Abstract
Background
Branched actin assembly is critical for both cell motility and membrane trafficking. The branched actin regulator, cortactin, is generally considered to promote cell migration by controlling leading edge lamellipodial dynamics. However, recent reports indicate that lamellipodia are not required for cell movement, suggesting an alternate mechanism.
Results
Since cortactin also regulates membrane trafficking and adhesion dynamics, we hypothesized that altered secretion of extracellular matrix (ECM) and/or integrin trafficking might underlie motility defects of cortactin-knockdown (KD) cells. Consistent with a primary defect in ECM secretion, both motility and lamellipodial defects of cortactin-KD cells were fully rescued by plating on increasing concentrations of exogenous ECM. Furthermore, cortactin-KD cell speed defects were rescued on cell-free autocrine ECM produced by control cells but not on ECM produced by cortactin-KD cells. Investigation of the mechanism revealed that whereas endocytosed FN is redeposited at the basal cell surface by control cells, cortactin-KD cells exhibit defective FN secretion and abnormal FN retention in a late endocytic/lysosomal compartment. Cortactin-KD motility and FN deposition defects were phenocopied by KD in control cells of the lysosomal fusion regulator Synaptotagmin-7. Rescue of cortactin-KD cells by expression of cortactin binding domain mutants revealed that interaction with Arp2/3 complex and actin filaments is essential for rescue of both cell motility and autocrine ECM secretion phenotypes whereas binding of SH3 domain partners is not required.
Conclusions
Efficient cell motility, promoted by cortactin regulation of branched actin networks, involves processing and resecretion of internalized ECM from a late endosomal/lysosomal compartment.
Introduction
Cell motility is a fundamental component of many physiological and pathological processes, including embryogenesis, wound healing, and cancer metastasis. Intrinsic cell motility cycles canonically consist of: protrusion of leading edge lamellipodia, formation of new adhesions, cell body contraction, and tail detachment. These cycles are modified by cellular interaction with extrinsic factors, including growth factors and extracellular matrix (ECM) [1]. A motility molecule that has received a great deal of attention is cortactin, due to its presence in leading edge lamellipodia and function as a regulator of the actin-nucleating Arp2/3 complex [2]. Numerous studies have shown that cortactin regulates cell migration in diverse cell types [3], including Drosophila border cells migrating in vivo [4]. In contrast, a few reports have found little or no effect of cortactin expression on cell migration, suggesting either cell type or microenvironmental influences [5, 6].
The underlying mechanism for cortactin regulation of cell motility has been variably attributed to regulation of Arp2/3-mediated branched actin dynamics in leading edge protrusions or to regulation of signaling [2, 7, 8]. However, a number of studies have found that cortactin is not essential for lamellipodial protrusion but rather affects lamellipodial dynamics [3]. In addition, lamellipodial protrusion has been shown to be dispensable for cell movement [9], suggesting that lamellipodial protrusion may serve primarily to direct the cell rather than drive the actual mechanics of intrinsic cell motility. By contrast, adhesion of cells to ECM is known to be critical for cell motility [1, 10].
Previously, we identified specific defects in both lamellipodial persistence (e.g. stability) and adhesion assembly in cortactin-knockdown (KD) cells [7] that were coordinately rescued along with cell motility by a minimal cortactin truncation protein that contained the Arp2/3 complex and F-actin binding sites. Interestingly, lamellipodial persistence defects are frequently associated with primary defects in integrin activity or expression [11–13], which suggests that the adhesion assembly defect of cortactin-KD cells might cause lamellipodial instability rather than vice versa. Since cortactin is known to regulate membrane trafficking [3], we hypothesized that the lamellipodial, adhesive, and motility defects observed in cortactin-deficient cells [3] could be a consequence of defective ECM secretion or altered integrin trafficking. Indeed, in this study we report that cortactin-KD cells exhibit decreased fibronectin (FN) secretion that leads to defects in cell motility and lamellipodial dynamics. Interestingly, investigation of the mechanism revealed that, dependent on cortactin interaction with branched actin networks, exogenous FN is internalized and resecreted from a late endocytic/lysosomal compartment to promote efficient cell motility.
Results
The goal of this study was to test the hypothesis that cortactin regulates cell motility by altering membrane trafficking of either ECM components or integrins. Such a mechanism would link two major cellular functions (migration and vesicular trafficking) described for cortactin and might explain divergent reports on the role of cortactin in cell motility.
Migration and lamellipodial defects of cortactin-KD cells are rescued on exogenous ECM
If cortactin primarily regulates motility through autocrine secretion of ECM, then the defective motility of cortactin-KD cells should be rescued by performing cell motility assays on ECM-coated surfaces. To test this possibility, serially diluted FN or collagen I was coated on the bottom of tissue culture plates for single cell (Figures 1A and 1C) or transwell membranes for transwell (Figures 1B and 1D) migration assays. As previously published [7], in the absence of exogenous ECM, cortactin-KD HT1080 fibrosarcoma cells migrated less efficiently than scrambled oligo-expressing control cells (scrambled) or KD cells rescued with shRNA-insensitive mouse cortactin cDNA (Rescue), whereas cells overexpressing cortactin (OE) cells migrated faster (Figures 1A and B, 0 μg/ml FN, see Figure S1A for cortactin protein abundance). However, plating cells on increasing concentrations of FN or collagen I led to full rescue of cortactin-KD motility defects to the level of control cells (Figures 1A-D) at higher ECM concentrations. Similarly, single cell motility defects of cortactin-KD MDA-MB-231 breast cancer cells were rescued by plating cells on the highest tested concentrations of FN or collagen I (Figure S1). There was much less effect of exogenous ECM on control and OE cell speed, suggesting a more optimal ECM-adhesion axis [10] on uncoated surfaces as might occur with autocrine secretion of ECM. These data suggest an extrinsic and not intrinsic motility defect in cortactin-KD cells.
