Abstract
Background
Although a subset of recent studies have suggested red blood cell (RBC) storage length is associated with adverse patient outcomes, others have shown no such relationship. Adults may be transfused with RBC units of different storage lengths, and existing studies do not take into consideration that fresh RBCs may alter responses to concurrently transfused stored RBCs. To test this possibility, we utilized a murine model and investigated transfusion outcomes of fresh, stored, or fresh plus stored RBCs.
Study Design and Methods
Fresh, 14-day stored, or fresh plus 14-day stored leukoreduced RBCs from HOD transgenic donors (with RBC specific expression of hen egg lysozyme, ovalbumin, and human Duffyb) were transfused into naïve C57BL/6 recipients. Serum cytokines and anti-HOD alloimmunization were evaluated following transfusion.
Results
In 6 of 6 experiments (n=90 mice total), a pro-inflammatory serum cytokine storm of interleukin-6, keratinocyte-derived chemokine/CXCL1, and monocyte chemoattractant protein-1 was observed in transfusion recipients of stored but not fresh RBCs, along with high degrees of anti-HOD alloimmunization. However, concurrent transfusion of fresh HOD RBCs along with stored HOD RBCs significantly decreased these adverse outcomes (p<0.05).
Conclusions
These results are consistent with fresh murine HOD RBCs losing protective properties during storage, and introduce a previously unrecognized variable in RBC storage studies. If translatable to humans, uniform “old blood” groups may be needed in future clinical studies to most accurately investigate the biological effects of older RBC units.
Introduction
FDA guidelines allow storage of human RBCs for up to 42 days before transfusion. A number of clinical studies have suggested that transfusion of “older” stored RBCs may lead to worse medical outcomes than fresher RBC units, including increases in multi-system organ failure, infection, deep vein thrombosis, inadequate oxygenation, and mortality 1–6. However, other studies have failed to find an association between older stored RBCs and adverse clinical outcomes 7–11. Thus, although it cannot be disputed that changes occur in RBCs over the course of storage 12, the effects of these changes on post-transfusion clinical outcomes are not entirely obvious.
There is no uniform definition of an “older” RBC unit, and different definitions of RBC age have been used in past studies, the majority of which have been retrospective in nature. Some investigators have defined the length of storage by median RBC unit age 13, some by oldest RBC unit age 7,10, and some by RBC unit age in hours 14. Complicating factors in these studies include that the most ill patients receive the largest number of blood products and thus are more likely to receive an older RBC unit. Furthermore, it is not known whether there is a certain RBC storage age beyond which adverse outcomes are seen (e.g. is a 22 day old unit safe but a 30 day old unit dangerous?). Thus, variation in the definition of an “older” RBC unit may have influenced the results of past studies.
The largest study to date investigating potential adverse outcomes of older RBCs was recently published by Koch et al in the NEJM 3. This retrospective study contained nearly 6000 patients receiving RBCs during surgery for coronary artery bypass grafting (CABG) or valve replacement. Patients were separated by whether they received RBC stored for 14 days or less or RBCs stored for more than 14 days; patients receiving RBCs of overlapping ages (e.g. both younger and older than 14 days) were excluded. Patients in the older RBC group had more adverse outcomes, including higher in hospital death rates (2.8%) compared to those in the fresher RBC group (1.7%). Although galvanizing, this study was limited not only by its retrospective nature but also by the fact that patients in the older RBC group had a higher incidence of left ventricular dysfunction, peripheral vascular disease, and mitral valve regurgitation 15.
