Abstract
Monte Carlo models of proton therapy treatment heads are being used to improve beam delivery systems and to calculate the radiation field for patient dose calculations. The achievable accuracy of the model depends on the exact knowledge of the treatment head geometry and time structure, the material characteristics, and the underlying physics. This work aimed at studying the uncertainties in treatment head simulations for passive scattering proton therapy. The sensitivities of spread-out Bragg peak (SOBP) dose distributions on material densities, mean ionization potentials, initial proton beam energy spread and spot size were investigated. An improved understanding of the nature of these parameters may help to improve agreement between calculated and measured SOBP dose distributions and to ensure that the range, modulation width, and uniformity are within clinical tolerance levels. Furthermore, we present a method to make small corrections to the uniformity of spread-out Bragg peaks by utilizing the time structure of the beam delivery. In addition, we re-commissioned the models of the two proton treatment heads located at our facility using the aforementioned correction methods presented in this paper.
1. Introduction
The accuracy of the planning and delivery of spread-out Bragg peak (SOBP) proton beam treatments is imperative since millimeter errors can lead to over-dosage of critical organs or under-dosage of the tumor if tight margins are being used (Goitein M 1978, Urie et al 1983, Urie et al 1986a, Urie et al 1986b, Hong et al 1996, Sisterson et al 1998). Monte Carlo methods have been used in proton therapy to help improve the accuracy of proton treatments (Paganetti et al 2004, Newhauser et al 2005, Paganetti 2006, Paganetti et al 2008, Titt et al 2008). The value of Monte Carlo simulations of proton treatments is realized by the accurate characterization of the radiation field at the exit of the treatment head, which depends on the ability to account for the synchronization of the proton beam with the treatment head geometries in order to produce clinically acceptable SOBP dose distributions.
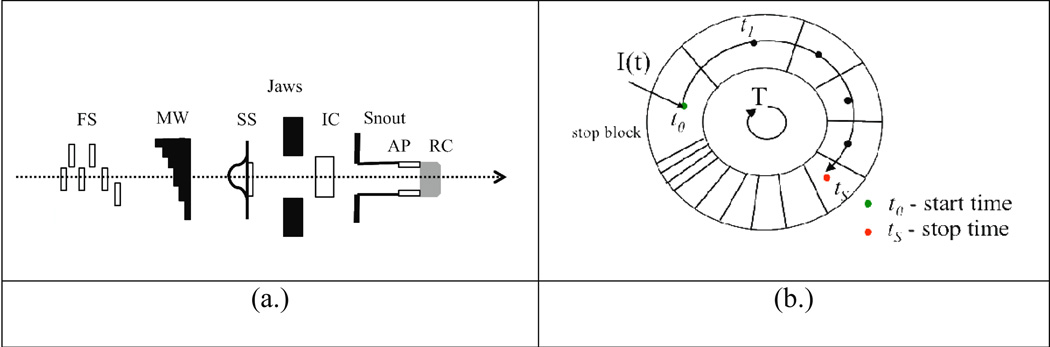
An SOBP dose distribution is produced by introducing materials along the proton beam path to modify the range of a proton Bragg peak as a function of time within the treatment head. This is usually done by the means of a range modulator wheel (MW). An illustration of a typical treatment head arrangement and a MW is presented in Figure 1. A series of steps is arranged on a MW track of increasing water-equivalent thickness to cause a pull back of peaks as a function of time for a constant wheel rotation. The superposition of these individual Bragg peaks creates a uniform SOBP dose distribution if the peaks are properly weighted. Due to the strong dependence of scattering on proton energy, the physical dimensions and materials of a track can only be optimized for a limited amount of ranges. However, it is possible to modulate the beam current intensity in synchronization with the MW rotation to further optimize the beam weights thus reducing the number of required tracks (Lu and Kooy 2006). Furthermore, it is possible to preferentially select the position on the MW to stop the beam current producing a desired modulation width for an SOBP dose distribution. This scenario is demonstrated in Figure 1b.
Figure 1.
Schematics of an SOBP proton beam treatment head. (a.) Diagram illustrating (not to scale) some of the essential components in the treatment head, including (r to l): first scatterers (FS), range modulator wheel (MW), second scatterer (SS), jaws, ionization chamber, snout, aperture, and range compensator. (b.) Diagram illustrating the arrangement of steps and the timing structure of the MW track.
The accuracy of the SOBP dose distribution is contingent on the time-dependent integration of both the treatment head configuration and the variable proton beam current. One important question that remains to be answered is what are the sensitivities of Monte Carlo models of SOBP proton therapy delivery systems to uncertainties in the beam-line parameters? There are uncertainties related to each Monte Carlo code that reduce the accuracy of the simulation. For example, the use of models to simplify transport during Monte Carlo simulations varies among different codes (e.g. condensed history method). Furthermore, the cross sections as well as their implementation may be subject to uncertainties and may also vary between codes (Zacharatou Jarlskog et al 2008). These uncertainties are not considered in this paper. Yet another source of uncertainty is produced during the modeling process of the treatment head. Most current models attempt to correctly model machine-specific components in the treatment head (i.e scattering components, modulator wheels, apertures, and compensators) using manufacture blueprints (Paganetti et al 2004, Newhauser et al 2005, Paganetti et al 2008, Titt et al 2008). Based on our experience, it is not uncommon that information provided by the manufacturer on these geometries is different than the real geometries in the commissioned treatment head. Not only the geometrical position but also physics constants of various materials might be uncertain. Uncertainties are also inherent in beam parameters because they are difficult to precisely measure. Moreover, uncertainties may also originate from the lack of knowledge on the arrangement of these materials as a function of time, even if the geometry and materials of the treatment head components are correctly implemented and the beam parameters are precisely known.
In this paper we investigate sensitivities of Monte Carlo models of SOBP proton therapy delivery systems to uncertainties in beam-line parameters. We also indentify a methodology to improve the accuracy of Monte Carlo models despite the aforementioned uncertainties. Finally, as an example, we apply this methodology to perform the full commissioning of two treatment head models representing gantries at the Francis H. Burr Proton Therapy Center at Massachusetts General Hospital (MGH). Several proton beam therapy centers are now operational or being planned and constructed, which promises the future need for Monte Carlo modeling of proton beam therapy treatment heads. The methodology and results presented in this paper will help accommodate the commissioning of new Monte Carlo simulated treatment heads. In addition, it might be helpful for individuals or manufacturers involved in designing proton therapy equipment.