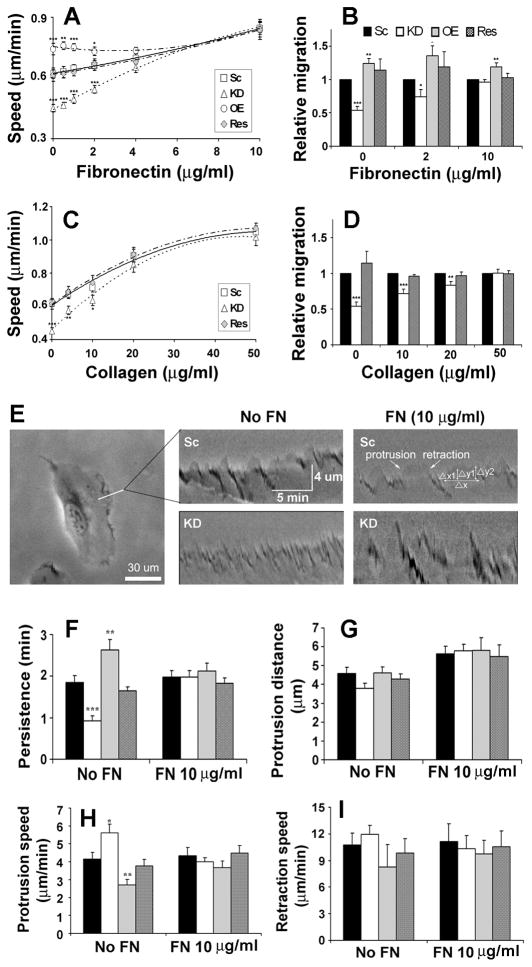
Figure 1. Cortactin-KD cell motility and lamellipodial defects are rescued on substrates coated with exogenous ECM.
(A and C) Single cell migration assays on fibronectin (FN) - (A) or collagen- (C) coated plates. N≥45 cells from 3 independent experiments for each cell line. (B and D) Transwell migration assays on fibronectin- (B) or collagen- (D) coated transwell filters. 4 different fields were observed in each of 3 independent experiments. (E) Representative kymographs on uncoated- or FN (10 μg/ml)-coated plates. Note definitions of lamellipodial persistence (length of time a protrusion lasts before retraction,Δx), protrusion distance (Δy2), protrusion speed (initial rate, Δx1/ Δy1). Scale bars indicate distance and time axes. Lamellipodial persistence (F), protrusion distance (G), protrusion speed (H), and retraction speed (I) are quantitated. n≥20 cells in≥3 independent experiments. Sc: scrambled oligo; KD: cortactin-knockdown; OE: cortactin-overexpressed; Res: rescued (KD cells reexpressing shRNA-insensitive mouse cortactin). *; p < 0.05, **; p < 0.01, ***; p < 0.001 compared to Sc. Error bars indicate SEM.
We previously reported that cortactin-KD cells have defective stability/persistence of lamellipodial protrusions that parallels the motility defects [7]. To analyze the effect of exogenous ECM on lamellipodial dynamics, membrane ruffling of cortactin-manipulated HT1080 cells plated on 0 or 10 μg/ml FN was recorded in time-lapse movies and analyzed by kymography [7, 14] (Figure 1E). Consistent with the migration assays, the lamellipodial persistence defect of cortactin-KD cells [7] was fully rescued on 10 μg/ml FN (Figure 1F). Exogenous FN also normalized the increased protrusion speed of cortactin-KD cells [7] to the level of controls (Figure 1H). Protrusion distance and retraction speed were not significantly different between cortactin manipulated cells in either the absence [7] or presence of exogenous FN (Figures 1G and 1I). Alterations in lamellipodial dynamics with OE cells were also equalized to the level of scrambled controls by plating on 10 μg/ml FN.
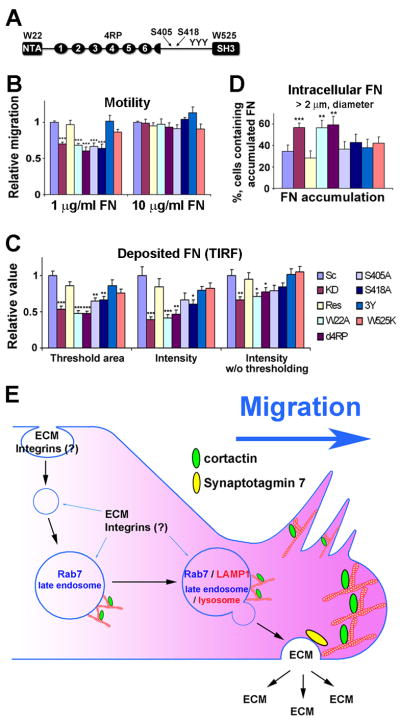
HT1080 cells are known to express integrin receptors α2β1 for collagen I, α3β1 for laminin, and α5β1 for FN [15]. Since cortactin-regulated motility was sensitive to the levels of the β1 integrin ligands collagen I and FN, we tested whether β1 expression was altered. The total cellular level of β1 integrin was similar between control and cortactin-KD cells by Western blot analyses (Figure 2A). In addition, flow cytometry revealed no differences in cell-surface expression of β1, α2, α3, and α5 integrins (Figures 2B and S2A). We also quantitated the presence of β1 integrin in adhesions in cortactin-manipulated cells plated on the non-integrin-binding attachment substrate poly-D-lysine (PDL), or 10 μg/ml FN by immunostaining fixed, permeabilized cells for the presence of total (AIIB2) or activated (12G10) β1 and imaging the cell-substrate interface with total internal reflection fluorescence (TIRF) microscopy. Consistent with the motility and lamellipodial dynamics results, cortactin-KD cells had reduced total and activated β1 present in adhesions when plated on PDL but not on FN (Figures 2 and S2). Analysis of β1 integrin endocytosis exhibited a similar profile, with an increase in early β1 internalization in cortactin-KD cells when plated on low but not high FN (2 and 5 min timepoints, Figure S2). We speculate that the increased endocytosis of β1 integrin in cortactin-KD cells plated on low concentrations of FN is likely due to defective adhesion formation (Figures 2 and S2, [7]), leading to a larger population of unengaged β1 integrins available for endocytosis [16]. Since both the low adhesion formation and the increase in early β1 endocytosis were rescued when cortactin-KD cells were plated on high FN concentrations, we hypothesize that the primary underlying defect in cortactin-KD cells is defective secretion of ECM.
Figure 2. Cortactin expression affects adhesion formation on low ECM substrates but not total or cell surface levels of β1 integrin.
