Abstract
Objectives
To examine changes in use of prescription opioids for the management of chronic non-cancer pain in HIV-infected patients and to identify patient characteristics associated with long-term use.
Methods
Long-term prescription opioid use (i.e. 120+ days supply or 10+ prescriptions during a year) was assessed between 1997 and 2005 among 6,939 HIV-infected Kaiser Permanente members and HIV-uninfected persons in the general health plan memberships.
Results
In 2005, 8% of HIV+ individuals had prevalent long-term opioid use, more than double the prevalence among HIV-uninfected individuals. However, the large increases in use from 1997 to 2005 in the general population were not observed for HIV-infected individuals. The strongest associations with prevalent use among HIV-infected individuals were female gender with a prevalence ratio [PR] of 1.8 (95% CI=1.3, 2.5); Charlson comorbidity score of 2 or more (compared with a score of 0) with a PR of 1.9 (95% CI=1.4, 2.8); injection drug use history with a PR of 1.8 (95% CI=1.3, 2.6); substance use disorders with a PR of 1.8 (95% CI=1.3, 2.5). CD4, HIV RNA, and AIDS diagnoses were associated with prevalent opioid use early in the antiretroviral therapy era (1997), but not in 2005.
Conclusions
Long-term opioid use for chronic pain has remained stable over time for HIV patients, while use increased in the general population. The prevalence of prescribed opioids in HIV patients was highest for certain subgroups, including women, and those with a comorbidity and substance abuse history.
Keywords: HIV/AIDS, chronic pain, prescription opioids, substance use disorders
INTRODUCTION
The introduction of combination antiretroviral therapy (ART) in 1996 has resulted in substantially decreased mortality and AIDS-related morbidity for HIV-infected individuals. As a result, the HIV-infected population in developed countries is rapidly aging, with those greater than 50 years of age one of the fastest growing demographics1-2. HIV patient care, therefore, has shifted to a chronic disease model focusing on lifelong use of therapy, management of side effects, and increasing focus on age-related comorbidities.
For many patients, lifelong infection and treatment is accompanied by high rates of pain and other clinical symptoms, with some studies indicating that up to two-thirds of patients on ART experience one or more clinical symptoms, most commonly gastrointestinal symptoms, body fat redistribution, myalgias, and paresthesias3-4. Pain in HIV-infected patients is generally chronic5, and results in significantly reduced quality of life6. Thus, effective chronic pain management is an important consideration for HIV-infected patients, and one option for chronic pain management that is increasingly common in the general population is long-term use of opioid analgesics7. However, long-term treatment of pain with opioid analgesics in HIV-infected patients is complex given concerns of possible drug misuse since many HIV-infected individuals have a prior drug abuse history8. Furthermore, there are known drug-drug interactions for all antiretroviral drug classes with methadone and buprenorphine 9-12, and also for other opioid analgesics including meperidine10 and fentanyl13 coadministered with ritonavir-boosted antiretrovirals.
The use of opioids for the treatment of chronic pain has increased markedly in the U.S.14, but there has been no evaluation of changes over time in opioid prescribing among HIV-infected individuals, nor a comparison of opioid prescribing in HIV-infected individuals compared with the general population. This paper compares trends in opioid prescribing between HIV-infected and HIV-uninfected individuals enrolled in integrated health plans between 1997-2005. We also evaluated whether demographic and HIV-associated clinical factors were associated with long-term prescription opioid use in HIV-infected individuals.
MATERIALS AND METHODS
Study setting, design and population
The analysis was performed within the CONsortium to Study Opioid Risks and Trends (CONSORT)15, which has the overall study goal to examine determinants and risks of long-term opioid therapy among for the treatment of chronic non-cancer pain. Eligible subjects included individuals at least 18 years of age without a history of cancer identified from two large integrated health plans: Kaiser Permanente Northern California (KPNC) and Group Health Cooperative (GHC) in Washington State. The CONSORT study period was 1997-2005, corresponding to availability of comprehensive electronic clinical data at both health plans. Both health plans serve employed persons, older populations enrolled in Medicare and lower-income persons insured by Medicaid and State health insurance programs for low-income populations. The health plans offer primary care as well as specialty services. Together, these health plans provide care to more than four million members, with demographic profiles similar to the catchment areas16-17. HIV-infected and HIV-uninfected CONSORT subjects within KPNC comprised the primary source population for analysis because of the larger population and additional risk factor data available including race/ethnicity and HIV transmission risk factors. GHC data was used principally for confirmation of opioid prescription calendar trends. The institutional review boards at each institution approved the study, providing waivers of informed consent.