2. Methods
2.1 Monte Carlo model of the proton beam treatment head
A detailed model of the Francis H. Burr proton beam treatment head was developed by Paganetti et al (2004) using the Monte Carlo code Geant4. All relevant components in the treatment head were modeled based on blueprints provided by the system manufacturer Ion Beam Applications S.A. (IBA, Louvain-la-Neuve, Belgium). Four-dimensional simulation of the dynamic components (i.e. range modulator wheel rotation) was fully implemented. The implementation of the temporal variation of the beam current (i.e. beam current modulation) was originally set to match the correct current modulation function in the treatment control system (Paganetti et al 2004). We have defined in our Monte Carlo models 24 beam current modulation functions for gantry 1 and 18 beam current modulation files for gantry 2. The beam current modulation corrections are incorporated in the treatment control system as well as in the Monte Carlo as look-up tables. A review of the nozzle design of this Monte Carlo proton nozzle can be found in Paganetti et al (2004).
All simulations were performed in Geant4 version 9.0 using the treatment head model. The snout size at the nozzle exit was set to 25 cm diameter for all fields. The depth dose curves were calculated along the beams’ central axis in a simulated water phantom using a scoring region radius of 3 cm and scoring depth of 0.1 cm. In order to achieve reasonable statistics for the depth dose curves at least 1×106 initial protons were simulated for each range and modulation width combination. All curves were calculated with enough protons so that the statistical uncertainty of each dose value at a given depth was less than 1%. All relevant physics processes were used for this study. Ionization and multiple scattered were handled with the standard electromagnetic physics option in Geant4. Inelastic processes were handled using the Binary cascade model. We chose to use a range cut of 0.05 mm for secondary particle production.
2.2 Sensitivities of Monte Carlo models of SOBP proton therapy delivery systems to uncertainties in beam-line parameters
Typically, a comparison of calculated SOBP dose distributions with measured data is done to commission Monte Carlo models of proton therapy treatment heads. The three fundamental characteristics of the SOBP dose distribution are range, modulation width, and uniformity. If the proton beam and modulator wheel is properly synchronized and the treatment head geometry is accurately modeled, the accuracy of these characteristics depends solely on material and beam parameters in the treatment head including: densities, mean ionization potentials, initial beam energy spread and beam spot size. The sensitivities of SOBP dose distributions on these parameters were tested using Monte Carlo simulations. Both full and partial SOBP dose distributions were simulated using prescribed ranges representing each of the available options. Since both treatment heads are very similar in design we will only refer to Gantry 1 for the sensitivity study.
2.2.1 Range Uncertainties
The range of a roton beam in a medium is primarily governed by proton interactions with atomic electrons and usually described by the proton’s rate of energy loss known as the stopping power, which can be determined by the well-known Bethe-Bloch equation. Several components in the treatment nozzle impact the range of protons in the treatment head including the first scatterer (FS), the MW, and second scatterer (SS) as shown in Figure 1, with the latter two components forming a unique combination for each option. These components are typically made of a combination of lead and plastic and are always option-dependent. More details about composition of each of these components are provided in Table 1. Note that for a given manufacturer it is unlikely that the design of the MW varies between different proton therapy centers. The two parameters in the Bethe-Bloch equation associated with possible uncertainties for predicting the correct range are the density and the mean ionization potential, which is also known as the I-value.
Table 1.
Materials that are present along the proton beam path for each option (A1–A8) of Gantry 1 at Francis H. Burr Proton Therapy Center. The relevant treatment head components are the FS, MW, and SS.
| Material | ||||
|---|---|---|---|---|
| Lead | Lexan | Carbon | ||
| FS | ||||
| A1 | MW | |||
| SS | ||||
| FS | ||||
| A2 | MW | |||
| SS | ||||
| FS | ||||
| A3 | MW | |||
| SS | ||||
| FS | ||||
| A4 | MW | |||
| SS | ||||
| FS | ||||
| A5 | MW | |||
| SS | ||||
| FS | ||||
| A6 | MW | |||
| SS | ||||
| FS | ||||
| A7 | MW | |||
| SS | ||||
| FS | ||||
| A8 | MW | |||
| SS | ||||
In this study, the densities and I-values were varied in Lexan, carbon and lead to test the sensitivity of range on these parameters. The densities of these materials were varied by ±1% and ±5% while the I-values of these materials were varied by ±10% and ±20%. The variations reflect the potential uncertainties in these parameters. Table 2 provides nominal densities and I-values for the materials considered. The range of each dose distribution was determined using the distal 90% of the SOBP. For these simulations, all eight options were considered.
Table 2.
Nominal densities and I-values for Lexan, carbon, and lead.
| density (g/cm3) | I-value (eV) | |
|---|---|---|
| Lexan | 1.20 | 73.1 |
| carbon | 1.82 | 78.0 |
| lead | 11.35 | 823 |
2.2.2 Uniformity Uncertainties
The uniformity of an SOBP dose distribution depends on the summation of the weighted Bragg peaks created by the integration of the MW and the proton beam current. Thus, treatment head parameters may also influence the uniformity of the SOBP dose distribution. Monte Carlo simulations allowed us to investigate the sensitivities of the SOBP uniformity on pertinent beam parameters such as the initial proton beam energy spread and spot size. These two parameters may directly influence the uniformity of the SOBP dose distribution by modifying the weights of selected Bragg peaks created by the MW without modifying the proton beam current. We hypothesized that changing the energy spread should increase or decrease the peak-to-plateau ratio of the pristine Bragg peaks that are constituents of an SOBP. In a similar manner, the beam spot may also influence the peak-to-plateau ratio of pristine Bragg peaks. Depending on the width of the MW step, the beam spot may cover more than one step. For these narrower steps and non-uniform beam current modulation, increasing or decreasing the assumed spot size will modify the weights of the pristine Bragg peaks and potentially influence the SOBPs uniformity. However, the overall sensitivity of SOBP dose distributions on these parameters has never been tested.