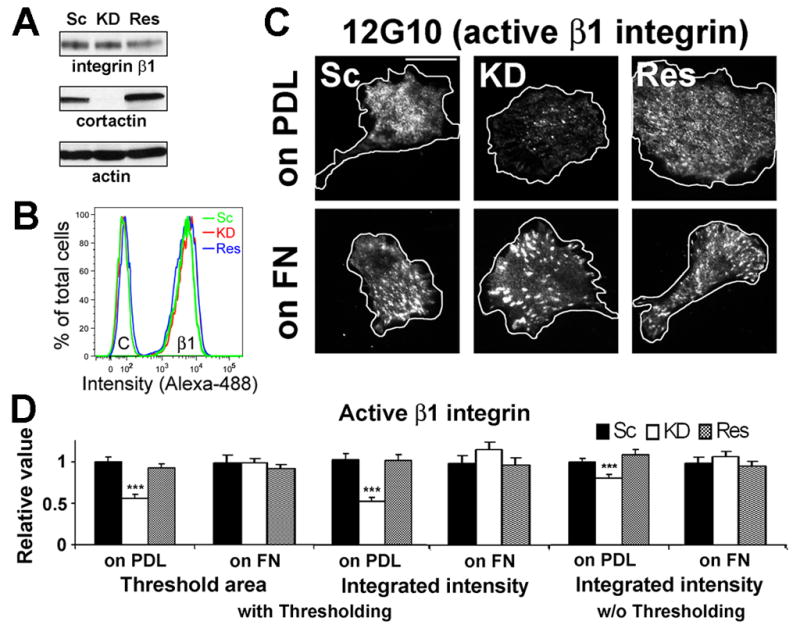
(A) Western blot analysis of total cell lysates from scrambled control (Sc), cortactin-KD (KD), and cortactin-rescued (Res) HT1080s shows no difference in total cell β1 integrin levels. Representative blot from n = 2. (B) Flow cytometry analysis shows no difference in β1 cell-surface expression. Representative profiles are shown. C; control IgG, β1; integrin β1. n=2. (C) Representative TIRF images of activated (visualized using 12G10 mAb staining) integrin β1 at basal surface of cells cultured on poly-D-lysine (PDL, 100 μg/ml)- or FN (10 μg/ml)-coated glass surfaces. Cell boundaries from DIC images (Fig S2) are outlined in white. Scale bar = 30 μm. (D) Quantitation of activated integrin β1-positive area/cell and intensity/cell calculated with or without (w/o) thresholding of TIRF images. n > 30 cells from 3 independent experiments. ***; p < 0.001. Error bars indicate SEM.
Motility defects of cortactin-KD cells are due to defective secretion of autocrine ECM
To directly test the effect of autocrine-produced ECM on cell motility, we prepared tissue culture plates coated with cell-free autocrine ECM from cortactin-manipulated HT1080 cells. Confluent cultures were grown for 48 h on 12-well plates before removal of cells with 20 mM ammonium hydroxide (NH4OH). NH4OH is minimally damaging to autocrine-produced ECM and leaves many biological properties intact, including adhesion, spreading and growth [17]. New cultures of cortactin-manipulated HT1080s were then plated on the autocrine-ECM-coated dishes for 2 h before testing in 5-hour single-cell motility assays. As predicted, cortactin-KD cell motility was fully rescued on autocrine ECM extracted from Scrambled and Rescue cells but not on cortactin-KD-derived autocrine ECM (Figure 3A). However, the motility of Scrambled and Rescue cells was similar regardless of the source of autocrine ECM, suggesting that cortactin-expressing cells can sufficiently modulate the ECM environment during the experiment for efficient migration. Cortactin-manipulated MDA-MB-231 cells responded similarly, with rescue of KD motility on autocrine-produced ECM from cells expressing cortactin (Figure 3B).
Figure 3. Autocrine-produced ECM from cortactin-expressing cells rescues the motility of cortactin-KD cells.
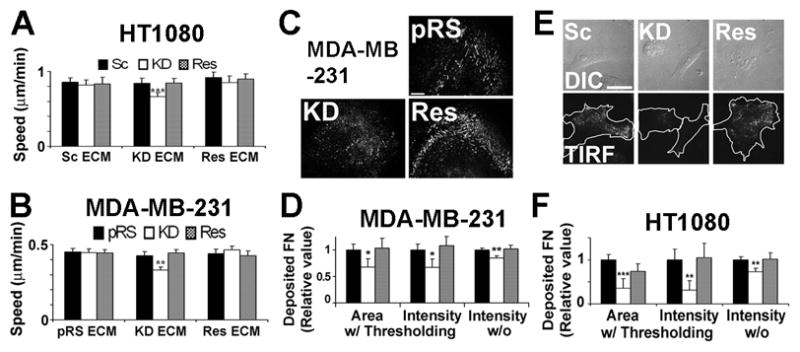
(A and B) Single cell migration assays of cortactin-manipulated HT1080 (A) and MDA-MB-231 (B) plated on cell-free autocrine ECM derived from control or KD cells as indicated. Sc: scrambled control; pRS: vector-expressing, KD: cortactin-KD, Res: cortactin-rescued. n>50 cells from 3 independent experiments. (C) Representative images of FN immunostaining of cell-free ECM derived from cortactin-manipulated MDA-MB-231 cells. Scale bar = 30 μm. (D) Quantitation of the area/frame and intensity/frame of MDA-MB-231-derived cell-free ECM images. N≥30 images from 3 independent experiments (E and F) Analysis of basally secreted FN in HT1080 cells. (E) Representative matched DIC and TIRF images from fixed, immunostained cortactin-manipulated HT1080 cells shows a deficit in basal FN underneath cortactin-KD cells. Scale bar = 30 μm. (F) Quantitation of FN-positive area/cell and intensity/cell from TIRF images. n 25 images from 3 independent experiments. *; p < 0.05, **; p < 0.01, ***; p < 0.001 compared to control cells. Error bars indicate SEM.
Immunofluorescent staining for FN in the autocrine-produced cell-free ECM revealed little detectable deposition in HT1080-produced ECM; however, FN staining was detectable in MDA-MB-231-produced ECM and there was less FN deposition in cortactin-KD cell-produced ECM (Figures 3C and 3D). To verify this finding in a second cell type, we obtained previously published control and cortactin-KD mouse embryonic fibroblasts (MEFs) [18] and prepared cell-free ECM. As expected, there was less FN detected in autocrine ECM prepared from cortactin-KD MEFs, compared with control-derived ECM (Figure S3). We speculate that the inability to visualize HT1080-produced cell-free FN represents either a signal-to-noise issue or greater extractability of HT1080 ECM from glass coverslips by NH4OH, since we visualize it easily below cortactin-manipulated HT1080 cells by TIRF microscopy (Figures 3E and 3F). Note also that the cell motility experiments were performed on autocrine-produced ECM-coated plastic and plastic adsorbs ECM more efficiently than glass [19]. These data indicate that cortactin is critical for FN secretion at the cell-substrate interface.