The Koch study, in combination with conflicting conclusions drawn from past retrospective studies, have led to the recent initiation of 4 large-scale, prospective, randomized trials investigating the effect of RBC storage duration on patient outcome. Koch and colleagues are conducting a prospective study entitled Red Cell Storage Duration and Outcomes in Cardiac Surgery, comparing post-operative morbid outcomes in 2800 cardiovascular surgery recipients of fresher (<14 days) or older (>20 days) RBCs 16. In addition, The National Heart Lung Blood Institute Red Cell Storage Duration Study (RECESS) is comparing the effects of fresher RBC units (≤10 days of storage) to older RBC units (≤21 days of storage) in cardiac surgery patients at 17 US hospitals 17. The Canadian Institutes of Health Research Age of Blood Evaluation (ABLE) study is comparing the effects of fresher RBC units (<8 days of storage) to standard issue RBC units (2–42 days of storage) in critically ill patients18. Lastly, The Age of Red Blood Cells in Premature Infants (ARIPI) trial is comparing fresher RBC units (<7 days of storage) to standard issue RBC units (<42 days) in low birth weight infants (<1250 g) 19. Patients on the “older” or “standard issue” arms of these studies may receive moderately old RBC units, RBC units near their outdates, or a mixture of fresher and older RBC units.
To control for precise RBC unit age and for underlying recipient disease status in a reductionist setting, we have developed a murine model of blood storage. We recently reported the characterization of this model, which uses the same storage solutions, pre-storage leukoreduction filters, and post-collection processing as is used for human RBCs20. Using this system, we have reported that transfusion of 14-day stored RBCs rapidly induces a pro-inflammatory recipient cytokine storm (e.g. interleukin-6 (IL-6), the murine IL-8 homologue keratinocyte-derived chemokine/CXCL1 (KC/CXCL1), and monocyte chemoattractant protein-1 (MCP-1)) 21. As cytokine storm is well-known to contribute to morbidity/mortality in some disease states 22–25, these findings may be of particular relevance to transfusion recipients. Moreover, stored murine RBCs are significantly more immunogenic than fresh RBCs, resulting in a substantial increase in recipient alloantibody formation 26, an issue of significance to patients requiring ongoing transfusion support.
Taking into account that most adult transfusion recipients require more than 1 unit of transfused RBCs at a time (often of different storage lengths), we have now utilized this reductionist murine system to test the combined effects of transfusing fresh and stored murine RBCs into the same recipient. Herein, we report that transfusion of fresh RBCs, concurrent with 14-day stored RBCs, decreases the cytokine storm and also decreases the alloantibody response seen following the transfusion of stored RBCs alone. The described studies thus introduce a previously unappreciated factor that may be relevant to human transfusion medicine, and one that may complicate the interpretation of studies investigating the effects of older versus fresher blood. Furthermore, uniform “old blood” groups may be needed in ongoing trials (including Red Cell Storage Duration and Outcomes in Cardiac Surgery, RECESS, ABLE, and ARIPI) to assess the biological effects of older RBC units.
Materials and Methods
Mice
C57BL/6 and FVB mice were purchased from Jackson Laboratories (Bar Harbor, ME) or the National Cancer Institute (Frederick, MD). HOD mice (containing RBC specific hen egg lysozyme, ovalbumin, and human Duffyb antigen sequences) 27 were bred by the Emory University Department of Animal Resources; all protocols were approved by the Emory University Institutional Animal Care and Use Committee. C57BL/6 mice were utilized as transfusion recipients, HOD mice were utilized as blood donors for transfusion of antigen positive RBCs, and RBCs from FVB mice were utilized as negative controls for flow cytometric crossmatching (FVB mice are on the same genetic background as HOD mice, but their RBCs lack the HOD antigen).
Murine Blood Collection, Processing, and Transfusion
Donor blood was collected in CPDA-1 with a final concentration of 14%, leukoreduced using a dry Pall neonatal leukoreduction filter (East Hills, NY), centrifuged at 250 x g, adjusted to an hematocrit of 75%, and stored in Eppendorf tubes at 4° C for 0 or 14 days prior to transfusion, as previously described 20. C57BL/6 recipients were transfused with 100 or 200 microliters (1 or 2 “units”) of fresh or stored leukoreduced HOD RBCs, or a combination of 1 unit of fresh plus 1 unit of stored RBCs.