Data sources
The CONSORT study uses automated health plan data, including information on pharmacy use, laboratory tests (CD4 and HIV virus levels), patient demographics (age and sex), enrollment, and clinical diagnoses (substance use disorders, depression diagnoses and other comorbidities)15. Information on medical encounters and pharmacy utilization were captured for all services provided directly by the health plans, and through claims data for any services covered which were provided outside of the health plan. The pharmacy databases were used to identify all dispensed opioid and antiretroviral prescriptions. The pharmacy files contain 1 record/drug dispensed, including generic drug name, strength, directions for use, date dispensed, quantity dispensed, days supply, prescriber identification number, and National Drug Code. Surveys at both plans indicated that pharmacy databases capture more than 90% of membership prescriptions16-17. Methadone for the treatment of opiate disorders would need to be obtained from outside community agencies since the health plans do not cover such treatments. During the study period, buprenorphine was also not provided for addiction treatment. Thus, these prescriptions identified in pharmacy databases for the study period would be for pain rather than for opioid addiction. Opioid prescriptions in both health plans are generally limited to a 30-day supply only.
Substance use disorders were defined as medical encounters with ICD-9 codes indicating drug and/or alcohol abuse or dependence. We previously found that a third of such diagnoses among KPNC members were received exclusively from Chemical Dependency or Psychiatry departments18. For this analysis, we computed a modified Charlson comorbidity score19, based on inpatient and outpatient diagnoses excluding those related to HIV or AIDS. The KPNC HIV Registry, as described previously2, includes all known cases of HIV infection since the early 1980’s. The GHC HIV registry includes cases identified since 1993. HIV infection status for all cases was confirmed by medical chart review or comparisons of case lists with HIV clinics. Race/ethnicity and HIV transmission risk factor data was additionally collected by chart review for KPNC only. Patients without verified HIV status were excluded from the respective HIV registries.
Prevalent long-term prescription opioid use
The primary outcome for this analysis was prevalent long-term prescription opioid use, defined in CONSORT using an opioid episode-based approach15. The beginning of an episode was defined by the date of dispensing of an opioid (oral or transdermal) without a previous dispensing in the prior 6 months. An episode ends with the last opioid dispensing with no additional dispensing in the following 6 months. The end date was defined as the date of last prescription plus days supply for that prescription. An episode was considered long-term, as described in detail previously15, if it was both longer than 90 days and associated with either a 120+ total days supply (i.e., sum of days supply for each opioid dispensed during an episode) or 10 or more opioid prescriptions dispensed within a given year. For each study year, 1997-2005, we identified prevalent episodes, defined as those ongoing at any time during the calendar year of interest. Prevalent episodes were identified among subjects enrolled for the entire year plus 182 days after the end of the year to ensure enough time to observe long-term use after an episode began.
Medication Use Profiles
Characteristics of HIV-infected and HIV-uninfected long-term opioid users were compared using a core set of variables (i.e., medication profiles) developed in CONSORT as described previously15, 18. The population for the medication profile analysis consisted of prevalent users in an episode of long-term opioid use on January 1, 2005, who also met the criteria for long-term use in 2005. Prevalent episodes may have started prior to January 1 2005, and extended beyond December 31, 2005. However, the medication profile measures correspond to only long-term use during 2005.
Several medication profile variables were based on the total days supply during a calendar year and types of medications prescribed, including average total days supply, % mainly Schedule II, % mainly long-acting Schedule II, and % with concurrent 180+ Days Supply Sedative-Hypnotics. The predominant opioid class used (e.g., mainly Schedule II) was defined as the opioid with the longest total days supply in the episode. Short-acting, Schedule II medications included morphine sulfate, codeine sulfate, hydromorphone, meperidine, fentanyl transmucosal, oxymorphone, and oxycodone. Long-acting, Schedule II medications included morphine sulfate sustained release (SR), fentanyl transdermal, levorphanol, oxycodone controlled release, methadone, hydromorphone SR, and oxymorphone SR. Other medication profile variables involved first calculating morphine equivalents for each opioid dispensed as follows: quantity x the strength (i.e., milligrams per unit dispensed) x drug-specific conversion factors15. Total morphine equivalents were calculated by adding the morphine equivalents for each opioid dispensed during the episode. Average prescribed dose is the total morphine equivalents divided by total days supply for the episode. Average daily dose is the total morphine equivalents divided by episode duration in days. Average daily dose is an estimate of mean daily consumption, while average prescribed dose approximates the maximum intended daily dose. Finally, high dose episodes were defined as those with an average daily dose of ≥20 mg, based on thresholds developed previously20.
Statistical methods
For each study year, 1997-2005, we first examined the annual prevalence of long-term opioid use episodes per 100 individuals, separately by health plan and by HIV-infection status. We then estimated the annualized percent change in prevalent opioid use across the nine-year study period with 95% confidence intervals obtained using a linear regression method described by Fay et al.21. This linearized annualized percent change estimates the constant annual (multiplicative) rate of change in prevalence over a fixed time period (e.g., 100 * [prevalencetime 2 − prevalencetime 1]/ prevalencetime 1). The annualized percent change was standardized to the 2005 age-sex distribution of the KPNC general population.