Cortactin regulates secretion of FN from an endocytic compartment
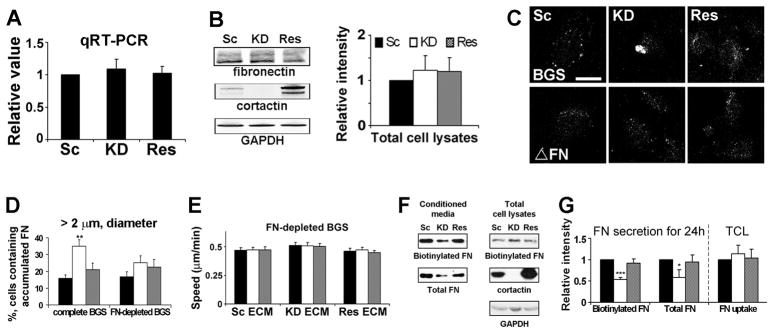
To determine whether changes in FN expression could explain the decreased FN secretion into cortactin-KD-produced autocrine ECM, we performed quantitative RT-PCR and Western blot analysis. No significant differences were observed in total cell levels of FN mRNA or protein between any of the cortactin-manipulated cell lines (Figures 4A and 4B), suggesting that cortactin may instead regulate FN trafficking. Indeed, examination of permeabilized cells immunostained for FN revealed that twice as many cortactin-KD cells contained large perinuclear FN-containing puncta compared with control cells (Figure 4C, top panels), suggesting an exit defect from a secretory compartment.
Figure 4. Internalized FN is used for motility.
(A) Quantitative real-time PCR. Relative FN mRNA abundance was measured by 2-ΔΔCt. n=3. (B) Representative Western blot images and analyses of FN protein abundance in total cell lysates. FN intensities were normalized by GAPDH intensities. n = 3. (C and D)To analyze intracellular accumulation of ECM, cortactin-manipulated HT1080 cells were cultured in media containing complete BGS (BGS) or FN-depleted BGS (ΔFN) and immunostained for FN. (C) Representative fluorescent confocal images. Scale bar = 30 μm. (D) % of cells with large intracellular FN-positive puncta (≥2 μm diameter). n ≥ 200 cells from 3 independent experiments. (E) Single cell migration on cell-free autocrine ECM with FN-depleted BGS-containing media (compare to Fig 3A). n≥50 cells from 3 independent experiments. (F) Representative Western blot images from pulse-chase assay performed using poly-HRP-conjugated streptavidin (“Biotinylated”) and FN antibody (“Total”). Total cell lysates were gathered after the 1 h pulse to show biotinylated FN uptake. Conditioned media were gathered 24 h after the pulse-chase. (G) Quantitation of (F). TCL = Total cell lysates and represents Biotinylated FN signal normalized by the GAPDH loading control. n = 3. *; p < 0.05, **; p < 0.01, ***; p < 0.001 compared to control cells. Error bars indicate SEM.
To identify that compartment, we first tested whether the major source of secreted FN was exogenous (serum contains significant amounts of FN [20]) as opposed to biosynthetic pathways. Up to this point, all experiments were performed in media containing regular serum. To determine the predominant pathway in our system, we repeated two critical experiments in media containing FN-depleted serum: immunostaining of intracellular FN and single cell motility on autocrine-produced ECM. For immunostaining, cells were plated on PDL-coated coverslips for 48 h in media containing 10% bovine growth serum (BGS) or 10% FN-depleted BGS (Figure S4) followed by fixation (Figures 4C and 4D). For motility experiments, cell-free autocrine ECM was prepared as before and cells were plated in media containing FN-depleted BGS, equilibrated for 2 h and then movies taken for 5 h (Figure 4E). Interestingly, intracellular FN accumulation in cortactin-KD cells was decreased and equivalent to control cells upon culturing in FN-depleted BGS (Figures 4C and 4D). Likewise, single cell motility of all cortactin-manipulated HT1080 cells was equivalent and dampened (by almost 2-fold) when the experiment was performed in FN-depleted BGS (Figure 4E, compare to Figure 3A). These data indicate that, dependent on cortactin, cells internalize and resecrete exogenous ECM to facilitate effective migration.
Since our motility experiments indicated that internalized FN is critical for cortactin-regulated motility, we performed a pulse-chase experiment to determine whether cortactin affects re-secretion of internalized FN into conditioned media. HT1080 cells were incubated with 20 μg/ml biotinylated FN for 1 h followed by washing and collection of CM after 24 h. Indeed, despite equivalent FN uptake, cortactin-KD cells secreted 2-fold less biotinylated FN into CM compared with controls (Figures 4F and 4G).
Cortactin regulates a late endosomal/lysosomal secretory compartment
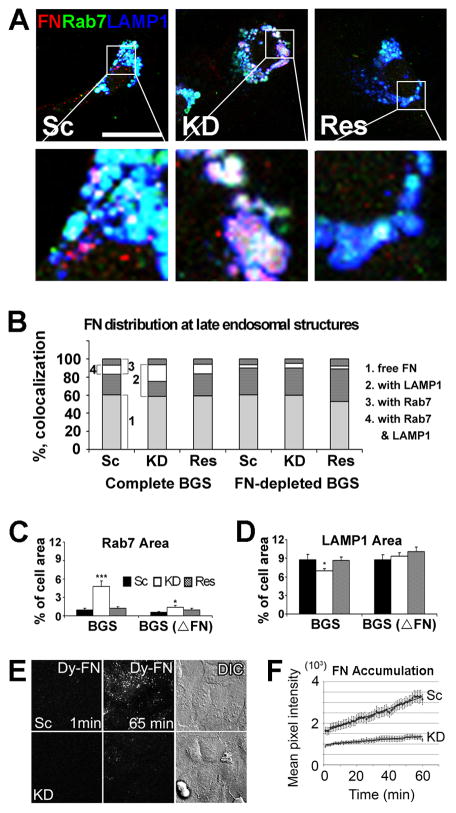
To determine which secretory compartment is regulated by cortactin, we colocalized internalized FN with vesicular markers associated with recycling endosomes, apicobasal trafficking, and secretory lysosomes [21]. Whereas there was little colocalization of FN with Rab8a or Rab11a (Figure S5A), a substantial proportion of the internalized FN colocalized with the late endosomal marker Rab7 and the lysosomal marker LAMP1 (Figures 5A and 5B). Interestingly, cortactin-KD cells exhibited a significant increase in colocalization of FN with Rab 7- and Rab7, LAMP1-positive compartments compared with control cells (Figures 5A, 5B, S5B, and S5C). There was also a large increase in the size of the Rab7 compartment in cortactin-KD cells (Figure 5C), suggesting a block in secretion from and/or maturation of late endosomes. There was also a small decrease in the size of the LAMP1-compartment (Figure 5D), suggesting a shift in the overall late endosomal/lysosomal population upon cortactin manipulation. Cortactin was also found to colocalize with Rab7- and LAMP1-positive vesicles (Figure S5D). Most KD phenotypes, including FN accumulation in Rab7-positive compartments, were rescued or diminished by removal of FN from the media (FN-depleted BGS condition, Figure 5B-D) consistent with an exogenous source of accumulated FN.