Flow Cytometry
Peripheral blood was collected 90 minutes after transfusion and cytokines were measured in serum by cytokine bead array assay (Mouse Flex Kit, BD Biosciences). Alloantibody responses to the HOD antigen were measured 2 weeks following transfusion by indirect immunofluorescence using HOD RBCs as targets and wild-type FVB RBCs to establish non-specific background. Antibody binding was detected by flow cytometry using goat anti-mouse immunoglobulin antibody (Becton Dickinson). Direct antiglobulin testing was performed by staining RBCs with goat anti-mouse immunoglobulin and gating on HOD RBCs using anti-Fy3 (MIMA 29, generously provided by Greg Halverson and Marion Reid of the NY Blood Center).
Statistical Analysis
Statistical analysis and graphing was performed with Graph Pad Prism software. One way ANOVA with Bonferroni post test or student’s t-test were performed, with a statistically significant value defined as p<0.05.
Results
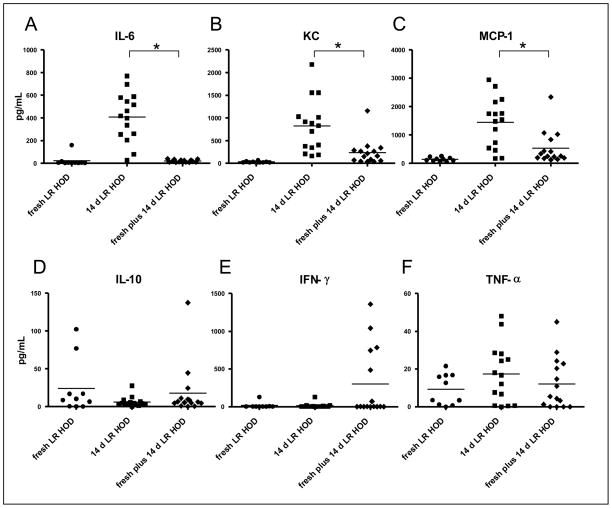
C57BL/6 mice were transfused with 1 or 2 units of fresh HOD RBCs, 1 or 2 units of 14-day stored HOD RBCs, or a combination of fresh and stored HOD RBCs (1 unit each, transfused minutes apart into opposite lateral tail veins). As previously observed in a different murine model of autologous transfusion 21, transfusion of 14-day stored HOD RBCs resulted in a recipient cytokine storm 90 minutes post-transfusion, with high serum levels of IL-6, the murine IL-8 homologue KC, and MCP-1. In contrast, only very low levels of cytokines were measured after transfusion of fresh HOD RBCs (Fig. 1), similar to baseline cytokine levels 21. However, in 3 of 3 experiments (n= 5 mice/group/experiment: 45 mice total) concurrent transfusion of fresh HOD RBCs along with stored HOD RBCs resulted in a substantial decrease in recipient serum IL-6, KC, and MCP-1 levels, compared to that seen following transfusion of stored blood alone (p<0.05). No statistically significant differences were observed in levels of IL-10, IFN-γ, or TNF-α. Of note, the mice with the highest levels of IFN-γ were all from the same cage, in the same experiment.
Figure 1. Concurrent transfusion of fresh HOD RBCs with stored HOD RBCs decreases the proinflammatory cytokine storm seen following the transfusion of stored HOD RBCs alone.
Wild-type C57BL/6 recipient mice were transfused with either 1 unit (i.e. 100 microliters of packed RBCs) of fresh, leukoreduced HOD RBCs, 1 unit of 14-day stored, leukoreduced HOD RBCs, or a combination of 1 unit of fresh, leukoreduced HOD RBCs and 1 unit of 14-day stored, leukoreduced HOD RBCs. In the combined group, the units were transfused minutes apart and into different tail veins. Recipient cytokine responses were evaluated in sera collected 90 minutes following transfusion. Concurrent transfusion of fresh plus stored HOD RBCs blunted the cytokine response of IL-6 (A), KC (B), and MCP-1 (C), as compared to stored RBCs alone, in a statistically significant manner (*p<0.05 by one way ANOVA with Bonferroni post-test). No statistically significant differences in recipient serum levels of IL-10 (C), IFN-γ (E), or TNF-α (F) were seen. A compilation of data from 3 independent experiments (4–5 mice/group/experiment) is shown.