Among HIV-infected individuals in KPNC, we described characteristics of HIV-infected members with and without prevalent long-term opioid therapy in 1997 and 2005. Next, we evaluated the association of patient characteristics and prevalent long-term prescription opioid use separately for years 1997 and 2005. Characteristics evaluated were age, sex, race/ethnicity, years known HIV-infected (as of January 1), HIV transmission by injection drug use, any prior use of antiretrovirals (as of January 1), CD4+ T-cell count and HIV RNA levels (most recent test in prior year), any prior diagnosis of AIDS (as of January 1), recent clinical depression diagnoses (within two years prior to January 1), Charlson comorbidity scores (within two years prior to January 1), and recent substance use disorders (within two years prior to January 1). Depression, Charlson comorbidity scores, and substance use disorder diagnoses were unavailable for analysis of 1997 data. Adjusted prevalence ratios (PR) were obtained from modified Poisson regression models with robust standard errors22 using Proc Genmod in SAS (Version 9.1, Cary, NC). Finally, we compared medication use profiles between HIV-infected and HIV-uninfected KPNC members with long-term prevalent opioid use. We presented unadjusted medication profile measures, but p-values comparing results to the KPNC general population by HIV infection status were age- and sex-adjusted.
RESULTS
Descriptive characteristics for KPNC HIV-infected individuals with and without long-term prevalent use in 1997 and 2005 are presented in Table 1. In total, 6,939 HIV-infected KPNC members were eligible for one or more calendar years between 1997-2005, with a median of four eligible years contributed per person. Univariate comparisons in 2005 indicated that prescription opioid users, compared with nonusers, were older, more often female, White, African-American, had more years known HIV-infected, more likely to report prior injection drug use, to have a prior depression diagnosis, have higher Charlson comorbidity scores, and less often Hispanic, other race/ethnicities, and men who have sex with men. Prevalent users, compared with nonusers, also had lower CD4+ T-cell counts, higher HIV RNA levels, and higher percentages with prior AIDS diagnoses and antiretroviral therapy experience. Similar results comparing users and nonusers were observed for 1997 (Table 1). All differences in these baseline measures between HIV-infected long-term opioid users and non-users were statistically significant at P<0.05.
Table 1. Characteristics of HIV-infected individuals with and without prevalent long-term opioid use at Kaiser Permanente Northern California, 1997 and 20051.
| Prevalent long-term opioid use in 1997 |
Prevalent long-term opioid use in 2005 |
|||
|---|---|---|---|---|
| Characteristic | Yes | No | Yes | No |
| N | 218 | 2878 | 332 | 3805 |
| Mean age (SD) | 42.6 (8.1) | 42.1 (9.1) | 48.0 (7.9) | 46.3 (9.6) |
| Male, % | 83.9 | 91.3 | 85.2 | 89.9 |
| Race/ethnicity, % | ||||
| White | 74.8 | 66.2 | 66.3 | 60.8 |
| African-American | 17.0 | 16.1 | 22.3 | 16.2 |
| Hispanic/Latino | 7.8 | 10.4 | 8.1 | 13.8 |
| Other | 0.5 | 4.0 | 0.9 | 6.2 |
| Unknown | 0.0 | 3.3 | 2.4 | 3.0 |
| Mean years known HIV-infected (SD) | 6.3 (3.6) | 5.2 (3.6) | 10.7 (5.9) | 9.3 (5.9) |
| HIV risk factor, % | ||||
| Men who have sex with men | 63.3 | 68.0 | 55.7 | 67.4 |
| Injection drug use | 14.2 | 6.1 | 15.4 | 5.5 |
| Heterosexual sex | 14.2 | 13.3 | 18.1 | 16.7 |
| Other | 1.4 | 0.1 | 0.9 | 0.1 |
| Unknown | 6.9 | 12.4 | 9.9 | 10.2 |
| Mean CD4+ T-cells/μL (SD)2 | 296 (223) | 372 (237) | 430 (233) | 464 (244) |
| Mean log HIV RNA copies/mL (SD)2 | 3.6 (0.9) | 3.5 (0.8) | 3.1 (0.8) | 3.1 (0.7) |
| Prior CDC AIDS, % | 67.4 | 41.9 | 64.5 | 53.8 |
| Antiretroviral therapy experienced, % | 77.5 | 56.1 | 84.9 | 77.2 |
| Charlson comorbidity index score3,4, % | ||||
| 0 | -- | -- | 64.5 | 80.4 |
| 1 | -- | -- | 22.6 | 14.0 |
| ≥2 | -- | -- | 13.0 | 5.6 |
| Depression prior 2 yrs, %4 | -- | -- | 41.1 | 26.4 |
| Substance use disorders prior 2 yrs, %4 | ||||
| Any substance use disorder | -- | -- | 25.2 | 10.1 |
| Opioid use disorder | -- | -- | 6.0 | 0.6 |
| Alcohol use disorder | -- | -- | 13.1 | 5.3 |
| Other drug use disorder | -- | -- | 17.7 | 6.8 |
SD, standard deviation
All differences in these baseline measures between HIV-infected long-term opioid users and non-users were statistically significant at P<0.05.
