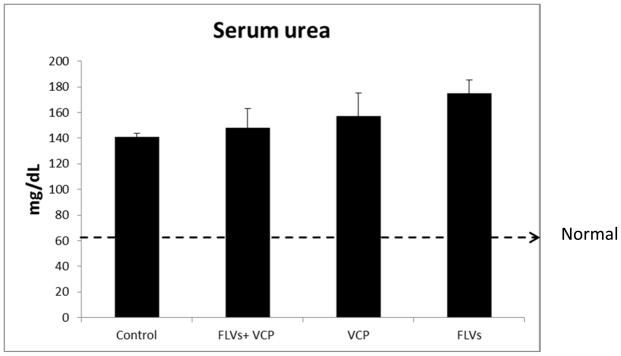
Serum creatinine and urea levels were quantified to evaluate renal function 24 h after IRI. We observed that in the animals treated with FLVs+VCP serum creatinine levels were significantly decreased (P<0.05) as compared to the control animals which received lactated Ringer’s solution (Fig. 1). In the control animals’ serum creatinine levels at 24 hours were increased 6 fold from baseline as compared to less than 3-fold in the FLVs+VCP treated group. Creatinine clearance in the FLVs+VCP group was 52% better than in controls. Animals treated either with FLVs or VCP alone also showed a modest decrease in creatinine of 26.51% and 20.85%, respectively, as compared to the control animals indicating minimal beneficial effect. However, therapy with VCP did not seem to affect the serum urea values which remained elevated suggesting poor urea clearance (Fig. 2).
Figure 1.
Animals treated with FLVs+VCP (Fusogenic lipid vesicles and Vaccinia virus complement control protein) maintain better renal function. Creatinine was analyzed from blood samples collected before inducing IRI and after 24h of reperfusion. Control animals which received lactated Ringer’s solution only, show 7 fold increase in serum creatinine as compared to <3 fold in the FLVs+VCP group (*P<0.05). Values are represented as means±SEM (n=6/group).
Figure 2.
There was no difference in the serum urea levels between the groups. Serum urea was measured similar to creatinine as described in Figure 1. Values are represented as means±SEM (n=6/group).
Histological assessment of severity of IRI in proximal tubules
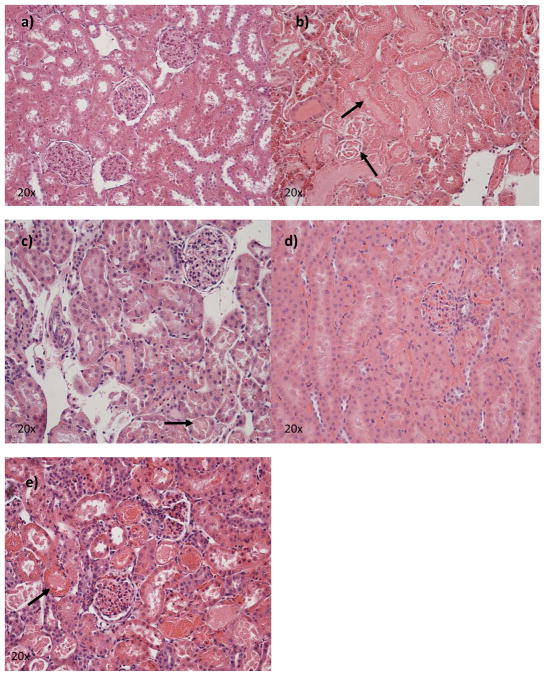
In order to assess the morphological changes associated with the different treatments we analyzed three histological sections one coronal and two sagittal. Figure 3 shows representative samples from each group. In the animals which received FLVs+VCP there was lesser degree of tubular damage in the cortex and cortico-medullary junction (Grade 3) as compared to the controls which showed severe damage (Grade 4). Control animals also showed extensive areas of focal necrosis. VCP therapy by itself showed decreased damage when compared to either of the groups above (Grade 2) however this did not correlate as well with the renal function, which was better maintained with FLVs+VCP treatment. Similar findings were noted in the FLVs therapy group (Grade 2).
Figure 3.