Figure 5. Cortactin promotes FN trafficking from a late endosomal/lysosomal secretory compartment.
(A–D) Colocalization of FN with vesicular compartments (A) Representative confocal images of FN (Red), Rab7 (late endosomes, Green), and LAMP1 (lysosomes, Blue) triple staining of cells cultured in complete BGS-containing media. White = triple colocalization, note in zooms. Scale bar = 30 μm. (B) Summarized graph of FN localization shows accumulation of FN in a Rab7/LAMP1 double positive compartment in KD cells. (C) Area of Rab7 staining out of total cell area (from single confocal slices) is plotted. (D) Area of LAMP1 confocal staining out of total cell area is plotted. Error bars indicate S.E.M from three independent experiments (n≥50 cells).*; p < 0.05, **; p < 0.01, ***; p < 0.001 compared to control cells. (E and F) Cortactin is necessary for accumulation of endocytosed DyLight550-FN at the cell-substrate interface. (E) Frames from TIRF live cell imaging sequences taken at 1 frame/min (see also Movies S1 (control) and S2 (KD)). The first frame of the movie (time 0) has been subtracted from each frame of the time sequence in order to detect only newly accumulated FN. Note efficient accumulation for Sc as compared to KD cells. DIC images illustrate confluence of cell monolayers. (F) Quantitation of live cell imaging data as in (E). FN accumulation was quantitated as mean fluorescence intensity beneath 10 individual cells from 4 independent experiments and was significantly different between Sc and KD cells (p<0.001, assessed by MatLab analysis of covariance comparing the 2 regression lines). Both Sc and KD cells exhibit constant FN accumulation (line function fitting confirmed by R2>0.9). Slope coefficient is 27.965 for Sc and 6.56 for KD. Error bars indicate SEM.
To provide additional evidence that FN derived from the media and processed by the endocytic system was deposited at the basal surface of cells, we incubated cells with DyLight-550-conjugated-FN and performed live cell imaging. By confocal microscopy, DyLight-FN localized to acidic organelles labeled with Lysotracker Green (Figure S5E). By TIRF microscopy, DyLight-FN was visible in moving vesicles and also appeared to be deposited at the basal surface of cells, as evidenced by progressive accumulation in elongated immobile structures that may represent newly-assembling adhesions (Movies S1 and S2). In addition, a few of the elongated structures disappeared before the end of the movie, indicating that either nascent FN deposits were re-endocytosed or that they represented tethered lysosomes that had not yet fused with the plasma membrane (Movies S1 and S2). Compared with control cells, there was a ~4-fold slower rate of total DyLight-FN accumulation at the base of cells in cortactin-KD cells (Figures 5E and 5F). Size and shape analyses of the movie data indicated that KD cells exhibited a significant reduction in the number of all types of FN structures appearing in the TIRF plane, including both large linear accumulations as well as small circular objects (Figures S5F and S5G, Movies S1 and S2). These data, in combination with our finding that in cortactin-KD cells FN accumulates in an enlarged Rab7-positive compartment (Figure 5A-C), suggest that cortactin is important for trafficking of endocytosed FN to the plasma membrane.
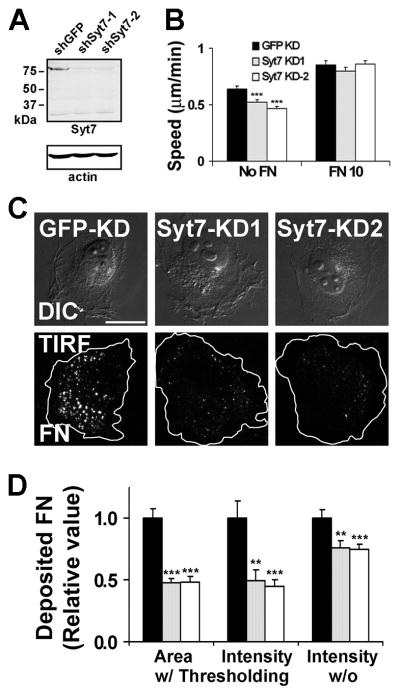
To test the general mechanism of lysosomal secretion in ECM deposition and cell motility, shRNA targeting the secretory lysosome fusion regulator Synaptotagmin-7 (Syt7) was expressed in HT1080 cells. Indeed, similar to cortactin-KD, Syt7-KD HT1080 cells exhibited decreased basal FN deposition and decreased motility on uncoated tissue culture dishes compared with control cells (Figure 6). Also similar to cortactin-KD cells, the motility of control and Syt7-KD cells was equivalent and increased on 10 μg/ml FN-coated dishes.
Figure 6. Loss of the lysosomal secretion regulator Synaptotagmin-7 (Syt7) also results in defective basal FN secretion and motility.
(A) Western blot of Syt7 expression in control (shGFP) and Syt7-KD (shSyt7-1, shSyt7-2) HT1080s. (B) Single cell migration assays on uncoated (No FN)- or FN (“FN 10”, 10 μg/ml)-coated plates. n≥45 cells for each cell line from 3 independent experiments. (C) Representative matched DIC and TIRF images from fixed, immunostained Syt7-manipulated HT1080 cells shows a deficit of basal FN underneath Syt7-KD cells. Cell boundaries are outlined in white in TIRF images. Scale bar = 30 μm. (D) Quantitation of basal FN-positive area/cell or intensity/cell with (w/) or without (w/o) thresholding. n > 30 cells from 3 independent experiments. **; p < 0.01, ***; p < 0.001 compared to shGFP control. Error bars indicate SEM.