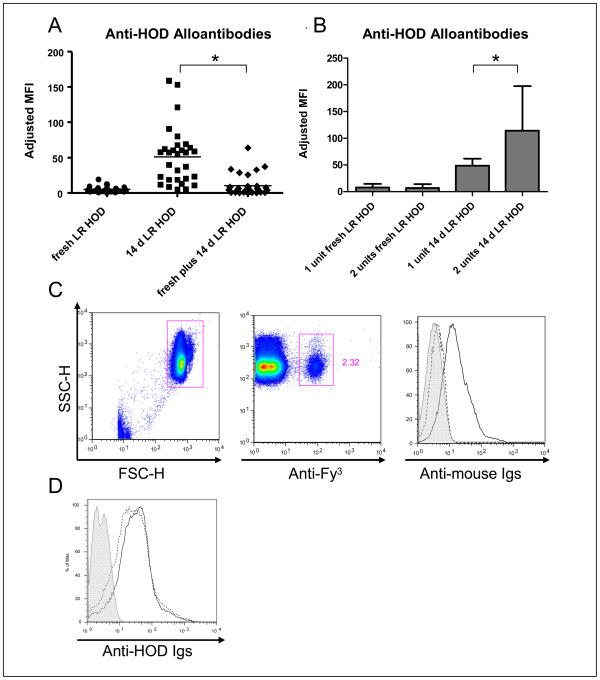
Two weeks post-transfusion, recipients were evaluated for alloantibody response(s) to transfused HOD RBCs by flow cytometric crossmatch. Sera were crossmatched with target HOD or control antigen negative (FVB) RBCs, with adjusted mean fluorescence intensity being determined by background binding (FVB) subtracted from target binding (HOD). Consistent with our prior observations, 14-day stored HOD RBCs were significantly more immunogenic than fresh HOD RBCs. However, in 6 of 6 experiments (5 mice/group/experiment: 90 recipients total) fresh HOD RBCs transfused concurrently with stored HOD RBCs significantly blunted the anti-HOD alloantibody response as compared to transfusion of stored RBCs alone (Fig. 2A).
Figure 2. Concurrent transfusion of fresh HOD RBCs with stored HOD RBCs decreases the enhanced alloantibody response seen following the transfusion of stored HOD RBCs alone.
Anti-HOD alloantibody responses in recipients were measured by flow cytometric crossmatch two weeks following the transfusion of either 1 unit of fresh, leukoreduced HOD RBCs, 1 unit of 14-day stored, leukoreduced HOD RBCs, or a combination of 1 unit of fresh, leukoreduced HOD RBCs and 1 unit of stored, leukoreduced HOD RBCs. Anti-HOD alloantibodies were assayed by indirect immunofluorescence; the adjusted mean fluorescence intensity (MFI) shown is the difference of the signal of sera crossmatched with control, antigen-negative target RBCs from the signal of sera crossmatched with target, transgenic HOD RBCs (A); a compilation of data from 6 independent experiments (4–5 mice/group/experiment) is shown, with a statistically significant difference in alloantibody level seen in the fresh plus stored RBC group, as compared to the stored RBC group (*p<0.05 by one way ANOVA with Bonferroni post-test). In 4 of these experiments, an additional group of mice was transfused with 2 units of stored, leukoreduced HOD RBCs or 2 units of fresh, leukoreduced RBCs. A compilation of the anti-HOD alloantibody response (mean + 1 sd) to 1 versus 2 units of fresh or stored HOD RBCs is shown (B); 2 units of stored RBCs induced significantly higher levels (*p<0.05) of anti-HOD alloantibodies than 1 unit. To determine whether the “fresh plus stored” group of transfusion recipients had less detectable anti-HOD in their sera due to the presence of circulating HOD antigen-positive RBCs binding anti-HOD antibody, a direct antiglobulin test (C) was performed 2 weeks following transfusion. A RBC gate (C, left panel) was established by forward and side scatter properties with circulating HOD-positive RBCs identified by anti-Fy3 (C, middle panel). Binding of antibodies to gated HOD RBCs was assessed using a goat anti-mouse immunoglobulin secondary antibody (C, right panel): the solid line is a positive control of HOD RBCs with bound polyclonal mouse anti-HEL antiserum; the dashed line is RBCs from a representative recipient of fresh plus stored HOD RBCs; the shaded area is the negative control of HOD RBCs incubated with anti-mouse immunoglobulin secondary antibody alone. To determine the degree to which circulating HOD RBCs were capable of binding anti-HOD antibody, mice who had been transfused with 14-day stored, leukoreduced HOD RBCs 2 weeks prior were transfused with 1 unit of fresh, leukoreduced HOD RBCs and anti-HOD alloantibody was measured prior to (D, solid line) and 1 hour after (D, dashed line) the transfusion of fresh HOD RBCs; shaded histogram represents sera crossmatched with control, antigen-negative RBCs.