Kidney samples were collected as described in the materials and methods section. Kidney injury was scored according to Jablonski’s criteria Grades 1–4. Representative histology pictures at 20x from: a) sham rats, b) control kidney (lactated Ringer’s solution perfusion) shows extensive damage of the tubules and glomeruli including necrosis (arrows), c) FLVs+VCP treated kidney shows preservation of normal architecture with moderate tubular damage (arrow) and lesser neutrophil infiltration, d) VCP treated animal shows minimal damage and decreased neutrophil infiltration, and e) FLVs treated kidney shows moderate injury and neutrophil infiltration.
Attenuation of IR induced increase in tissue neutrophils
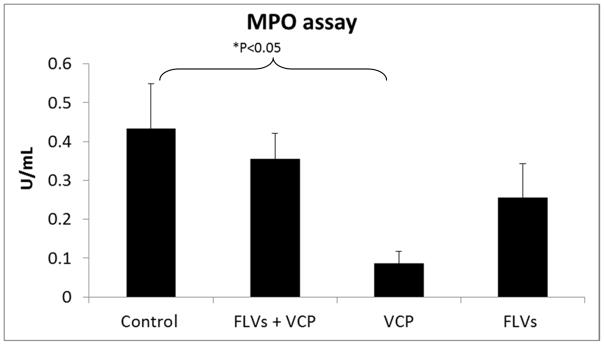
MPO level in the tissue is a specific index of neutrophil accumulation. MPO assay was conducted to determine if neutrophil recruitment to the sites of inflammation was decreased by inhibiting complement activation and deposition with FLVs+VCP treatment. In the animals treated with VCP alone there was a significant reduction in the MPO activity (P<0.05), and in the FLVs+VCP and FLVs group we observed a non-significant attenuation (Fig. 4). In contrast, control group showed increased MPO activity indicating marked neutrophil influx.
Figure 4.
Effect of treatments on MPO activity as a marker for neutrophil infiltration into the tissues. VCP therapy by itself shows significantly reduced MPO activity (P<0.05) and treatment with FLVs+VCP and FLVs shows a downward trend. Control group shows increased levels indicating marked neutrophil influx. Values are represented as means±SEM (n=6/group).
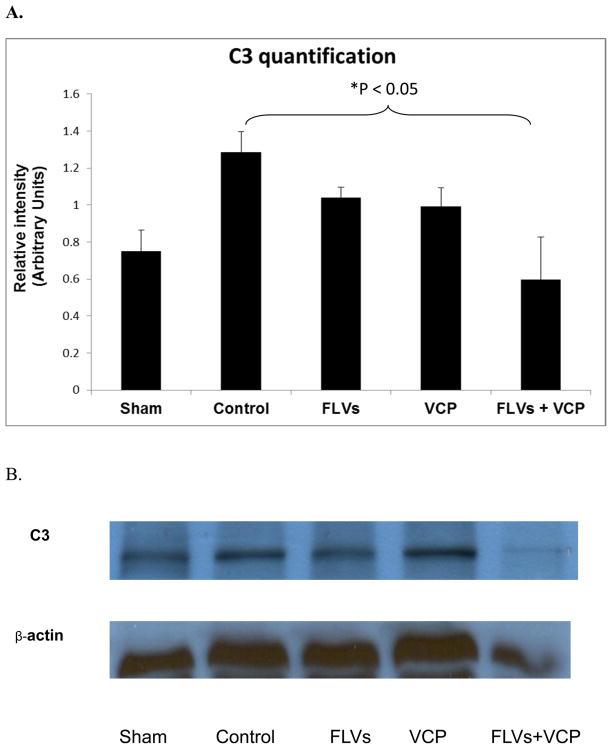
Western blot analysis of C3 deposition
To investigate if treatment with FLVs+VCP decreases activation/production of C3 and deposition of C3b we performed western blot analysis. C3a and C3b are active products produced by cleavage of C3 by C3 convertase. We measured the level of C3 and found that the control group expressed high levels of C3 whereas treatment with FLVs+VCP decreased these levels significantly (Fig. 5). This result demonstrated that FLVs+VCP treatment was able to suppress C3 production and deposition in the tissues.