Most recent test in 365 days prior to January 1
Modified Charlson comorbidity scores based on inpatient or outpatient diagnoses, excluding HIV/AIDS.
Prior Charlson comorbidity scores, depression diagnoses, and substance use disorders were assessed in two years prior to start of calendar year and not available for 1997.
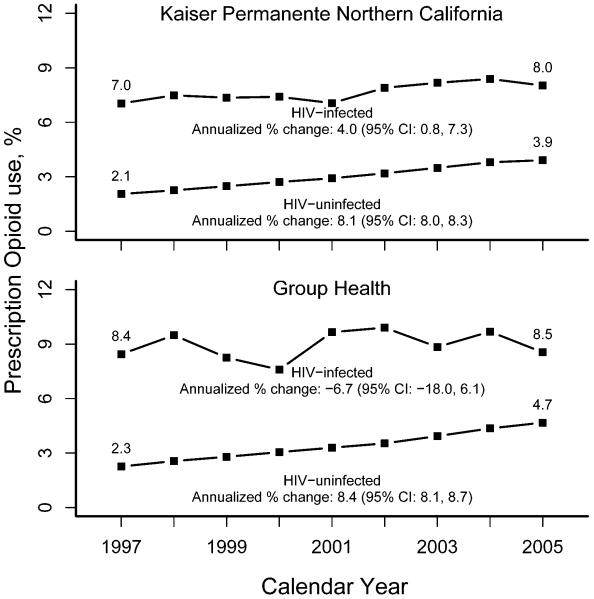
The age and sex distribution comparing HIV-infected individuals in KPNC and GHC were similar (Table 2). HIV-uninfected members in both health plans were older and more likely female compared with HIV-infected members of the same health plan, particularly among prevalent users. Annual prevalence for long-term opioid prescription use is presented in the Figure for HIV-infected and HIV-uninfected individuals. In KPNC, the unadjusted prevalence of long-term opioid use increased only slightly among HIV-infected individuals from 7.0% in 1997 to 8.0% in 2005, corresponding to an age- and sex-adjusted annualized percent change of 4.0% (95% CI: 0.8-7.3). Among KPNC HIV-uninfected individuals, the unadjusted prevalence increased from 2.1% in 1997 to 3.9% in 2005, corresponding to an age- and sex-adjusted annualized percent change of 8.4% (95% CI: 8.1-8.7).. The difference in trends comparing HIV-infected with HIV-uninfected individuals was statistically significant (p<0.05). The pattern was similar at GHC, where prevalence increased only slightly between 1997 and 2005 from 8.4% to 8.5% for HIV-infected individuals but increased 2.3% to 4.7% in HIV-uninfected individuals. The difference in overall trends at GHC by HIV infection status were also statistically significant (p<0.05).
Table 2. Demographics of HIV-infected and HIV-uninfected individuals with and without long-term prevalent opioid use in 2005.
| N | Mean age1 | % Male1 | |
|---|---|---|---|
|
Kaiser Permanente
Northern California | |||
| HIV+ with prevalent use | 332 | 48.0 | 85.2 |
| HIV+ without prevalent use | 3,805 | 46.3 | 89.9 |
| HIV− with prevalent use | 70,712 | 57.1 | 36.3 |
| HIV− without prevalent use | 1,745,291 | 47.6 | 47.7 |
| Group Health Cooperative | |||
| HIV+ with prevalent use | 30 | 48.7 | 90.0 |
| HIV+ without prevalent use | 321 | 45.7 | 94.4 |
| HIV− with prevalent use | 11,593 | 56.1 | 35.3 |
| HIV− without prevalent use | 236,447 | 49.9 | 45.4 |
Differences in age and sex between long-term opioid users and non-users were statistically significant at P<0.05 for both HIV+ and HIV− individuals.
Figure.
Unadjusted prevalence (%) of HIV-infected and HIV-uninfected individuals with prevalent long-term opioid use, 1997-2005, in Kaiser Permanente Northern California and Group Health Cooperative. Age-sex adjusted annualized percent change presented using the Kaiser Permanente Northern California general membership in 2005 as the standard population.
As shown in Table 3 for 1997, statistically significant associations (P<0.05) with prevalent long-term opioid use were observed for female sex with a PR of 2.0 (95% CI=1.3, 3.0); history of injection drug use with a prevalence ratio (PR) of 2.1 (95% CI=1.5, 3.2); recent higher HIV virus levels with a PR of 1.2 per log unit increase (95% CI=1.0, 1.4); and prior AIDS with a PR of 2.0 (95% CI=1.2, 3.1). In 2005, significant associations were observed for age 40-49 years (compared with 18-39 years) with a PR of 1.6 (95% CI=1.1, 2.4); female gender with a PR of 1.8 (95% CI=1.3, 2.5); 10 or more years known HIV-infected (compared with <5 years) with a PR of 1.5 (95% CI=1.1, 2.2); history of injection drug use with a PR of 1.9 (95% CI=1.4, 2.7); Charlson comorbidity scores ≥2 (compared with 0) with a PR of 1.9 (95% CI=1.4, 2.8) and scores of 1 (compared with 0) with a PR of 1.6 (95% CI=1.2, 2.1); Depression with a PR of 1.3 (95% CI=1.0, 1.7); and, substance use disorders with a PR of 1.9 (95% CI=1.4, 2.6).