The recipients of the fresh plus stored groups received twice as much HOD antigen as the recipients in the fresh or stored only groups. Thus, to determine if the observed antibody blunting was due to either excess HOD antigen or increased RBC volume being immunosuppressive, further experiments were completed in which recipients received 1 or 2 units of 14-day stored HOD RBCs. In 3 of 3 experiments (5 mice/group/experiment: 30 recipients total), recipients of 2 units of 14-day stored HOD RBCs made significantly more anti-HOD than did recipients of 1 unit (Fig. 2B), p<0.05. Thus, the decreased anti-HOD in the fresh plus stored group was not simply due to recipients being exposed to more HOD antigen or greater RBC volume.
While fresh HOD RBCs circulate for many weeks after transfusion, 14-day stored HOD RBCs are cleared more rapidly 26. Thus, two additional experiments were performed to investigate whether the low serum levels of alloantibodies detected in the fresh plus stored group were an artifact of antibodies being removed from serum through binding to increased numbers of HOD RBCs in circulation. Direct antiglobulin tests (DATs) were performed in recipients of fresh plus stored HOD RBCs, using directly conjugated anti-Fy3 to gate on recipient circulating HOD RBCs and goat anti-mouse immunoglobulin to look for bound antibody. In 2 of 2 experiments (n=10 recipients total), there was no significant anti-HOD bound to residual circulating HOD RBCs at any time point evaluated (7, 10, or 14 days post-transfusion) (Fig. 2C shows a representative DAT). This negative result was not due to an inability of the assay to detect anti-HOD binding, as controls utilizing HOD RBCs incubated with polyclonal anti-HEL serum were DAT positive.
Moreover, to determine the amount of anti-HOD that fresh HOD RBCs were capable of removing from the serum, fresh HOD RBCs were transfused into mice with high-titer anti-HOD (recipients of 14-day stored RBCs). Anti-HOD was measured in recipient sera prior to the transfusion of fresh HOD RBCs and again at 1 hour and 24 hours post-transfusion. In 2 of 2 experiments, only subtle changes in serum anti-HOD levels were detected in 9 of 10 alloimmunized recipients following transfusion of HOD RBCs (e.g. indirect antiglobulin tests of serum anti-HOD levels were only slightly decreased after transfusion of target RBCs) (Fig. 2D shows a representative example). Based upon these additional control studies, we reject the interpretation that the fresh HOD RBCs simply titrate out anti-HOD from the serum and conclude that fresh HOD RBCs truly do inhibit the enhanced alloantibody responses seen following the transfusion of stored HOD RBCs alone.