Figure 5.
A. Graph of Western blot analysis comparing the deposition of C3 in kidneys from all groups (n=3/group). Excessive C3 deposits in the kidney homogenates was significantly decreased in the FLVs+VCP treated group as compared to control (P<0.05). B. Representative blot from a complete set of experiments.
Discussion
Reperfusion of ischemic organs can lead to significant damage with loss of function and this injury has been attributed in part to excessive complement activation that occurs during this period. Complement-induced injury is further exacerbated by the shedding of endogenous complement control proteins from endothelial cells during reperfusion.[33–35] In organ transplantation complement activation can occur after prolonged periods of ischemia followed by reperfusion or when organs are harvested from non-heart beating donors. Delayed graft function has a higher incidence in both the above clinical scenarios and is an independent predictor of acute graft loss. In view of the predominant role complement plays in IRI, targeting these serine proteases is an option to reduce this injury. In this study, our results show that an endothelial membrane targeted therapy against C3 has the potential to inhibit complement activation and deposition thereby reducing the extent of damage the organ sustains during IRI.
In this study, we used a novel approach that enhances the endothelium’s complement control in renal vasculature with VCP a potent exogenous C3 convertase inhibitor. We chose VCP because recent evidence indicates involvement of alternate, classical and lectin pathways in renal IRI in different animal models,[3;21] and since VCP has the ability to inhibit all three major complement pathways at the C3 convertase step, it was the ideal agent. The ability of VCP administered systemically to offer protection from the adverse effects of complement activation has been demonstrated in acute settings such as cranial trauma, spinal cord injury and xenotransplantation.[36–38] However, tissue retention is a limiting factor which can vary with the dosage and route of administration, as a result in clinical situations requiring continued complement suppression it warrants repeated and high dose administration.[39] Kotwal et al, reported using 125 mg/kg of VCP by intraperitoneal injection in one group of animals undergoing unilateral renal IRI and 4 mg/kg in another group undergoing bilateral renal IRI.[40] This was followed by an additional dose given intravenous to achieve complete complement suppression. In contrast, we found that using our technique a significantly smaller single dose (0.8 mg/kg) applied directly to the kidney was sufficient to anchor an effective therapeutic level of VCP. In addition, due to the therapy being localized it prevents systemic suppression of complement thereby maintaining normal host defenses against potential infectious threats.
When assessing the damage caused by IRI in an organ, the most important parameters to be considered are organ survival and function. In the present study, renal function test was considered as the best indicator of organ damage particularly serum creatinine because this is widely used as an indirect measure of glomerular filtration rate even in clinical situations. The other features of renal IRI include morphological changes which are most evident in the proximal convoluted tubules. In our study, animals treated with FLVs+VCP and VCP without FLVs had a modest improvement in histological grade in comparison with controls, which had severe necrosis. Although VCP therapy without FLVs showed less morphological changes it contrasted with renal function when compared with the FLVs+VCP treated group. This beneficial effect of VCP without FLVs may possibly be due to VCP’s heparin binding domain which allows binding to polyanions present on the surface of the endothelium thereby serving as a complement regulator.[41;42] Based on Jablonski criteria although the FLVs+VCP treated animals showed grade 3 injuries they maintained better renal function than the controls and VCP group. In some cases a direct relationship between histological findings and organ function can be difficult to establish because of the large compensatory ability of the kidney.[30;43;44] In the present study, the scoring system used was confined to the presence of necrosis in individual cells or across segments of proximal tubules. Weight et al. developed an alternative scoring system which looked at several individual parameters both in the cortex and medulla.[45] In their study animals which underwent >60 minutes of ischemia had significant morphological changes associated with worsening renal function. However, if the present (Jablonski) scoring were applied to their study the histology scores would have been normal or grade 1.[45] In general, there is a direct correlation between the severity of lesion and poor renal function as the ischemia period increases. However when assessing the efficacy of any new therapy all the other variables should also be considered, and in such situation, we feel that organ function and survival are more valid parameters.