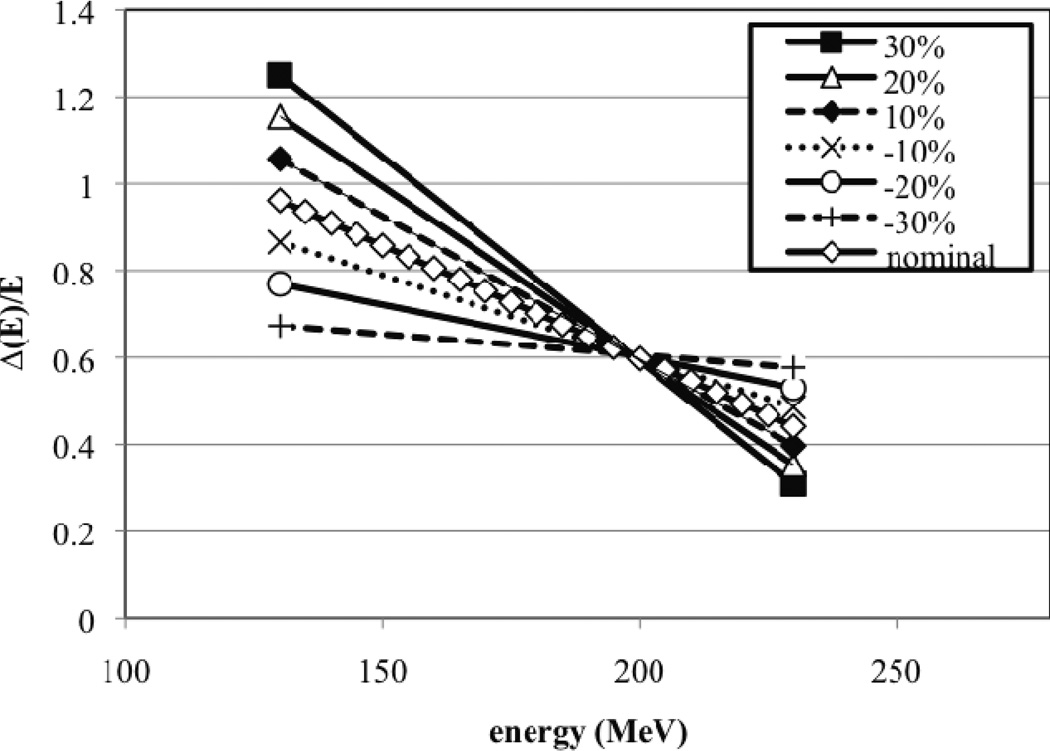
In our Monte Carlo model we define the weighted energy spread (ΔE/E) of the proton beam entering the treatment head by a linear function that decreases with beam energy. Note, we assume a Gaussian energy spread function ΔE. The assumption of a linear relationship is an approximation, since one might expect a sharper increase at lower energies. At our facility, the energy spread reaches its maximum at around 160 MeV due to the settings of the beam absorber and beam shaping slits in the energy selection system at the cyclotron exit along with the magnetic beam steering. The relationship between beam energy and energy spread is not known accurately (probably within 20% uncertainty) as the beam’s energy spread is not easy to measure. Six additional linear energy spread functions (ΔE/E) with different slopes were defined to test the sensitivity of beam energy spread on SOBP uniformity. The nominal energy spread equation along with the six modified equations are plotted in Figure 2. The percent change is reflected in the energy spread of the lowest energy point, i.e. 130 MeV. Similarly, beam spot size is measured at our facility using a segmented ionization chamber at the treatment head entrance. The nominal beam spot size was defined by a Gaussian function with a full-width-at-half-maximum (FWHM) of 0.65 cm.
Figure 2.
The nominal and six modified energy spread functions (ΔE/E) for the beam at nozzle entrance. The modified energy spread functions were each normalized to the nominal spread function at 200 MeV. This choice of normalization was based on our prior knowledge that the SOBP dose distribution for the option corresponding to this energy was uniform.
The sensitivity of the uniformity on the energy spread and spot size was tested. The uniformity was defined as the percent difference between the maximum and minimum dose on the SOBP plateau region. Only the dose values between 5% and 95% of the SOBP plateau region were considered to avoid the inclusion of statistical fluctuations near large dose gradients. For a perfectly flat SOBP the percent change would obviously be zero. To test the sensitivity of the uniformity on the energy spread we use the six linear equations presented in Figure 2. Likewise, to test the sensitivity of the beam spot size on the SOBP uniformity, the FWHM of the spot size was modified by ±5% and ±20%.
2.2.3. Modulation Width Uncertainties
A change in uniformity of an SOBP dose distribution also impacts the modulation width. Therefore, in this study we also investigate the sensitivity of modulation of the initial beam energy spread and spot size.
2.3 A correction method to improve SOBP uniformity using beam current modulation optimization
In the previous section we discussed the potential limitation for accurately modeling SOBP dose distributions produced by Monte Carlo models due to uncertainties in the treatment head configuration. If there is a discrepancy between the measured and simulated range of an SOBP dose distribution, it is possible to correct for this discrepancy by adjusting beam line material parameters (e.g. density) as long as these adjustments are within the tolerances specified by the manufacturer. However, if the SOBP uniformity is inaccurate then a more rigorous tuning method might be needed, since even a slight disagreement between the modulator wheel rotation and beam current in the Monte Carlo model could result in non-uniform SOBP dose distributions (Lu et al 2007). Interestingly, the latter can be advantageous because the beam current modulation function can be slightly tuned in the Monte Carlo model to obtain accurate SOBP dose distributions. The importance of accounting for the time-dependent integration of treatment head geometry with beam current in Monte Carlo treatment head models has been demonstrated (Paganetti et al 2004).
In the following discussion, a tuning method is presented that is based on concepts provided in several previous papers on beam current modulation (Lu and Kooy 2006, Lu et al 2007, Lu 2008). A general formulism that expresses the relationship between the depth dose distribution D(x) and the beam current modulation function I(t) is given by,
| (1) |
where C(x,t) is the transformation representing the specific configuration of the treatment head settings. The upper limit S in the integral is simply the stop time of the proton beam. Equation (1) is the general form for the dose distribution at a given depth in terms of the treatment head configuration and the beam current modulation function (Lu and Kooy 2006).