Table 3. Factors associated with prevalent long-term opioid use in 1997 and 2005 in Kaiser Permanente.
| 1997 |
2005 |
|||||
|---|---|---|---|---|---|---|
| PR | (95% CI) | P | PR | (95% CI) | P | |
| Age | ||||||
| ≥50 years | 0.9 | (0.6, 1.4) | 0.76 | 1.3 | (0.9, 2.1) | 0.20 |
| 40-49 years | 0.8 | (0.6, 1.2) | 0.31 | 1.6 | (1.1, 2.4) | 0.025 |
| 18-39 years (reference) | 1 | 1 | ||||
| Female | 2.0 | (1.3, 3.0) | 0.001 | 1.8 | (1.3, 2.5) | <0.001 |
| White Race/ethnicity | 1.4 | (1.0, 2.0) | 0.069 | 1.3 | (1.0, 1.7) | 0.083 |
| Years known HIV+ | ||||||
| ≥10 | 1.4 | (0.9, 2.2) | 0.111 | 1.4 | (1.0, 2.1) | 0.053 |
| 5-9.9 | 1.1 | (0.8, 1.5) | 0.75 | 1.1 | (0.7, 1.7) | 0.66 |
| <5 (reference) | 1 | 1 | ||||
| History of injection drug use | 2.1 | (1.5, 3.2) | <0.001 | 1.8 | (1.3, 2.6) | 0.001 |
| Prior use of antiretrovirals | 1.5 | (0.9, 2.5) | 0.085 | 1.1 | (0.7, 1.8) | 0.62 |
| CD4+ T-cell counts (per 100) | 1.0 | (0.9, 1.1) | 0.70 | 1.0 | (0.9, 1.0) | 0.138 |
| HIV virus levels (per log) | 1.2 | (1.0, 1.4) | 0.027 | 1.1 | (0.9, 1.3) | 0.36 |
| Prior AIDS diagnosis | 2.0 | (1.2, 3.1) | 0.004 | 1.2 | (0.9, 1.6) | 0.250 |
| Charlson comorbidity index2,3 | ||||||
| ≥2 | 1.9 | (1.4, 2.8) | <0.001 | |||
| 1 | 1.6 | (1.2, 2.1) | 0.003 | |||
| 0 (reference) | 1.0 | (1.0, 1.0) | . | |||
| Prior depression diagnosis3 | 1.3 | (1.0, 1.7) | 0.058 | |||
| Prior substance use disorders3 | 1.8 | (1.3, 2.5) | <0.001 | |||
PR, prevalence ratio; CI, confidence interval
Adjusted prevalence ratios obtained from modified Poisson regression models using Proc Genmod in SAS. Models are adjusted for all variables in table.
Modified Charlson comorbidity scores based on inpatient or outpatient diagnoses, excluding HIV/AIDS.
Prior Charlson comorbidity scores, depression diagnoses, and substance use disorders were assessed in two years prior to start of calendar year and not available for 1997.
Finally, we compared medication use profiles for 204 HIV-infected and 37,377 HIV-uninfected individuals with a prevalent long-term opioid use episode in 2005 (Table 4). HIV-infected individuals had higher average prescribed and daily doses, and higher percentages of high dose, mainly Schedule II drugs, mainly long-acting Schedule II drugs, and 180+ days supply sedative hypnotics. However, with adjustment for age and sex, none of these differences remained statistically significant.
Table 4. Profiles of prevalent long-term opioid episodes at Kaiser Permanente Northern California, 2005.
| p-value |
||||
|---|---|---|---|---|
| Characteristics | HIV+ | HIV− | Unadjusted | Adjusted1 |
| Number of persons | 204 | 37,377 | ||
| Average Prescribed Dose2 | 79.9 | 54.1 | 0.003 | 0.45 |
| Average Daily Dose2 | 90.8 | 47.7 | <0.001 | 0.23 |
| Average Total Days Supply | 299.4 | 284.1 | 0.18 | 0.95 |
| Percent High Dose3 | 57.4 | 48.9 | 0.016 | 0.92 |
| % Mainly Schedule II4,5 | 27.9 | 17.7 | <0.001 | 0.59 |
| % Mainly Long-Acting Schedule II5 | 23.5 | 13.0 | <0.001 | 0.42 |
| % with 180+ Days Supply Sedative-Hypnotics | 37.3 | 30.2 | 0.031 | 0.14 |
P-value is age- and sex-adjusted to 2005 KPNC general population.
Average prescribed dose is the total morphine equivalents for an episode divided by total days supply. Average daily dose is the total morphine equivalents for an episode divided by episode duration in days. Average daily dose is an estimate of mean daily consumption, while average prescribed dose approximates the maximum intended daily dose.