Discussion
Together, these data demonstrate that concurrent transfusion of fresh HOD RBCs along with stored HOD RBCs blunts both the recipient pro-inflammatory cytokine storm and also blunts the alloimmunization response seen following the transfusion of stored HOD RBCs alone. These unexpected results suggest that fresh murine HOD RBCs possess immune dampening properties, and that these properties are lost at some point prior to 14 days of storage. The implications of these findings, should they reflect a general property of RBCs in humans, may be of significance to both interpreting clinical trials in particular and to transfusion management of patients in general.
Adverse outcomes of stored human RBC units have been described to include blood infection, thrombosis, pneumonia, renal failure, myocardial infarction, decreased systemic oxygenation, increased number of ventilator days, increased length of hospital stay, and death. The largest trial to date 3 examined a composite outcome of 17 different clinical outcomes, as well as 30-day mortality. However, none of the studies have examined recipient cytokine responses directly. Cytokines (including IL-1, IL-6, IL-8, TNF-α) are known to have both direct and indirect effects on a number disease processes 22–25,28. Hence, cytokine storm pathology is likely to have significant effects on medical outcomes, especially in patients with underlying co-morbidities. The cytokines examined in these experiments include those previously noted to be altered after transfusion of stored murine RBCs21 as well as those felt to be pertinent negatives; a more thorough analysis of recipient cytokine profiles following transfusion may offer additional insight into the presumed immune dampening effects of fresh HOD RBCs.
RBC alloimmunization was chosen as an outcome in these studies because it can be both medically and logistically problematic. The development of antibodies against an RBC antigen can result in hemolysis of the transfused unit 29. Furthermore, the presence of multiple alloantibodies may delay the provision of compatible units of blood for transfusion 30. Although rates of alloimmunization can be variable 31–33, all RBC transfusion recipients are potentially at risk for this complication.
The described findings may potentially be relevant to the interpretation of past clinical studies investigating effects of stored blood. More than half of these studies have suggested older RBCs are more detrimental than fresher RBCs, though study design and definition of RBC age vary significantly. Some studies have utilized the mean age of RBC units for analysis. A mean RBC age of 19 days would be reported for both a patient who received 2 units that were stored for 19 days and also for a patient who received a 3-day old and a 35-day old unit. Of note, the largest retrospective study to date that detected increased adverse outcomes in recipients of older RBC units 3 used neither mean nor median age as a measure, and had a true segregation based upon RBC unit ages. In contrast, many of the smaller studies that did not detect a difference used either mean or median age. Thus, a full understanding of the biology described herein, and its analysis within the context of human transfusion, are necessary.
Our findings may also potentially have relevance to the design of the ongoing Red Cell Storage Duration and Outcomes in Cardiac Surgery study, as well as the RECESS, ABLE, and ARIPI studies. Indeed, it is possible that a transfusion recipient on the “standard issue” arm of the ABLE study may receive a 3-day old unit along with a 42-day old unit, with the 3-day old unit potentially abrogating adverse effects seen had the 42-day old unit been transfused alone. Furthermore, realizing the time line by which a human RBC may lose immune dampening properties is not known, a cardiac patient in the Red Cell Storage Duration and Outcomes in Cardiac Surgery study or the RECESS study could receive a 22-day old unit along with a 42-day old unit, with the 22-day old unit potentially abrogating adverse effects of the 42-day old unit. These scenarios could lead to underestimation of the sequelae of older RBCs units.