Another important finding in this study was a significantly decreased amount of C3 deposition in the kidney as confirmed by western blotting assay. This was in agreement with the other findings seen with better maintenance of renal function and histological findings. Recently, local synthesis of complement as the main cause of renal injury has been gaining importance.[12;46] Farrar et al. reported that when C3 deficient renal isografts are transplanted into C3 positive recipients minimal injury in transplanted kidneys occurs.[1] In contrast, when C3 positive kidneys are transplanted into C3 positive or C3 negative recipients extensive damage is observed. Based on this data they concluded that local synthesis of complement contributed significantly while the circulating complement had no role in renal IRI.[1] When this is taken into consideration with our findings of decreased C3 deposition in tissues, it appears that enhancing complement control on the endothelial barrier may be reducing C3 expression in the interstitium suggesting a potential modulatory mechanism by the complement suppression in the vascular compartment during IRI.
Recruitment of pro-inflammatory cells showed a non-significant downward trend in the FLVs+VCP and FLVs groups, and did not achieve significance as in similar studies.[28;47] However, VCP treatment by itself was effective in reducing the number of infiltrating neutrophils as compared to the vehicle control. Perhaps a larger sample size for this variable would have shown statistical significance for all groups vs. controls.
More recently the focus in treatment for complement mediated IRI has shifted towards development of targeted therapy for reasons mentioned earlier. Some of these agents include sCR1sLe, a combination drug targeting complement receptor 1 and selectin, and a membrane localizing agent ATP070. sCR1sLe has been shown to reduce IRI after lung transplantation and cardio-protective following myocardial infarction.[48;49] APT070 is membrane localizing agent and a derivative of complement receptor type 1. It has been shown to successfully reduce complement mediated injury and increase graft survival in kidney transplantation.[50] In another study APT070 was able to reduce local and remote injury by suppressing the systemic inflammatory response associated with intestinal IRI, however, it did not alter the mortality rate in these animals.[47] Despite these studies no product has been released in the market yet.[51]
Our novel approach has shown that anti complement therapy can be localized, firmly adherent to the endothelium and successfully reduce renal dysfunction following IRI. The partial improvement seen in histopathology and MPO activity could be further improved. In our study, we used a warm ischemia of 45 min and this time constraint did not allow us to perform optimal incubation of FLVs and VCP as we did in highly successful cell based experiments.[52] We feel that our modest findings could have resulted from shorter duration of FLVs and VCP incubation, which led to reduced metal tether incorporation, and thus less VCP on the cell membranes. Our goal in future studies is to address this issue with better formulation of FLVs which will enable rapid incorporation of tethers with a shorter incubation period.
In summary, the role of complement is being increasingly recognized in both acute and chronic pathological conditions. Since complement also plays an important role in the host’s defense mechanism recent research is directed towards developing drugs capable of targeting specific tissues in diseased states. An ideal agent has to be an agent incapable of eliciting an acute immune response, have high specificity, and also retain systemic immunity intact. Our approach with modified VCP containing histidine tag fulfills these criteria. We conclude that enhancement of complement control at the vascular endothelial barrier with VCP appears to modulate complement activation and/or production during the first 24h- reducing vascular and renal dysfunction following IRI. This endothelium targeted therapy can be particularly useful in kidney transplantation where the harvested organs can be perfused ex-vivo concomitant to normal flushes with preservation solutions prior to storage and transport.
Acknowledgments
Grant sponsors:
This work was funded in part by a grant from the National Institutes of Health (NIH) R41HL079855, and by EndoProtech, Inc.
Footnotes
Financial disclosure
The authors Drs. Claudio Maldonado and Gustavo Perez-Abadia declare that they have conflict of interest.
The authors Sathnur Pushpakumar, Chirag Soni, Rong Wan, Nathan Todnem, Phani K Patibandla, Tathyana Fensterer, Qunwei Zhang, John H. Barker, do not have any conflict of interest.
