Abstract
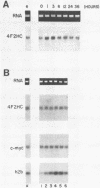
The murine 4F2 molecule is a 125 kilodalton disulfide-linked heterodimeric cell-surface glycoprotein which has been shown to be involved in the processes of cellular activation and proliferation (1). To elucidate the structure, expression, and regulation of the 4F2 molecule, a murine 4F2 heavy chain (4F2HC) cDNA has been isolated and structurally characterized. The murine 4F2HC is a 526 amino acid (aa) type II membrane glycoprotein which is composed of a 75 aa N-terminal intracytoplasmic region, a single hydrophobic putative transmembrane domain, and a 428 aa C-terminal extracellular domain. Comparison with the human 4F2HC cDNA reveals the highest degree of sequence identity within the transmembrane and intracytoplasmic domains. Northern blot analyses have demonstrated that the 4F2HC gene is expressed at relatively high levels in adult testis, lung, brain, kidney, and spleen, and at significantly lower levels in adult liver and cardiac and skeletal muscle. Studies designed to elucidate the pattern of regulation of the murine 4F2HC gene have demonstrated that it is induced during the process of cell activation, but is subsequently expressed at constant levels throughout the cell cycle in exponentially growing cells.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alterman R. B., Ganguly S., Schulze D. H., Marzluff W. F., Schildkraut C. L., Skoultchi A. I. Cell cycle regulation of mouse H3 histone mRNA metabolism. Mol Cell Biol. 1984 Jan;4(1):123–132. doi: 10.1128/mcb.4.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Azzarone B., Malpièce Y., Zaech P., Moretta L., Fauci A., Suarez H. Analysis of the expression of the 4F2 surface antigen in normal and neoplastic fibroblastic human cells of embryonic and adult origin. Exp Cell Res. 1985 Aug;159(2):451–462. doi: 10.1016/s0014-4827(85)80018-5. [DOI] [PubMed] [Google Scholar]
- Bender T. P., Kuehl W. M. Murine myb protooncogene mRNA: cDNA sequence and evidence for 5' heterogeneity. Proc Natl Acad Sci U S A. 1986 May;83(10):3204–3208. doi: 10.1073/pnas.83.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Bron C., Rousseaux M., Spiazzi A. L., MacDonald H. R. Structural homology between the human 4F2 antigen and a murine cell surface glycoprotein associated with lymphocyte activation. J Immunol. 1986 Jul 15;137(2):397–399. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Thompson C. B., Eisenman R. N. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. 1985 Mar 28-Apr 3Nature. 314(6009):366–369. doi: 10.1038/314366a0. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Hemler M. E., Strominger J. L. Characterization of antigen recognized by the monoclonal antibody (4F2): different molecular forms on human T and B lymphoblastoid cell lines. J Immunol. 1982 Aug;129(2):623–628. [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. Protooncogene expression during the cell cycle. Lab Invest. 1986 Apr;54(4):365–376. [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J Immunol. 1984 Jun;132(6):2857–2861. [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Formation of the 3' end of histone mRNA by post-transcriptional processing. Nature. 1984 Mar 8;308(5955):203–206. doi: 10.1038/308203a0. [DOI] [PubMed] [Google Scholar]
- La Bella F., Brown E. H., Basilico C. Changes in the levels of viral and cellular gene-transcripts in the cell cycle of SV40 transformed mouse cells. J Cell Physiol. 1983 Oct;117(1):62–68. doi: 10.1002/jcp.1041170110. [DOI] [PubMed] [Google Scholar]
- Leiden J. M., Dialynas D. P., Duby A. D., Murre C., Seidman J., Strominger J. L. Rearrangement and expression of T-cell antigen receptor genes in human T-lymphocyte tumor lines and normal human T-cell clones: evidence for allelic exclusion of Ti beta gene expression and preferential use of a J beta 2 gene segment. Mol Cell Biol. 1986 Sep;6(9):3207–3214. doi: 10.1128/mcb.6.9.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T., June C. H., Thompson C. B., Leiden J. M. Regulation of 4F2 heavy-chain gene expression during normal human T-cell activation can be mediated by multiple distinct molecular mechanisms. Mol Cell Biol. 1988 Sep;8(9):3820–3826. doi: 10.1128/mcb.8.9.