For this work we provide a specific from of equation (1) that defines the depth dose distribution produced by measured pristine Bragg peaks in the actual treatment system as,
| (2) |
The function I0(t) was previously determined from measured pristine Bragg peaks during the commissioning process of our facility using an in-house software called DoseTools (Lu and Kooy 2006). We should note that measured data was taken in a water tank using a Markus-type ionization chamber (Lu and Kooy 2006). Reports of uncertainties for measurements of this type range from 2–4% (Newhauser et al 2002). Whereas, the depth dose distribution produced by the Monte Carlo model using the same beam current modulation function can be expressed by,
| (3) |
Using the aforementioned measured Bragg peaks it is possible to adjust the beam current function in DoseTools to fit the same depth dose distribution that is produced by the Monte Carlo model, i.e.,
| (4) |
We can now solve for an adjusted beam current function that can be used in the Monte Carlo to produce the original (i.e. uniform) depth dose distribution,
| (5) |
Using equations (2–5), a solution for the optimized Monte Carlo beam current function I2(t) can be determined, i.e.
| (6) |
This expression for the optimized Monte Carlo beam current function should produce uniform SOBP dose distributions for any treatment head arrangement. Obviously, one would expect the difference between I0(t) and I2(t) to be small.
Commissioning was performed for our Monte Carlo models of the treatment heads in Gantry 1 and Gantry 2 to test the feasibility of this correction method. We compared the calculated range, modulation width, and uniformity to the expected values for each sub option. For this study we followed the tolerance levels defined by Engelsman et al (2009) for the range and modulation width, with an additional criteria that considered the SOBP uniformity. The tolerance defined for the range is between (prescribed range-1 mm) and (prescribed range+2 mm), which preferentially errs to higher dose to normal tissue. However, we have to subtract 1 mm because of an off-set from the treatment control system considering that the SOBP range is typically 1 mm shorter than the range of the deepest peak within the SOBP leading to a tolerance between (prescribed range-2 mm) and (prescribed range+1 mm). A tolerance of prescribed width±3 mm was set for the modulation width at the proximal 98% isodose level. A third criteria was set for the uniformity of the SOBPs. The uniformity tolerance on the SOBP plateau was set to be between 0±2%, which follows the uniformity requirement used at our facility. The nominal material parameter values presented in Table 2 were used for commissioning.
3. Results and Discussion
3.1 Sensitivities of SOBP simulations to uncertainties in beam or material parameters in the treatment head
3.1.1 Range Uncertainties
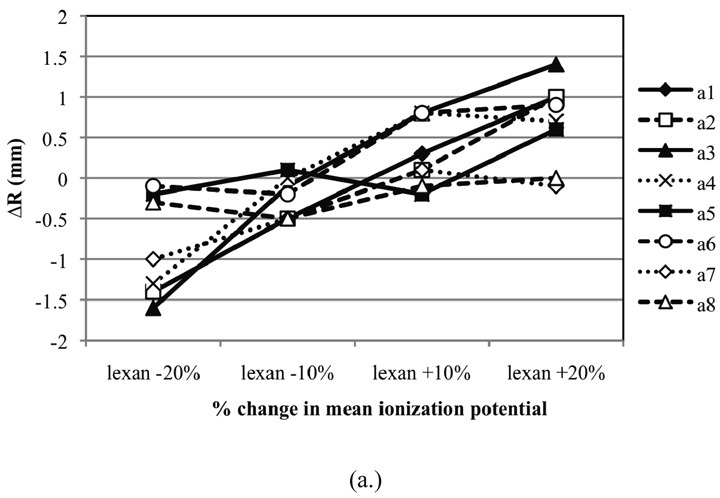
Figure 3 (a.) shows the absolute difference of the simulated SOBP range to the measured (nominal) range as a function of changes in the density of Lexan for eight of the deliverable options from the Monte Carlo model of the treatment nozzle in Gantry 1. Slight changes in density clearly influence the range of the SOBP. In addition, the sensitivity of the density changes is dependent on the option since each option uses a unique combination of a FS, MW, and SS (see Table 1). For example, the MW track for option A1, which is composed of a large amount of Lexan, is much more sensitive to density changes compared to the MW track option A7, which is composed of only a small amount Lexan. Figure 3 (b.) provides the absolute difference of the modified SOBP range to the nominal range as a function of changes in the density of carbon. It appears that slight changes in the density of carbon does not have a noticeable effect within the statistical uncertainty of the Monte Carlo simulation. This can be attributed to the fact that carbon is only present on the MW track of two options (A4 and A6) and the amount of carbon material present on these tracks compared to other materials is insignificant. That is, the water-equivalent thickness of carbon on the MW is much less than the water-equivalent thickness of lead on the MW. Alternatively, changes in the density of lead have a much more prominent effect on the range of the SOBPs, as can be seen in Figure 3 (c.). The relative influence of density change on SOBP range for each option is slightly greater for Lexan compared to lead due to the larger areal density of Lexan along each track. In addition, Lexan and lead are also located on the FS and SS in the treatment head. For example, despite the fact that Lexan does not appear on the MW track for option A4, this option is still sensitive to changes in the density of Lexan since it uses a SS that consists of a large amount Lexan compared to other scattering material.
Figure 3.
The absolute difference of the modified SOBP range to the nominal range (R) as a function of change in the density of (a.) Lexan (b.) carbon and (c.) lead for eight of the deliverable options from the treatment head (see Table 1 for materials defined for each option).
It has been reported that material density is a parameter that is prone to error when provided in manufacturer blue prints of radiation therapy hardware (Sheikh-Bagheri and Rogers 2002). Density values for lead provided in the literature or given by vendors vary in value by less than a few percent. Conversely, the densities of Lexan and carbon are generally more inconsistent, mainly due to uncertainties inherent in the fabrication process of plastics. Therefore, the variation in the density of lead, Lexan and carbon could influence the accuracy of the Monte Carlo simulation. The range accuracy of a Monte Carlo model could be improved by simply adjusting material densities within the beam line, as long as these adjustments are within the tolerances provided by the manufacturer. Of course, requesting smaller tolerances in the manufacturer specifications of material parameters would also help improve the accuracy of the model.