Interaction of cortactin with the Arp2/3 complex and actin filaments is critical for motility and secretion phenotypes
To determine underlying molecular interactions that mediate cortactin regulation of FN secretion and cell motility, we performed rescue experiments in which cortactin proteins with mutations in the binding sites for Arp2/3 complex (W22A), actin filaments (Δ4RP), or SH3 domain binding partners (W525K) or in Src (3Y) or Erk (S405A, S418A) phosphorylation sites [7, 22–24] were reexpressed in KD cells. Interestingly, both cell motility and FN deposition defects of KD cells were rescued by reexpression of W525K and 3Y mutants (Figures 7 and S6). Although not statistically significant, the rescue by W525K was frequently not to the level of that by wild type cortactin or 3Y. Conversely, KD defects were not rescued by W22A, Δ4RP, S405A or S418A in either assay. Analysis of intracellular accumulation of large FN puncta yielded similar results except that the Erk mutants also rescued KD defects in this assay (Figures 7 and S6). Our interpretation of these results is that the most critical activity of cortactin in FN secretion and cell motility is to regulate branched actin networks. A secondary (nonessential) activity may be to recruit or otherwise interact with SH3 binding partners at those networks.
Figure 7. Binding of cortactin to Arp2/3 complex and actin filaments is critical for cell motility and ECM secretion.
(A) Cortactin domain structure marked with mutation sites: W22, critical Arp2/3 complex binding residue; NTA, N-terminal acidic domain; ovals, cortactin repeat domains; 4RP, F-actin binding domain; S405 and S418, Erk phosphorylation sites; YYY, Srk phosphorylation sites; SH3, Src homology 3 domain; and W525, mutation of this residue prevents binding to the SH3 domain. (B) Quantitation of transwell migration assays of cortactin mutant-rescued cells on low (1 μg/ml) or high (10 μg/ml) FN coating concentrations. 4 fields/filter were observed in each of 4 independent experiments. (C) Quantitation of area/cell and intensity/cell of deposited FN from TIRF images (Representative DIC and TIRF images shown in Fig. S6B). n≥25 images per cell line from 3 experiments). (D) The percentage of cells with large intracellular FN accumulations (≥2 μm diameter puncta) was quantitated from wide-field images (See Fig. S6C for representative images). n≥80 cells from 3 independent experiments. *; p < 0.05, **; p < 0.01, ***; p < 0.001 compared to Res. Error bars indicate SEM. (E) Model of cortactin regulation of ECM deposition and cell motility. ECM (and potentially other motility molecules such as integrins) is internalized and resecreted from a late endosomal/lysosomal compartment to support cell motility. Cortactin regulation of branched actin networks is likely to be critical for either the generation or docking of ECM-containing secretory carriers derived from a Rab7/LAMP1-positive compartment whereas Syt7 regulates vesicle fusion at the plasma membrane. The proposed model in which cortactin-mediated secretion of ECM leads to adhesion formation, lamellipodial stability, and cell motility links known cortactin functions but provides an alternate mechanism to the current model in which lamellipodial dynamics drives cell motility.
Discussion
Cortactin regulates cell migration; however diverse underlying mechanisms have been proposed. In this study, we report the novel finding that cortactin regulates extrinsic rather than intrinsic mechanisms of cell motility by promoting ECM secretion from a late endosomal/lysosomal compartment (see model, Figure 7E). Consistent with lysosomal secretion of ECM as a critical regulatory point for cell motility, cortactin-KD motility and secretion defects were phenocopied by KD of the late endosomal/lysosomal fusion regulator Syt7. Cortactin functions in motility and secretion were dependent on interaction with branched actin networks but not the SH3 domain.
Cortactin has been intensively studied as a motility factor since the discovery that it can serve as a cofactor for Arp2/3 activation as well as a stabilizer of actin branches [25, 26]. Numerous studies have demonstrated that cortactin promotes cell motility as well as proteolytic invasion through ECM [3]. However, conflicting results between studies suggest context-dependent cortactin functions. For example, several studies have reported no effect of cortactin loss on cell migration [6] or lamellipodial architecture [8]. Interestingly, in those reports the cell migration and electron microscopy studies were respectively performed using cells plated on 10 μg/ml [6] and 50 μg/ml [8] FN. Consistent with those results, we find that cortactin-KD cell defects in motility and lamellipodial dynamics are fully rescued to the level of control cells upon plating on similar concentrations of ECM. Our discovery that cortactin regulates cell motility by promoting ECM deposition provides a unifying mechanism that explains disparate literature findings and links reported cortactin functions in motility and membrane trafficking.
Through mutant/rescue studies, we tested the requirements for key molecular interactions in cortactin regulation of FN trafficking and cell motility. Consistent with our previous report [7], we find that cortactin binding to the Arp2/3 complex and actin filaments is essential for rescue of all phenotypes. The other sites examined were in the C-terminus and regulate binding of additional partners. Neither the SH3 domain, which binds most known cortactin binding partners, or the Src/Abl phosphosites, which create an SH2 binding domain when phosphorylated [3] and are thought to negatively regulate the SH3 domain [23], were required for rescue of any cortactin-KD phenotype. These data were somewhat surprising because several SH3-domain-binding partners are good candidates for regulating vesicle trafficking, including the pinchase Dynamin2, the cdc42 GEF Fgd1, and the Arf GAP ASAP1 [3]. By contrast, intact Erk phosphorylation sites were required for rescue of motility [27] and FN deposition, suggesting that Erk phosphorylation may regulate more cortactin functions than just SH3 domain accessibility [23, 28]. Based on the requirement for intact Arp2/3- and F-actin-binding domains but not for SH3 domain binding partners, we speculate that the truly essential role of cortactin in ECM trafficking is to regulate branched actin networks at late endosomes or at docking sites for secretory endosomal/lysosomal carriers. Binding of SH3-domain partners by cortactin might contribute to cortactin-regulated vesicular trafficking events (e.g. fission or fusion) but may not be essential if a stable actin network in conjunction with membrane-bound signaling factors is sufficient to assemble the required multiprotein trafficking complexes.
A somewhat surprising finding from this study is that cortactin-KD does not inhibit endocytosis of β1 integrin, or apparently, FN. A number of studies have examined the role of cortactin in endocytosis and most have found KD or inhibition of cortactin reduces endocytic rates [29–36]. However, the vast majority of those studies examined clathrin-dependent endocytosis and β1 integrin has been reported to undergo clathrin-independent, lipid raft-dependent endocytosis [16, 37]. Although cortactin has also been reported to regulate clathrin-independent endocytosis [32, 34], β1 integrin has not been previously examined as a cargo and there are multiple clathrin-independent pathways [37]. Furthermore, cortactin likely does not affect all endocytic events since dextran uptake is apparently unperturbed by cortactin inhibition [30].