Defined as average daily dose of 20+ mg morphine equivalents
Short-acting, Schedule II=morphine sulfate; codeine sulfate; hydromorphone; meperidine; fentanyl transmucosal; oxymorphone; oxycodone
Long-acting, Schedule II=morphine sulfate sustained release (SR); fentanyl transdermal; levorphanol; oxycodone controlled release; methadone; hydromorphone SR; oxymorphone SR
DISCUSSION
In this study, we determined that long-term prevalent opioid prescription use was more common among HIV-infected individuals compared with the general population. However, prevalence was increasing dramatically in the general population but was stable in the HIV-infected population. The strongest predictors of prevalent long-term opioid use in the HIV-infected population were female gender, HIV transmission by injection drug use, prior comorbidities and substance use disorders. Certain characteristics including older age and longer years known HIV-infected were only associated with prevalent long-term opioid use in 2005, while clinical characteristics, including higher HIV virus levels and a diagnosis of AIDS were only associated with prevalent opioid use in 1997. Depression diagnoses, which were common among HIV-infected individuals were also significantly associated with prevalent opioid use. To our knowledge, this is the first analysis of trends in the use of long-term prescription opioid use for the management of chronic pain in HIV-infected patients. The trends observed among study subjects in Northern California were supported by similar trends among the study subjects in Washington State.
The higher prevalence of prescription opioids in HIV-infected individuals is likely due in large part to the higher HIV-related illness burden resulting in increased chronic pain23. However, it is notable that we observed a less rapid increase in opioid prescribing for HIV-infected patients compared with a doubling of the prevalence for the general population. It has been suggested that the increases in the general population may be due to either more pain-related diagnoses overall, or more likely, better patient and provider attention to pain and pain management7. The more stable prevalence over time in HIV patients may reflect opposing pressures on pain management and etiology over time. First, this analysis started near the beginning of the ART era when HIV/AIDS was transformed from a terminal illness to a chronic disease24. Therefore, earlier in the ART era, opioid management was more likely part of palliative, end-of-life care and consequently more liberal. Over time, the clinical management of HIV patients overall has improved, including substantial reductions in HIV-related complications, and improved tolerability of newer generation antiretrovirals12.
To offset this tendency for a decreased prevalence of prescription opioids over time, there are likely other factors resulting in a tendency for an increased prevalence. First, a consequence of the improved survival in the ART era for HIV patients is the higher burden of non-AIDS-related comorbidities25-26, resulting in the potential greater need for pain-related management. In support of this, we confirmed here that a higher comorbidity burden was a strong predictor of long-term prevalent opioid use. Second, HIV patients are also likely benefiting from improvements in pain management. Although we could not directly assess this possibility, it is noteworthy that older age and more years HIV experience were associated with higher prevalent opioid use in 2005, but not 1997. These results likely reflect the growing numbers of patients in these subgroups, accompanied by an increasing inclination for the treatment of pain in HIV patients with the longest exposure to HIV and/or prolonged exposure to antiretrovirals. Together, these opposing pressures on pain etiology and management may explain the stable trends in use of pain medication for HIV patients relative to the large increases observed for the population-at-large.
Long-term use of prescription opioids was strongly associated with HIV transmission by injection drug use, and substance use disorders diagnosed within the prior two years. These two factors were related in our data, but included little overlap. To illustrate, in 2005, of those with a substance use disorder in the prior two years, 16% had an HIV transmission risk factor of injection drug use, compared with only 5% among those without prior substance use disorders. Thus, most HIV-infected individuals identified here with a prior substance use disorder did not have a recorded HIV exposure transmission of injection drug use. This is relevant since such diagnoses if made in the Chemical Dependency or Psychiatry departments, may not have been known to prescribing physicians outside these departments, including HIV clinicians. Diagnoses are not disclosed to such prescribing physicians because of Federal 42CFR Part 2 privacy regulations, which prohibit any disclosure of chemical dependency visits or diagnoses (or in the Psychiatry department when Chemical Dependency is affiliated with Psychiatry), to other departments in the same health plan27-28. Thus, careful screening by the prescribing physician for the potential abuse of prescribed opioids or existence of a substance abuse problem is needed, particularly in HIV-infected individuals who are more likely to have a substance abuse history compared with the general population.
The association of drug abuse with prescription opioid use is consistent with earlier studies among HIV-infected individuals8, 29-31, as well as recent studies comparing prescription opioid use between those with and without substance use disorders in the general population18, 32. Those with diagnosed substance use disorders may have reduced pain tolerance23, 33, or perhaps increased prevalence of other comorbid illnesses34, resulting in more frequent use of prescription opioids at higher dosage levels. The higher prevalence of prescription opioid use in HIV patients with substance use disorders may also reflect increased risk of misuse of prescription opioids in this population. Tsao et al.31, for example, evaluated 2267 HIV-infected individuals in 1996-1997 in the HIV Cost and Services Utilization study (HCSUS) and found an increased risk of pain and aberrant opioid use among those with a drug abuse history (n=870) compared with those without a drug abuse history (n=1397). Those with a history of drug abuse also persistently reported aberrant opioid use during follow-up. Another study of 190 HIV-infected individuals seen in a single U.S. medical clinic indicated that HIV-infected individuals with a history of IDU were more likely to have been prescribed narcotics inappropriately compared with other men who have sex with men or heterosexuals8.