References
- 1.Farrar CA, Zhou W, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J. 2006;20:217–226. doi: 10.1096/fj.05-4747com. [DOI] [PubMed] [Google Scholar]
- 2.Yamada K, Miwa T, Liu J, Nangaku M, Song WC. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol. 2004;172:3869–3875. doi: 10.4049/jimmunol.172.6.3869. [DOI] [PubMed] [Google Scholar]
- 3.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170:1517–1523. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- 4.Carter AM, Prasad UK, Grant PJ. Complement C3 and C-reactive protein in male survivors of myocardial infarction. Atherosclerosis. 2009;203:538–543. doi: 10.1016/j.atherosclerosis.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Distelmaier K, Adlbrecht C, Jakowitsch J, Winkler S, Dunkler D, Gerner C, Wagner O, Lang IM, Kubicek M. Local complement activation triggers neutrophil recruitment to the site of thrombus formation in acute myocardial infarction. Thromb Haemost. 2009;102:564–572. doi: 10.1160/TH09-02-0103. [DOI] [PubMed] [Google Scholar]
- 6.Ducruet AF, Zacharia BE, Hickman ZL, Grobelny BT, Yeh ML, Sosunov SA, Connolly ES., Jr The complement cascade as a therapeutic target in intracerebral hemorrhage. Exp Neurol. 2009;219:398–403. doi: 10.1016/j.expneurol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis AG, Kohl G, Ma Q, Devarajan P, Kohl J. Pharmacological targeting of C5a receptors during organ preservation improves kidney graft survival. Clin Exp Immunol. 2008;153:117–126. doi: 10.1111/j.1365-2249.2008.03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de GH, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39:481–484. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM. III Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol. 2002;169:4620–4627. doi: 10.4049/jimmunol.169.8.4620. [DOI] [PubMed] [Google Scholar]
- 10.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 12.Li K, Sacks SH, Zhou W. The relative importance of local and systemic complement production in ischaemia, transplantation and other pathologies. Mol Immunol. 2007;44:3866–3874. doi: 10.1016/j.molimm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Barnum SR. Complement biosynthesis in the central nervous system. Crit Rev Oral Biol Med. 1995;6:132–146. doi: 10.1177/10454411950060020301. [DOI] [PubMed] [Google Scholar]
- 14.Legoedec J, Gasque P, Jeanne JF, Scotte M, Fontaine M. Complement classical pathway expression by human skeletal myoblasts in vitro. Mol Immunol. 1997;34:735–741. doi: 10.1016/s0161-5890(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 15.McPhaden AR, Whaley K. Complement biosynthesis by mononuclear phagocytes. Immunol Res. 1993;12:213–232. doi: 10.1007/BF02918254. [DOI] [PubMed] [Google Scholar]
- 16.Zhao YX, Andoh A, Shimada M, Takaya H, Hata K, Fujiyama Y, Bamda T. Secretion of complement components of the alternative pathway (C3 and factor B) by the human alveolar type II epithelial cell line A549. Int J Mol Med. 2000;5:415–419. doi: 10.3892/ijmm.5.4.415. [DOI] [PubMed] [Google Scholar]
- 17.Thurman JM, Lucia MS, Ljubanovic D, Holers VM. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 2005;67:524–530. doi: 10.1111/j.1523-1755.2005.67109.x. [DOI] [PubMed] [Google Scholar]
- 18.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 19.Moller-Kristensen M, Wang W, Ruseva M, Thiel S, Nielsen S, Takahashi K, Shi L, Ezekowitz A, Jensenius JC, Gadjeva M. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol. 2005;61:426–434. doi: 10.1111/j.1365-3083.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- 20.de VB, Walter SJ, Peutz-Kootstra CJ, Wolfs TG, van Heurn LW, Buurman WA. The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia-reperfusion injury. Am J Pathol. 2004;165:1677–1688. doi: 10.1016/S0002-9440(10)63424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellano G, Melchiorre R, Loverre A, Ditonno P, Montinaro V, Rossini M, Divella C, Battaglia M, Lucarelli G, Annunziata G, Palazzo S, Selvaggi FP, Staffieri F, Crovace A, Daha MR, Mannesse M, van WS, Schena FP, Grandaliano G. Therapeutic Targeting of Classical and Lectin Pathways of Complement Protects from Ischemia-Reperfusion-Induced Renal Damage. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.090276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu F, Chauhan AK, Fernandes SM, Walsh MT, Wagner DD, Davis AE. III The effect of C1 inhibitor on intestinal ischemia and reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1042–G1049. doi: 10.1152/ajpgi.90460.2008. [DOI] [PubMed] [Google Scholar]