Figure 4 provides the absolute difference of the simulated SOBP range to the measured range as a function of change in the I-value of Lexan, carbon, and lead. Here we demonstrate slight changes in the range due to I-value changes in beam line materials. Small changes in the I-values do not significantly impact the range of the SOBP dose distribution because the I-value is only defined in the denominator of the logarithmic term of the relative stopping power formula. However, it appears that larger uncertainties may produce range changes that are clinically significant. This finding may be important since, the largest uncertainty in the stopping power for clinically used proton beam energies extends from the I-value (Andreo 2009, Gottschalk 2010). I-values for all materials can be determined by measurement or theoretical calculation. Typically, the I-value is adjusted to agree with measurement for elements where data exists, and is interpolated, using theory as a guide, where data does not exist. However, it is clear from the literature that theory and measurement do not always agree, possibly due to binding effects (Ahlen 1980). In Ahlen (1980), variations in published I-values are presented for most elements. Differences in published I-values for given elements can exceed 10% in some cases (Ahlen 1980). Andreo (2009) investigated the effect of variation in I-values of water on pristine Bragg peaks where the uncertainties may exceed 15%. The author noted a shift in Bragg peaks of up to 5 mm. Therefore, the beam line material I-values may also be altered within the reported tolerances when tuning of a Monte Carlo model is needed. We would also like to emphasize that improvements in the estimates of I-values for beam line materials would help to improve the accuracy of the Monte Carlo model.
Figure 4.
The absolute difference of the modified SOBP range to the nominal range (R) as a function of change in the I-value of (a.) Lexan (b.) carbon and (c.) lead for eight of the deliverable options from the treatment head (see Table 1 for materials defined for each option).
3.1.2 Uniformity and Modulation Width Uncertainties
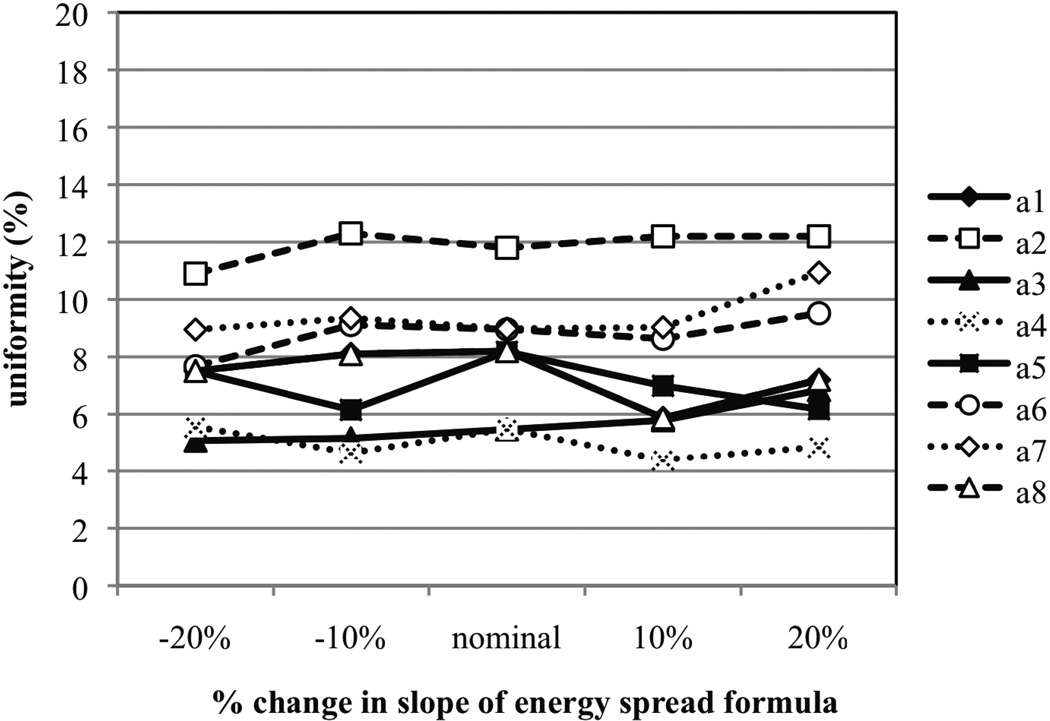
The sensitivity of SOBP uniformity on the initial energy spread was studied by modifying the linear equation that defines ΔE/E as a function of beam energy at the treatment head entrance. Six different linear ΔE/E equations were studied, which are provided in Figure 2. Figure 6 provides the uniformity as a function of different percent changes in the beam energy spread formula. It appears that a change in beam energy spread does not have a noticeable effect on the uniformity of the SOBP. It is know that for individual peaks the peak-to-plateau ratio of the pristine peak is influenced by the change in energy spread. For example, increasing the energy spread decreases the peak-to-plateau ratio due to the broadening of the Bragg peak (Paganetti et al 2004). However, for the realistic energy spread changes considered in this paper the effect is too subtle to have a significant impact on the uniformity of the SOBP (as shown in Figure 6).
Figure 6.
The uniformity plotted as a function of percent change in slope of the energy spread formula. The uniformity is defined as the percent change in dose from the proximal to distal portion of the SOBP plateau region.