Although the murine studies were carefully controlled and are highly reproducible, we present the results with complete recognition of the caveats of the model system. The murine RBCs were stored in the same anti-coagulant preservative solution (CPDA-1) used for humans but were not stored in bags with DEHP [Di(2-ethylhexyl)phthalate] plasticizer, and murine RBCs have a natural life-span that is substantially shorter at baseline than human RBCs. The RBCs used in these studies were stored for 14-days, which, when indexed for the murine 55 day RBC life span, approximates a human RBC unit near its 42-day outdate. However, 14-day stored murine HOD RBCs have a lower 24 hour post-transfusion recovery than typical 42-day stored human RBCs. In addition, the rate of transfusion is more rapid in the murine system than in most non-emergent human transfusion settings. Finally, like most human RBCs, HOD RBCs contain the human Duffyb antigen. This antigen has been shown to bind interleukin-8, MCP-1, and RANTES 34–38. The role of the Duffy antigen in the described findings is not entirely clear; however, as this antigen is also found on most human RBCs, its presence is consistent with protein biology of human RBCs.
In summary, the presented data suggest that HOD murine RBCs lose protective effects with storage. Additional studies are underway to determine whether this effect is generalizable to donor strains of other genetic backgrounds, to antigens other than HOD, and to outcomes other than recipient cytokine response and alloimmunization. Being cognizant that murine biology may not reflect human biology, these data introduce a previously unrecognized factor in the evaluation of the effects of fresh versus stored RBCs, and raise the question of whether human RBCs also lose immune dampening effects with storage. If so, human trials involving true “fresh” and “old” RBC arms, with a limited storage length distribution within each group, may be warranted to evaluate the biological effects of older stored transfused RBCs.
Acknowledgments
This work was supported in part by grants from the NIH (HL092959) and the American Society of Hematology (to JEH)
Footnotes
The authors declare they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION
Contributors: JEH, EAH, KEH, SLS, and JCZ designed the research, analyzed the data, and wrote the manuscript; JEH, EAH, and KEH performed the research.
References
- 1.Bennett-Guerrero E, Stafford-Smith M, Waweru PM, et al. A prospective, double-blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion. 2009;49(7):1375–1383. doi: 10.1111/j.1537-2995.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 2.Eikelboom JW, Cook RJ, Liu Y, Heddle NM. Duration of red cell storage before transfusion and in-hospital mortality. Am Heart J. 2010;159(5):737–743. e731. doi: 10.1016/j.ahj.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 3.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 4.Leal-Noval SR, Jara-Lopez I, Garcia-Garmendia JL, et al. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology. 2003;98(4):815–822. doi: 10.1097/00000542-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13(5):R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinicalconsequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 7.Vamvakas EC, Carven JH. Length of storage of transfused red cells and postoperative morbidity in patients undergoing coronary artery bypass graft surgery. Transfusion. 2000;40(1):101–109. doi: 10.1046/j.1537-2995.2000.40010101.x. [DOI] [PubMed] [Google Scholar]
- 8.Yap CH, Lau L, Krishnaswamy M, Gaskell M, Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86(2):554–559. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Hebert PC, Chin-Yee I, Fergusson D, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100(5):1433–1438. doi: 10.1213/01.ANE.0000148690.48803.27. table of contents. [DOI] [PubMed] [Google Scholar]
- 10.Taylor RW, O’Brien J, Trottier SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34(9):2302–2308. doi: 10.1097/01.CCM.0000234034.51040.7F. quiz 2309. [DOI] [PubMed] [Google Scholar]
- 11.Walsh TS, McArdle F, McLellan SA, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32(2):364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 12.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96(2):93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 13.Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion. 1999;39(7):701–710. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- 14.Wasser MN, Houbiers JG, D’Amaro J, et al. The effect of fresh versus stored blood on postoperative bleeding after coronary bypass surgery: a prospective randomized study. Br J Haematol. 1989;72(1):81–84. doi: 10.1111/j.1365-2141.1989.tb07656.x. [DOI] [PubMed] [Google Scholar]
- 15.Dzik W. Fresh blood for everyone? Balancing availability and quality of stored RBCs. Transfus Med. 2008;18(4):260–265. doi: 10.1111/j.1365-3148.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 16.Koch CG. [Accessed 2-1-11.];Red Cell Storage Duration and Outcomes in Cardiac Surgery. http://wwwclinicaltrialsgov/NCT00458783.