A key finding of this study is that FN that is internalized, processed, and resecreted via a late endocytic/lysosomal compartment is reused as a motility substrate. Lysosomally processed FN may be particularly potent as a substrate, since partial degradation of FN can yield fragments with increased adhesiveness [38], and the lysosomal proteinase Cathepsin produces biologically active fragments of FN [39]. To directly test this potential mechanism, we knocked down the lysosomal fusion regulator Syt7. Indeed, KD of Syt7 phenocopied cortactin-KD and inhibited both basal FN secretion and cell motility. Interestingly, a recent report implicated Syt7-mediated lysosomal secretion in leukocyte chemotaxis while another study described regulation of epithelial cell migration by the late endosomal/lysosomal v-SNARE VAMP7 [40, 41], suggesting that this mechanism of motility regulation may be general. Both studies showed localization of lysosomal markers at lamellipodia, consistent with exocytosis occurring at the leading edge of migrating cells.
Although we focused on fibronectin secretion in this study, it is likely that the same vesicular carriers contain other motility-regulating molecules (Figure 7E). Indeed, integrins may be co-secreted with their ligands, since α5β1 integrin-fibronectin cotrafficking through multivesicular bodies to lysosomes was recently shown to be important for cell migration [42]. Consistent with that idea, in our β1 integrin endocytosis assays the only change in KD cells that was not rescued by plating on high FN was intracellular accumulation of β1 integrin at the late 15 min timepoint, suggesting a block in late endosomal trafficking. It is also possible that newly synthesized motility molecules may be secreted via the same pathway since post-Golgi packaging can occur in endosomes and secretory lysosomes [43, 44].
In light of our findings, how do we consider the role of cortactin throughout the cell? Cortactin is one of the best markers of branched actin at various cellular sites and is particularly popular as a marker for branched-actin-rich lamellipodia and invadopodia [2]. Given the affinity of cortactin for Arp2/3 complex [25] and newly-polymerized actin [7], and the cooperative regulation of branched actin assembly noted by many investigators [26, 45–48], it seems likely that cortactin does regulate branched actin in lamellipodia and elsewhere. In fact, cortactin has been shown to affect cell edge protrusion during initial cell spreading on ECM-coated substrates [18], suggesting that cortactin does indeed regulate branched actin dynamics within cellular protrusions [7, 8, 49]. However, we found that interaction of cortactin with Arp2/3 complex and F-actin was also required for FN secretion suggesting that regulation of exocytosis is a key consequence of cortactin action on branched actin networks that promotes migration and invasion [3].
Experimental Procedures
Cell culture and gene manipulation
HT1080 cell lines, knockdown and rescue constructs, motility and lamellipodial dynamics assays were previously described [7]. Cortactin mutants were gifts from Dr. Thomas Parson (University of Virginia) and cloned into LZRS retroviral vector. A lentiviral shRNA expression system, pLKO.1 was used to knock down Synaptotagmin 7 (Open Biosystems, RHS3979-9577052 for KD-1 and RHS3979-9577053 for KD-2) or GFP (Sigma-Aldrich, SHC005) in HT1080 cells.
Antibodies and reagents
Anti-β1 (12G10 for flow cytometry and M-106 for Western blots), anti-α2 (P1E6), anti-α3 (P1B5), anti-α5 (P1D6) integrin antibodies, normal mouse IgG1 isotype control for FACS (sc-3877), anti-FN rabbit polyclonal (H-300 used in the triple staining with Rab7 and LAMP1 antibodies), and anti-Rab7 (goat, A-16) antibodies were from Santa Cruz Biotechnology. Anti-cortactin mouse monoclonal (4F11, Upstate) and rabbit polyclonal (H-191, Santa Cruz Biotechnology), anti-β-actin (Ac-74, Sigma), anti-GAPDH (14C10, Cell signaling), anti-FN mouse monoclonal (610077, used in TIRF and intracellular accumulation assays), anti-EEA1 (610456), and anti-CD107a (LAMP1, mouse, 555798) (BD Transduction Laboratories) antibodies were purchased. Anti-β1 integrin rat monoclonal antibody (AIIB2) was a gift from Dr. Roy Zent (Vanderbilt University). Anti-Rab8a and anti-Rab11a antibodies were gifts from Dr. James Goldenring (Vanderbilt University). Anti-Synaptotagmin 7 antibody (105 173) was from Synaptic Systems. AlexaFluor-conjugated secondary antibodies were from Invitrogen. Human plasma FN was from GIBCO BRL and human collagen type I was from Sigma. DyLight 550 NHS Ester (62262) and EZ-Link Sulfo-NHS-LC-LC-Biotin (21338) were from Thermo Scientific and conjugated to FN through manufacturer’s instructions. LysoTracker Green DND-26 (L-7526) was from Molecular Probes.
Statistical analyses
All statistical analyses were performed using the student t-test and calculated by Microsoft Office Excel software except for the live TIRF imaging, for which statistical analysis was performed using MatLab.
Supplemental Experimental Procedures are included in Supplemental Information.
Supplementary Material
Acknowledgments
We thank members of the Weaver laboratory and Dr. Geri Kreitzer for helpful comments. This work was supported by NIH 1R01GM075126 and ACS RSG-118085 to AMW and NIH R01-GM78373 to IK. Some experiments involved use of the VUMC Flow Cytometry Core.