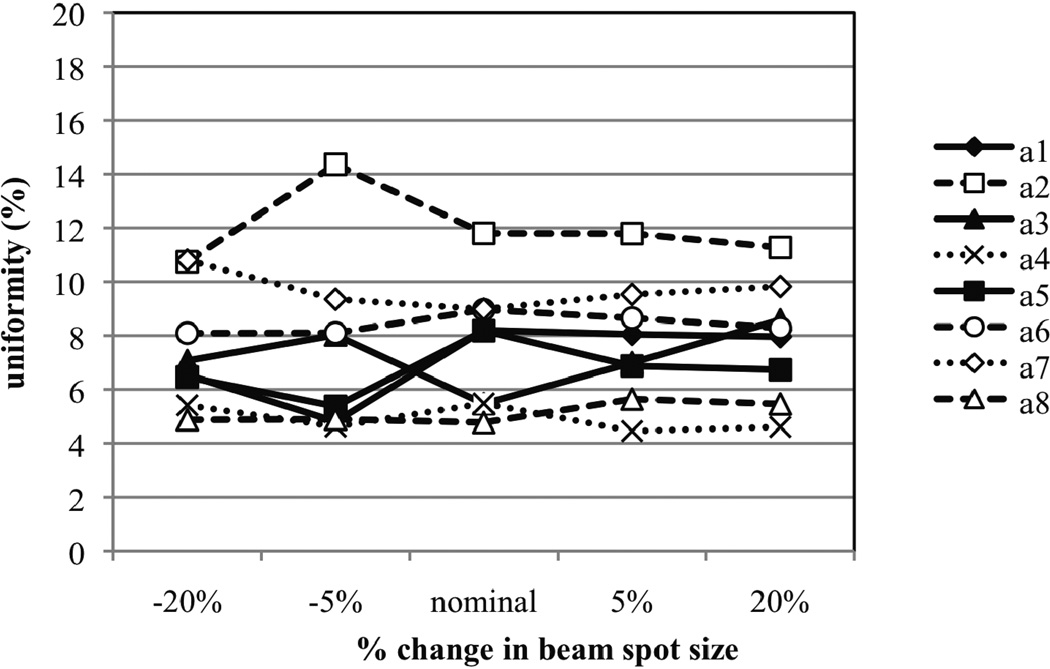
Figure 7 provides the uniformity of the SOBP dose distribution in different options as a function of percent change in the FWHM of the beam spot size at the treatment head entrance. Again, the beam spot size does not have a noticeable effect on the uniformity. The beam spot size has little influence on deeper peaks due to the large step widths for these peaks on the modulator wheel. For shallower peaks in the SOBP it is more likely that the beam spot size will overlap multiple steps causing the pristine peaks to broaden. However, similar to the effect of changes in beam energy spread of the beam on the SOBP dose distribution, spot size effects on shallower peaks are too subtle to produce significant changes to the uniformity of the SOBP. As a result, it appears that both beam energy spread and spot size are poor candidates for correcting the uniformity of the SOBP produced by Monte Carlo models. An alternative correction method is presented in the next section.
Figure 7.
The uniformity plotted as a function of percent change in beam spot size. The uniformity is defined as the percent change in dose from the proximal to distal portion of the SOBP plateau region.
We were also unable to discern an effect of changes in spot sizes or energy spread under consideration on the modulation width. Actually, this result is opposite than what we originally hypothesized. For example, an increase in spot size gives more weight to the most proximal Bragg peak relative to deeper peaks. It might even result in protons passing through a step in the MW (towards steps with greater thicknesses) that would not be normally exposed. Even if the effect of beam spot overlap cancels out with the summation of Bragg peaks, this is not the case for the last step where a bigger spot size might involve an additional step. A slight change in the weight of the peaks produced by these narrower steps could produce a significant change in the modulation width because the slope of the depth-dose distribution proximal to the SOBP is very flat. However, insensitivity of spot size on modulation width might be due to the fact that our facility uses wheel steps with relatively large width (360 degrees for one full set of steps as shown in Figure 1). Other facilities might use propellers with multiple blades (e.g. 4 blades using just 90 degrees for a full set of steps). This results in smaller step widths and would increase the effect of a changing beam spot size considerably. Based on these observations we believe that the uniformity is more sensitive to uncertainties in the modeling of the timing structure of the treatment head.
3.2 Commissioning the Monte Carlo model by optimizing beam current modulation functions
Using the methodology described in Section II.C., we optimized the beam current function in the Monte Carlo model to correct for discrepancies between measured and simulated SOBPs and improve the accuracy of our model. The improvements in the dose distribution are presented in Figure 8 where distributions produced by the optimized beam current modulation function are compared with those produced from the initial beam current functions. We only present data for the middle energy ranges of each option in Gantry 1 and for full modulation. However, since the beam current modulation functions are option dependent we performed beam current modulation corrections for all 24 sub-options (beam current modulation tables) in Gantry 1 and all 18 sub-options in Gantry 2.
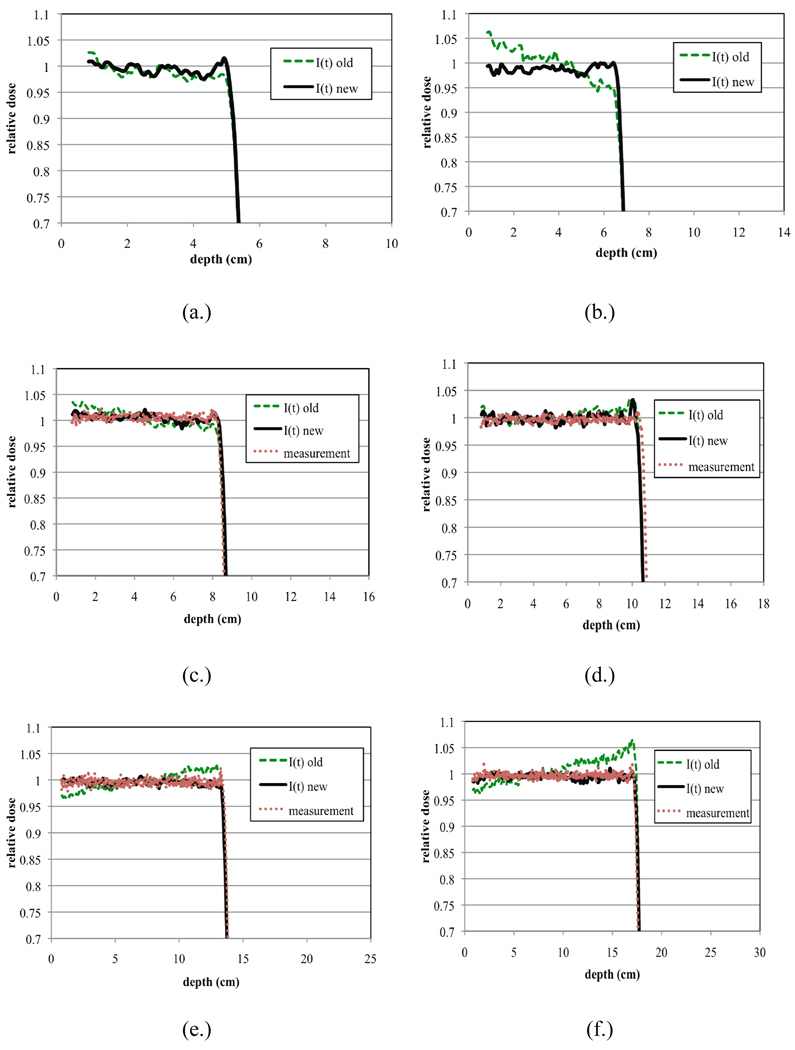
Figure 8.