Clinical factors associated with prevalent long-term opioid use were high HIV virus levels, prior AIDS, prior depression and other comorbidity diagnoses. HIV virus levels and AIDS were associated with opioid use in 1997 but not in 2005, which again likely reflects the improving prognosis for HIV/AIDS patients over time and availability of better tolerated therapy. Prior studies have not evaluated such factors with respect to opioid prescribing, although some have reported that these same clinical factors were associated with reported clinical symptoms among HIV-infected persons4. Other demographic factors associated with prevalent opioid use in our study were female gender, older age, and more years known HIV-infected.
In the absence of controlled trials evaluating the benefits and risks of long-term opioid use for chronic pain, it is difficult to know to what extent observed rates of long-term opioid use reflect excessive use of prescribed opioids in certain subgroups. Conversely, others have concluded that pain in HIV-infected patients may be undertreated29-30, 35. Although our study did not address the appropriateness of opioid prescription use directly, it is noteworthy that we found no differences by HIV infection status, after accounting for age and sex, in opioid dosing, or use of Schedule II drugs, suggesting that prescribing is similar in these populations. However, in a study of U.S. Veterans seen between 1998-2001, Hermos et al.36 reported that oxycodone/acetaminophen prescription use in HIV+ patients was more likely to be long-term rather than short- or intermediate-term, and more often associated with higher doses, suggesting a potential high risk prescribing pattern. Thus, further analysis of the appropriate management of chronic pain in HIV-infected individuals is warranted.
There were study limitations. First, depression, substance use disorder diagnoses, and other medical comorbidity data were ascertained from automated diagnostic data, and may have been underreported, although not likely to be different between those with and without prescription opioid use. Furthermore, these variables were not available for analysis of factors associated with prescription opioid use in 1997. However, exclusion of these variables from the final multivariable model in 2005 had no effect on the magnitude of PRs for other variables (data not shown). An additional limitation was the lack of information regarding indications for opioid prescribing, including types of pain experienced. Such information would be valuable for a refined understanding of the etiology and treatment of chronic pain in HIV patients. An additional limitation is the lack of generalizability to those without health insurance, or to other healthcare systems that are not guided by an integrated health care delivery model. However, the comparable trends observed within two separate health plan systems suggest highly generalizable results for other similar healthcare systems with comprehensive medical coverage.
In summary, we determined that HIV-infected individuals have a higher prevalence of long-term prescription opioid use compared with the general population, although prevalence in the HIV-infected population was stable whereas general population rates were increasing rapidly. HIV disease severity and HIV treatment experience were not predictive of opioid use in the most recent study year, suggesting improvements over time in the tolerability of antiretrovirals. We also determined that certain subgroups had increased long-term opioid use, particularly those with a history substance use disorders. These results are relevant to strategies for management of chronic pain in HIV-infected individuals. Specifically, careful screening for substance use problems is warranted when considering long-term medically prescribed opioids in this population.
ACKNOWLEGEMENTS
This research was supported by grant numbers R01 DA022557 and R37 DA 10572 from NIDA and K01AI071725 from NIAID. We acknowledge Wendy Leyden, MPH for her assistance with data analysis, and Steven Carzasty for helpful discussions during manuscript preparation.