Footnotes
Supplemental information includes 6 figures, 2 Videos, and Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Weaver AM. Cortactin in tumor invasiveness. Cancer Letters. 2008;265:157–166. doi: 10.1016/j.canlet.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: A multifunctional regulator of cellular invasiveness. Cell Adh Migr. 2011:5. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somogyi K, Rorth P. Cortactin modulates cell migration and ring canal morphogenesis during Drosophila oogenesis. Mech Dev. 2004;121:57–64. doi: 10.1016/j.mod.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Perrin BJ, Amann KJ, Huttenlocher A. Proteolysis of Cortactin by Calpain Regulates Membrane Protrusion during Cell Migration. Mol Biol Cell. 2006;17:239–250. doi: 10.1091/mbc.E05-06-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka S, Kunii M, Harada A, Okabe S. Generation of cortactin floxed mice and cellular analysis of motility in fibroblasts. Genesis. 2009;47:638–646. doi: 10.1002/dvg.20544. [DOI] [PubMed] [Google Scholar]
- 7.Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–1285. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 8.Lai FP, Szczodrak M, Oelkers JM, Ladwein M, Acconcia F, Benesch S, Auinger S, Faix J, Small JV, Polo S, et al. Cortactin promotes migration and platelet-derived growth factor-induced actin reorganization by signaling to Rho-GTPases. Mol Biol Cell. 2009;20:3209–3223. doi: 10.1091/mbc.E08-12-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupton SL, Anderson KL, Kole TP, Fischer RS, Ponti A, Hitchcock-DeGregori SE, Danuser G, Fowler VM, Wirtz D, Hanein D, et al. Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J Cell Biol. 2005;168:619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Borm B, Requardt RP, Herzog V, Kirfel G. Membrane ruffles in cell migration: indicators of inefficient lamellipodia adhesion and compartments of actin filament reorganization. Exp Cell Res. 2005;302:83–95. doi: 10.1016/j.yexcr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Goldfinger LE, Han J, Kiosses WB, Howe AK, Ginsberg MH. Spatial restriction of alpha4 integrin phosphorylation regulates lamellipodial stability and alpha4beta1-dependent cell migration. J Cell Biol. 2003;162:731–741. doi: 10.1083/jcb.200304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinz B, Alt W, Johnen C, Herzog V, Kaiser H. Quantifying lamella dynamics of cultured cells by SACED, a new computer-assisted motion analysis. Exp Cell Res. 1999;251:234–243. doi: 10.1006/excr.1999.4541. [DOI] [PubMed] [Google Scholar]
- 15.Schifferli KP, Henrich CJ. Analysis of integrin expression and function in HT1080 cells using inhibitory anti-integrin antibodies. Focus. 1996;18:13–15. [Google Scholar]
- 16.Shi F, Sottile J. Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci. 2008;121:2360–2371. doi: 10.1242/jcs.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruk PA, Auersperg N. A line of rat ovarian surface epithelium provides a continuous source of complex extracellular matrix. In Vitro Cell Dev Biol Anim. 1994;30A:217–225. doi: 10.1007/BF02632043. [DOI] [PubMed] [Google Scholar]
- 18.Lapetina S, Mader CC, Machida K, Mayer BJ, Koleske AJ. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J Cell Biol. 2009;185:503–519. doi: 10.1083/jcb.200809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boettiger D. Quantitative measurements of integrin-mediated adhesion to extracellular matrix. Methods Enzymol. 2007;426:1–25. doi: 10.1016/S0076-6879(07)26001-X. [DOI] [PubMed] [Google Scholar]
- 20.Hayman EG, Ruoslahti E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J Cell Biol. 1979;83:255–259. doi: 10.1083/jcb.83.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu VW, Prekeris R. Transport at the recycling endosome. Curr Opin Cell Biol. 2010;22:528–534. doi: 10.1016/j.ceb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinley AW, Weed SA, Weaver AM, Karginov AV, Bissonette E, Cooper JA, Parsons JT. Cortactin Interacts with WIP in Regulating Arp2/3 Activation and Membrane Protrusion. Curr Biol. 2003;13:384–393. doi: 10.1016/s0960-9822(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, Parsons JT. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uruno T, Liu J, Zhang P, Fan Yx Y, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 26.Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 27.Kelley LC, Hayes KE, Ammer AG, Martin KH, Weed SA. Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PLoS One. 2010;5:e13847. doi: 10.1371/journal.pone.0013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley LC, Hayes KE, Ammer AG, Martin KH, Weed SA. Revisiting the ERK/Src cortactin switch. Commun Integr Biol. 2011;4:205–207. doi: 10.4161/cib.4.2.14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao H, Chen J, Krueger EW, McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the "constitutive" endocytosis of transferrin. Mol Cell Biol. 2010;30:781–792. doi: 10.1128/MCB.00330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engqvist-Goldstein AE, Zhang CX, Carreno S, Barroso C, Heuser JE, Drubin DG. RNAi-mediated Hip1R silencing results in stable association between the endocytic machinery and the actin assembly machinery. Mol Biol Cell. 2004;15:1666–1679. doi: 10.1091/mbc.E03-09-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grassart A, Meas-Yedid V, Dufour A, Olivo-Marin JC, Dautry-Varsat A, Sauvonnet N. Pak1 phosphorylation enhances Cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic. 2010;11:1079–1091. doi: 10.1111/j.1600-0854.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 33.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Sauvonnet N, Dujeancourt A, Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of {gamma}c cytokine receptor. J Cell Biol. 2005;168:155–163. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Yu D, Zeng XC, Zhou K, Zhan X. Receptor-mediated endocytosis involves tyrosine phosphorylation of cortactin. J Biol Chem. 2007;282:16086–16094. doi: 10.1074/jbc.M701997200. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Zhou K, Hao JJ, Liu J, Smith N, Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J Cell Sci. 2005;118:807–817. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]
- 37.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valenick LV, Hsia HC, Schwarzbauer JE. Fibronectin fragmentation promotes alpha4beta1 integrin-mediated contraction of a fibrin-fibronectin provisional matrix. Exp Cell Res. 2005;309:48–55. doi: 10.1016/j.yexcr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 39.Humphries MJ, Ayad SR. Stimulation of DNA synthesis by cathepsin D digests of fibronectin. Nature. 1983;305:811–813. doi: 10.1038/305811a0. [DOI] [PubMed] [Google Scholar]
- 40.Colvin RA, Means TK, Diefenbach TJ, Moita LF, Friday RP, Sever S, Campanella GS, Abrazinski T, Manice LA, Moita C, et al. Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat Immunol. 2010;11:495–502. doi: 10.1038/ni.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proux-Gillardeaux V, Raposo G, Irinopoulou T, Galli T. Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biol Cell. 2007;99:261–271. doi: 10.1042/BC20060097. [DOI] [PubMed] [Google Scholar]
- 42.Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerod L, Stenmark H. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell. 2010;19:148–159. doi: 10.1016/j.devcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 45.Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, Condeelis J. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010;123:3662–3673. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci U S A. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol. 2002;12:1270–1278. doi: 10.1016/s0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- 49.Cai L, Makhov AM, Schafer DA, Bear JE. Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell. 2008;134:828–842. doi: 10.1016/j.cell.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.