- 23.Fattouch K, Bianco G, Speziale G, Sampognaro R, Lavalle C, Guccione F, Dioguardi P, Ruvolo G. Beneficial effects of C1 esterase inhibitor in ST-elevation myocardial infarction in patients who underwent surgical reperfusion: a randomised double-blind study. Eur J Cardiothorac Surg. 2007;32:326–332. doi: 10.1016/j.ejcts.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Storini C, Rossi E, Marrella V, Distaso M, Veerhuis R, Vergani C, Bergamaschini L, De Simoni MG. C1-inhibitor protects against brain ischemia-reperfusion injury via inhibition of cell recruitment and inflammation. Neurobiol Dis. 2005;19:10–17. doi: 10.1016/j.nbd.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Homeister JW, Satoh PS, Kilgore KS, Lucchesi BR. Soluble complement receptor type 1 prevents human complement-mediated damage of the rabbit isolated heart. J Immunol. 1993;150:1055–1064. [PubMed] [Google Scholar]
- 26.Lehmann TG, Koeppel TA, Munch S, Heger M, Kirschfink M, Klar E, Post S. Impact of inhibition of complement by sCR1 on hepatic microcirculation after warm ischemia. Microvasc Res. 2001;62:284–292. doi: 10.1006/mvre.2001.2342. [DOI] [PubMed] [Google Scholar]
- 27.Pratt JR, Hibbs MJ, Laver AJ, Smith RA, Sacks SH. Effects of complement inhibition with soluble complement receptor-1 on vascular injury and inflammation during renal allograft rejection in the rat. Am J Pathol. 1996;149:2055–2066. [PMC free article] [PubMed] [Google Scholar]
- 28.de VB, Matthijsen RA, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75:375–382. doi: 10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]
- 29.Goga l, Perez-Abadia G, Pushpakumar S, Cramer D, Yan J, Todnem N, Anderson G, Soni C, Barker J, Maldonado C. Cell Membrane Modification for Rapid Display of Bi-Functional Peptides: A Novel Approach to Reduce Complement Activation. The Open Cardiovascular Medicine Journal. 2010;4:157–165. doi: 10.2174/1874192401004010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Lentsch AB, Kato A, Saari JT, Schuschke DA. Augmented metalloproteinase activity and acute lung injury in copper-deficient rats. Am J Physiol Lung Cell Mol Physiol. 2001;281:L387–L393. doi: 10.1152/ajplung.2001.281.2.L387. [DOI] [PubMed] [Google Scholar]
- 32.Sen U, Tyagi N, Kumar M, Moshal KS, Rodriguez WE, Tyagi SC. Cystathionine-beta-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cells. Am J Physiol Cell Physiol. 2007;293:C1779–C1787. doi: 10.1152/ajpcell.00207.2007. [DOI] [PubMed] [Google Scholar]
- 33.Tedesco F, Fischetti F, Pausa M, Dobrina A, Sim RB, Daha MR. Complement-endothelial cell interactions: pathophysiological implications. Mol Immunol. 2000;37:91. doi: 10.1016/s0161-5890(00)00036-5. [DOI] [PubMed] [Google Scholar]
- 34.Fischetti F, Tedesco F. Cross-talk between the complement system and endothelial cells in physiologic conditions and in vascular diseases. Autoimmunity. 2006;39:417–428. doi: 10.1080/08916930600739712. [DOI] [PubMed] [Google Scholar]
- 35.Moseley E, Goddard M, Stoica S, Large S, Wallwork J, Atkinson C. Complement regulators are downregulated by ischemia reperfusion in heart transplantation. The Journal of Heart and Lung Transplantation. 2005;24:S153. [Google Scholar]
- 36.Anderson JB, Smith SA, van WR, Chien S, Kotwal GJ. Vaccinia viruscomplement control protein ameliorates hyperacute xenorejection by inhibiting xenoantibody binding. Transplant Proc. 2002;34:3277–3281. doi: 10.1016/s0041-1345(02)03692-8. [DOI] [PubMed] [Google Scholar]