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. T., Baserga R., Mercer W. E. Adenovirus type 2 activates cell cycle-dependent genes that are a subset of those activated by serum. Mol Cell Biol. 1985 Nov;5(11):2936–2942. doi: 10.1128/mcb.5.11.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumadue J. A., Glick A. B., Ruddle F. H. Cloning, sequence analysis, and expression of the large subunit of the human lymphocyte activation antigen 4F2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9204–9208. doi: 10.1073/pnas.84.24.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K., Bron C. Cell surface glycoproteins involved in the stimulation of interleukin 1-dependent interleukin 2 production by a subline of EL4 thymoma cells. I. Functional characterization by monoclonal antibodies. J Immunol. 1985 Dec;135(6):3944–3950. [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M., Quackenbush E. J., Letarte M. Inhibition of Na+/Ca2+ exchanger activity in cardiac and skeletal muscle sarcolemmal vesicles by monoclonal antibody 44D7. J Biol Chem. 1986 Jan 5;261(1):92–95. [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Persson H., Gray H. E., Godeau F. Growth-dependent synthesis of c-myc-encoded proteins: early stimulation by serum factors in synchronized mouse 3T3 cells. Mol Cell Biol. 1985 Nov;5(11):2903–2912. doi: 10.1128/mcb.5.11.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posillico J. T., Wilson R. E., Srikanta S. S., Eisenbarth G. S., Letarte M., Quackenbush E. J., Quaranta V., Kajaji S., Brown E. M. Monoclonal antibody-mediated modulation of parathyroid hormone secretion by dispersed parathyroid cells. Arch Surg. 1987 Apr;122(4):436–442. doi: 10.1001/archsurg.1987.01400160062009. [DOI] [PubMed] [Google Scholar]
- Quackenbush E. J., Linsley P., Letarte M. Mouse L cells express a molecular complex carrying the human epitopes recognized by monoclonal antibodies 44D7 and 44H7 after DNA-mediated gene transfer. J Immunol. 1986 Jul 1;137(1):234–239. [PubMed] [Google Scholar]
- Quackenbush E., Clabby M., Gottesdiener K. M., Barbosa J., Jones N. H., Strominger J. L., Speck S., Leiden J. M. Molecular cloning of complementary DNAs encoding the heavy chain of the human 4F2 cell-surface antigen: a type II membrane glycoprotein involved in normal and neoplastic cell growth. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6526–6530. doi: 10.1073/pnas.84.18.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleckman B. P., Peterson A., Jones W. K., Foran J. A., Greenstein J. L., Seed B., Burakoff S. J. Expression and function of CD4 in a murine T-cell hybridoma. Nature. 1987 Jul 23;328(6128):351–353. doi: 10.1038/328351a0. [DOI] [PubMed] [Google Scholar]
- Snyder M., Davidson N. Two gene families clustered in a small region of the Drosophila genome. J Mol Biol. 1983 May 15;166(2):101–118. doi: 10.1016/s0022-2836(83)80001-1. [DOI] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Suomalainen H. A. The monoclonal antibodies Trop-4 and 4F2 detect the same membrane antigen that is expressed at an early stage of lymphocyte activation and is retained on secondary lymphocytes. J Immunol. 1986 Jul 15;137(2):422–427. [PubMed] [Google Scholar]
- Swan D. G., Hale R. S., Dhillon N., Leadlay P. F. A bacterial calcium-binding protein homologous to calmodulin. Nature. 1987 Sep 3;329(6134):84–85. doi: 10.1038/329084a0. [DOI] [PubMed] [Google Scholar]
- Teixeira S., Di Grandi S., Kühn L. C. Primary structure of the human 4F2 antigen heavy chain predicts a transmembrane protein with a cytoplasmic NH2 terminus. J Biol Chem. 1987 Jul 15;262(20):9574–9580. [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Expression of the c-myb proto-oncogene during cellular proliferation. 1986 Jan 30-Feb 5Nature. 319(6052):374–380. doi: 10.1038/319374a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yagita H., Masuko T., Hashimoto Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation-associated cell surface antigen system in rats and humans. Cancer Res. 1986 Mar;46(3):1478–1484. [PubMed] [Google Scholar]
- Yagita H., Masuko T., Takahashi N., Hashimoto Y. Monoclonal antibodies that inhibit activation and proliferation of lymphocytes. I. Expression of the antigen on monocytes and activated lymphocytes. J Immunol. 1986 Mar 15;136(6):2055–2061. [PubMed] [Google Scholar]