Plots showing the improvements in the SOBP plateau uniformity once the beam current modulation functions in the Monte Carlo were optimized. Shown here are eight different plots (a–h) representing dose distributions produced for the middle options of A1–A8. The lines labeled I(t) old (dashed lines) represent the dose distributions produced by our Monte Carlo model from the old beam current modulation function (see equation 3). The lines labeled I(t) new (solid lines) represent the dose distributions produced by our Monte Carlo model from the new beam current modulation function (see equation 5). Finally the red dotted lines represent measured dose distributions. Note that measurement data was unavailable for options A1 and A2.
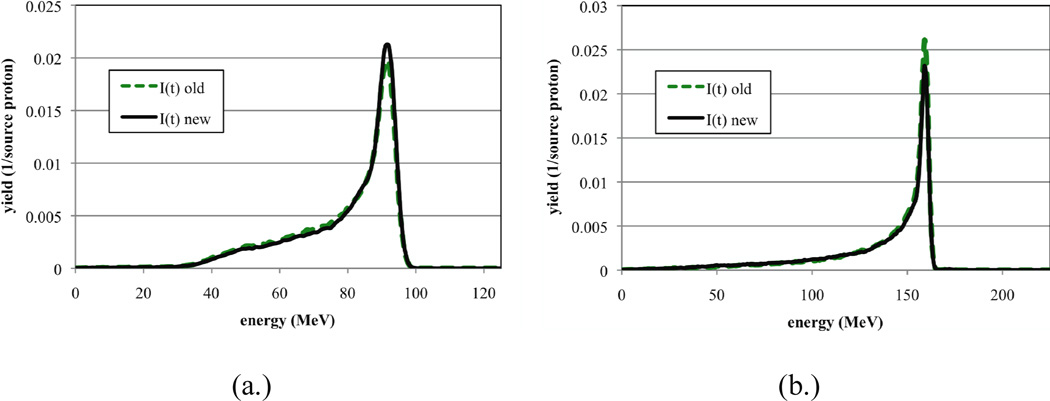
It is important to note that the tuning procedure presented in this paper does not significantly change the characteristics of the proton beam. In Figure 9 we present the energy distribution of protons recorded at the exit of the treatment head using both I(t) old and I(t) new. Two different options (i.e. A2 and A6) were chosen for this comparison. As demonstrated in the figures there are only slight differences between the energy distributions produced by the two different beam current modulation functions. One should make sure that the beam characteristics are not significantly altered when applying this tuning procedure.
Figure 9.
Energy distributions recorded at the exit of the treatment head produced from I(t) old and I(t) new for A2 and A6. All distributions are normalized per source proton.
All commissioning results in terms of range, modulation width and uniformity for Gantry 1 are presented in Figures 10a–c illustrating how fine-tuning the beam current modulation results in excellent agreement between simulated and measured clinical field parameters. Figure 10a plots the calculated ranges for each of the deliverable 24 sub-options. Also shown is the range tolerance. The data for all sub-options are within the clinical tolerances. Figure 10b shows the calculated modulation widths for each sub-option. The clinical tolerance of 3 mm or 3% regarding the location of the 98% isodose level was satisfied for each sub-option. Figure 10c shows the uniformity (in %) for each sub-option of Gantry 1. Again, the uniformity of each dose distribution is within the clinical tolerance.