- 37.Pillay NS, Kellaway LA, Kotwal GJ. Vaccinia virus complement control protein significantly improves sensorimotor function recovery after severe head trauma. Brain Res. 2007;1153:158–165. doi: 10.1016/j.brainres.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds DN, Smith SA, Zhang YP, Mengsheng Q, Lahiri DK, Morassutti DJ, Shields CB, Kotwal GJ. Vaccinia virus complement control protein reduces inflammation and improves spinal cord integrity following spinal cord injury. Ann N Y Acad Sci. 2004;1035:165–178. doi: 10.1196/annals.1332.011. [DOI] [PubMed] [Google Scholar]
- 39.Jha P, Smith SA, Justus DE, Kotwal GJ. Prolonged retention of vaccinia virus complement control protein following IP injection: implications in blocking xenorejection. Transplant Proc. 2003;35:316–3162. doi: 10.1016/j.transproceed.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 40.Ghebremariam YT, Engelbrecht G, Tyler M, Lotz Z, Govender D, Kotwal GJ, Kahn D. Vaccinia virus complement control protein (VCP) improves kidney structure and function following ischemia/reperfusion injury in rats. J Surg Res. 2010;159:74–754. doi: 10.1016/j.jss.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 41.Ganesh VK, Smith SA, Kotwal GJ, Murthy KH. Structure of vaccinia complement protein in complex with heparin and potential implications for complement regulation. Proc Natl Acad Sci U S A. 2004;101:892–8929. doi: 10.1073/pnas.0400744101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Murthy KH, Smith SA, Ganesh VK, Judge KW, Mullin N, Barlow PN, Ogata CM, Kotwal GJ. Crystal structure of a complement control protein that regulates both pathways of complement activation and binds heparan sulfate proteoglycans. Cell. 2001;104:30–311. doi: 10.1016/s0092-8674(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 43.Brenner BM. Nephron adaptation to renal injury or ablation. Am J Physiol. 1985;249:F32–F337. doi: 10.1152/ajprenal.1985.249.3.F324. [DOI] [PubMed] [Google Scholar]
- 44.Hales CN. Suicide of the nephron. Lancet. 2001;357:136–137. doi: 10.1016/S0140-6736(00)03553-4. [DOI] [PubMed] [Google Scholar]
- 45.Weight SC, Furness PN, Nicholson ML. New model of renal warm ischaemia-reperfusion injury for comparative functional, morphological and pathophysiological studies. Br J Surg. 1998;85:166–1673. doi: 10.1046/j.1365-2168.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 46.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:58–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 47.Souza DG, Esser D, Bradford R, Vieira AT, Teixeira MM. APT070 (Mirococept), a membrane-localised complement inhibitor, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2005;145:102–1034. doi: 10.1038/sj.bjp.0706286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stammberger U, Hamacher J, Hillinger S, Schmid RA. sCR1sLe ameliorates ischemia/reperfusion injury in experimental lung transplantation. J Thorac Cardiovasc Surg. 2000;120:107–1084. doi: 10.1067/mtc.2000.111175. [DOI] [PubMed] [Google Scholar]
- 49.Zacharowski K, Otto M, Hafner G, Marsh HC, Jr, Thiemermann C. Reduction of myocardial infarct size with sCR1sLe(x), an alternatively glycosylated form of human soluble complement receptor type 1 (sCR1), possessing sialyl Lewis x. Br J Pharmacol. 1999;128:94–952. doi: 10.1038/sj.bjp.0702889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel H, Smith RA, Sacks SH, Zhou W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J Am Soc Nephrol. 2006;17:110–1111. doi: 10.1681/ASN.2005101116. [DOI] [PubMed] [Google Scholar]
- 51.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:126–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goga L, Pushpakumar SB, Perez-Abadia G, Olson P, Anderson G, Soni CV, Barker JH, Maldonado C. A novel liposome-based therapy to reduce complement-mediated injury in revascularized tissues. J Surg Res. 2011;165:e5–e57. doi: 10.1016/j.jss.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]