- 17.Steiner ME, Assmann SF, Levy JH, et al. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7) Transfus Apher Sci. 2010;43(1):107–116. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perseghin P, Balduzzi A, Galimberti S, et al. Red blood cell support and alloimmunization rate against erythrocyte antigens in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2003;32(2):231–236. doi: 10.1038/sj.bmt.1704114. [DOI] [PubMed] [Google Scholar]
- 19.Fergusson D, Hutton B, Hogan DL, et al. The age of red blood cells in premature infants (ARIPI) randomized controlled trial: study design. Transfus Med Rev. 2009;23(1):55–61. doi: 10.1016/j.tmrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;48(9):1446–1453. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinarello CA, Pomerantz BJ. Proinflammatory cytokines in heart disease. Blood Purif. 2001;19(3):314–321. doi: 10.1159/000046960. [DOI] [PubMed] [Google Scholar]
- 23.Economou E, Tousoulis D, Katinioti A, et al. Chemokines in patients with ischaemic heart disease and the effect of coronary angioplasty. Int J Cardiol. 2001;80(1):55–60. doi: 10.1016/s0167-5273(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 24.Guilherme L, Cury P, Demarchi LM, et al. Rheumatic heart disease: proinflammatory cytokines play a role in the progression and maintenance of valvular lesions. Am J Pathol. 2004;165(5):1583–1591. doi: 10.1016/S0002-9440(10)63415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra VC, Ramgolam VS, Bender JR. Cytokines and cardiovascular disease. J Leukoc Biol. 2005;78(4):805–818. doi: 10.1189/jlb.0405182. [DOI] [PubMed] [Google Scholar]
- 26.Hendrickson JE, Hod EA, Spitalnik SL, Hillyer CD, Zimring JC. Storage of murine red blood cells enhances alloantibody responses to an erythroid-specific model antigen. Transfusion. 2010;50(3):642–648. doi: 10.1111/j.1537-2995.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desmarets M, Cadwell CM, Peterson KR, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114(11):2315–2322. doi: 10.1182/blood-2009-04-214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westphal E, Rohrbach S, Buerke M, et al. Altered interleukin-1 receptor antagonist and interleukin-18 mRNA expression in myocardial tissues of patients with dilatated cardiomyopathy. Mol Med. 2008;14(1–2):55–63. doi: 10.2119/2007-00058.Westphal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talano JA, Hillery CA, Gottschall JL, Baylerian DM, Scott JP. Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease. Pediatrics. 2003;111(6 Pt 1):e661–665. doi: 10.1542/peds.111.6.e661. [DOI] [PubMed] [Google Scholar]
- 30.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76(7):1431–1437. [PubMed] [Google Scholar]
- 31.Schonewille H, van Zijl AM, Wijermans PW. The importance of antibodies against low-incidence RBC antigens in complete and abbreviated cross-matching. Transfusion. 2003;43(7):939–944. doi: 10.1046/j.1537-2995.2003.t01-1-00435.x. [DOI] [PubMed] [Google Scholar]
- 32.Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48(10):2069–2076. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 33.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. New England Journal of Medicine. 1990;322(23):1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 34.Reid ME, CL-F . The Blood Group Antigen Facts Book. 2. Amsterdam: Elsevier Academic Press; 2004. [Google Scholar]
- 35.Reid ME, Mohandas N. Red blood cell blood group antigens: structure and function. Seminars in Hematology. 2004;41(2):93–117. doi: 10.1053/j.seminhematol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Mangalmurti NS, Xiong Z, Hulver M, et al. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113(5):1158–1166. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JS, Wurfel MM, Matute-Bello G, et al. The Duffy antigen modifies systemic and local tissue chemokine responses following lipopolysaccharide stimulation. JImmunol. 2006;177(11):8086–8094. doi: 10.4049/jimmunol.177.11.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darbonne WC, Rice GC, Mohler MA, et al. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest. 1991;88(4):1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]