Footnotes
The authors report no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mack KA, Ory MG. AIDS and older Americans at the end of the Twentieth Century. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S68–75. doi: 10.1097/00126334-200306012-00003. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg MJ, Leyden W, Horberg MA, et al. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med. 2007;167:684–691. doi: 10.1001/archinte.167.7.684. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg MJ, Gore ME, French AL, et al. Prevalence of clinical symptoms associated with HAART in the Women’s Interagency HIV Study. Clin Infect Dis. 2004;39:717–724. doi: 10.1086/423181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverberg MJ, Jacobson LP, French AL, et al. Age and racial/ethnic differences in the prevalence of reported symptoms in human immunodeficiency virus-infected persons on antiretroviral therapy. J Pain Symptom Manage. 2009;38:197–207. doi: 10.1016/j.jpainsymman.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus KS, Kerns RD, Rosenfeld B, et al. HIV/AIDS-related pain as a chronic pain condition: implications of a biopsychosocial model for comprehensive assessment and effective management. Pain Med. 2000;1:260–273. doi: 10.1046/j.1526-4637.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- 6.Kowal J, Overduin LY, Balfour L, et al. The role of psychological and behavioral variables in quality of life and the experience of bodily pain among persons living with HIV. J Pain Symptom Manage. 2008;36:247–258. doi: 10.1016/j.jpainsymman.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison RE, Brint JM, Smith WR, et al. Appropriate and inappropriate prescribing of narcotics for ambulatory HIV-positive patients. J Gen Intern Med. 1994;9:301–305. doi: 10.1007/BF02599175. [DOI] [PubMed] [Google Scholar]
- 9.McCance-Katz EF, Moody DE, Smith PF, et al. Interactions between buprenorphine and antiretrovirals. II. The protease inhibitors nelfinavir, lopinavir/ritonavir, and ritonavir. Clin Infect Dis. 2006;43(Suppl 4):S235–246. doi: 10.1086/508188. [DOI] [PubMed] [Google Scholar]
- 10.Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–1059. doi: 10.1345/aph.1K615. [DOI] [PubMed] [Google Scholar]
- 11.Bruce RD, Altice FL, Gourevitch MN, et al. Pharmacokinetic drug interactions between opioid agonist therapy and antiretroviral medications: implications and management for clinical practice. J Acquir Immune Defic Syndr. 2006;41:563–572. doi: 10.1097/01.qai.0000219769.89679.ec. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services [Accessed 2/4/11];Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2011 http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 13.Olkkola KT, Palkama VJ, Neuvonen PJ. Ritonavir’s role in reducing fentanyl clearance and prolonging its half-life. Anesthesiology. 1999;91:681–685. doi: 10.1097/00000542-199909000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby JV, Smith DH, Johnson ES, et al. Kaiser Permanente Medical Care Program. In: Strom BL, editor. Pharmacoepidemiology. 4th ed. Wiley; New York: 2005. pp. 241–259. [Google Scholar]
- 17.Saunders KW, Davis RL, Stergachis A, Group Health Cooperative . In: Pharmacoepidemiology. 4th ed. Strom BL, editor. Wiley; New York: 2005. pp. 223–239. [Google Scholar]
- 18.Weisner CM, Campbell CI, Ray GT, et al. Trends in prescribed opioid therapy for non-cancer pain for individuals with prior substance use disorders. Pain. 2009;145:287–293. doi: 10.1016/j.pain.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Braden JB, Sullivan MD, Ray GT, et al. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31:564–570. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fay MP, Tiwari RC, Feuer EJ, et al. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62:847–854. doi: 10.1111/j.1541-0420.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 22.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Tsao JC, Dobalian A, Stein JA. Illness burden mediates the relationship between pain and illicit drug use in persons living with HIV. Pain. 2005;119:124–132. doi: 10.1016/j.pain.2005.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detels R, Muñoz A, McFarlane G, et al. Multicenter AIDS Cohort Study Investigators Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA. 1998;280:1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 25.Braithwaite RS, Justice AC, Chang CC, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med. 2005;118:890–898. doi: 10.1016/j.amjmed.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2007;44:179–187. doi: 10.1097/01.qai.0000247229.68246.c5. [DOI] [PubMed] [Google Scholar]
- 27.Beckerman JZ, Pritts J, Goplerud E, et al. A delicate balance: behavioral health, patient privacy, and the need to know. California HealthCare Foundations; [Accessed 2/4/11]. 2008. http://www.chcf.org/~/media/Files/PDF/A/PDF%20ADelicateBalanceBehavioralHealthAndPrivacyIB.pdf. [Google Scholar]
- 28.Beckerman JZ, Pritts J, Leifer JC, et al. [Accessed 2/4/11];Health information privacy, patient safety, and health care quality: issues and challenges in the context of treatment for mental health and substance use. Health Care Policy Report 16. 2008 http://ihcrp.georgetown.edu/pdfs/pritts0208.pdf.
- 29.Fantoni M, Ricci F, Del Borgo C, et al. Central Italy PRESINT Group Multicentre study on the prevalence of symptoms and symptomatic treatment in HIV infection. J Palliat Care. 1997;13:9–13. [PubMed] [Google Scholar]
- 30.Larue F, Fontaine A, Colleau SM. Underestimation and undertreatment of pain in HIV disease: multicentre study. Bmj. 1997;314:23–28. doi: 10.1136/bmj.314.7073.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsao JC, Stein JA, Dobalian A. Pain, problem drug use history, and aberrant analgesic use behaviors in persons living with HIV. Pain. 2007;133:128–137. doi: 10.1016/j.pain.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edlund MJ, Martin BC, Devries A, et al. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26:1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Borgo C, Izzi I, Chiarotti F, et al. Multidimensional aspects of pain in HIV-infected individuals. AIDS Patient Care STDS. 2001;15:95–102. doi: 10.1089/108729101300003690. [DOI] [PubMed] [Google Scholar]
- 34.Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45:1593–1601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormack JP, Li R, Zarowny D, et al. Inadequate treatment of pain in ambulatory HIV patients. Clin J Pain. 1993;9:279–283. doi: 10.1097/00002508-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Hermos JA, Young MM, Gagnon DR, et al. Characterizations of long-term oxycodone/acetaminophen prescriptions in veteran patients. Arch Intern Med. 2004;164:2361–2366. doi: 10.1001/archinte.164.21.2361. [DOI] [PubMed] [Google Scholar]