Figure 10.
Commissioning results presented for Gantry 1. Shown are plots for (a.) the calculated ranges, (b.) calculated modulations widths, and (c.) uniformity for each of the 24 deliverable sub-options (three per option A1–A8). The dashed lines indicate the clinical tolerances.
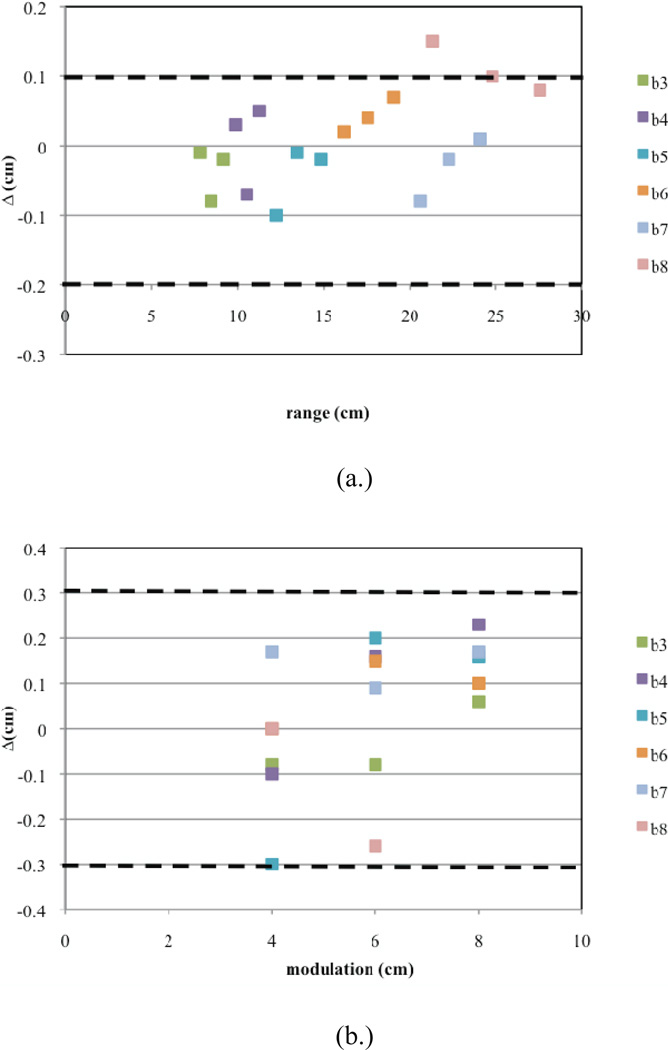
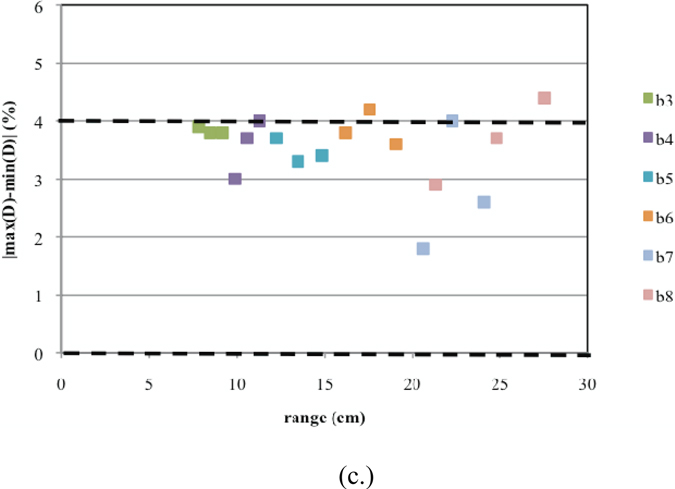
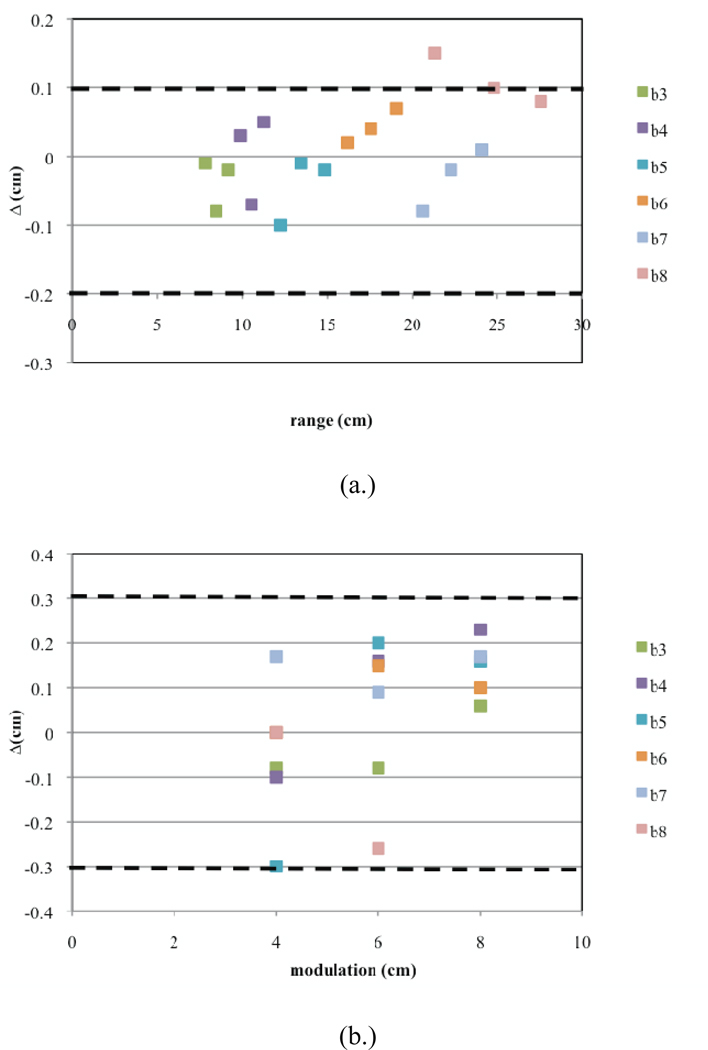
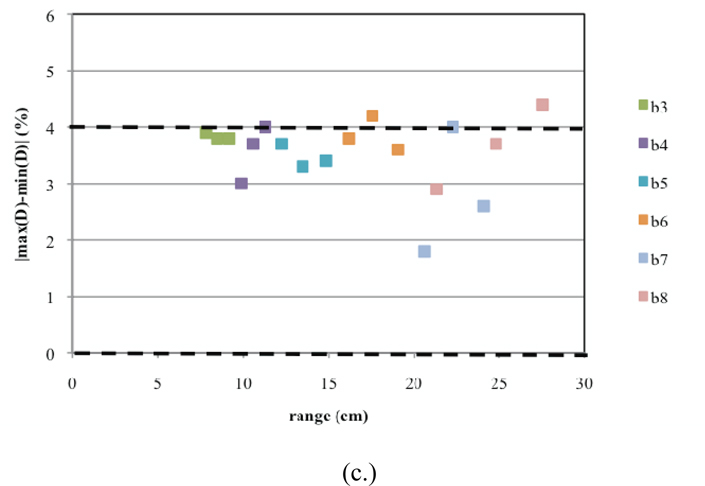
Similarly, the commissioning results for Gantry 2 are presented in Figures 11a–c. Here, for one SOBP dose distribution, the Monte Carlo results predict a slight overshoot (Figure 11a). Furthermore, there are two sub-options that have uniformities slightly greater than 4% (Figure 11c). These discrepancies could not be corrected using beam current modulation. This could be due to significant differences between the designed and built geometry for treatment head devices used in these options. However, these slight differences in the uniformity will cancel out when more than one field is used for treatment.
Figure 11.
Commissioning results presented for Gantry 2. Shown are plots for (a.) the calculated ranges, (b.) calculated modulations widths, and (c.) uniformity for each of the 18 deliverable sub-options (three per option B3–B8). The dashed lines indicate the clinical tolerances.
4. Conclusion
The purpose of this paper was to present methods that can be used to assist in the development of Monte Carlo models of SOBP delivery systems. Monte Carlo models of SOBP delivery systems can be used in treatment planning for challenging clinical cases. In order to simulate the proton field generated by a treatment head in proton therapy, an accurate model of the entire delivery system is required. Even if the modeling of the treatment head is based on manufacturer’s blueprints, there are limitations in accuracy due to uncertainties in the geometries and materials (i.e. based on manufacturer drawings of the dimensions and positions of various objects as well as on material parameters) and beam parameters (i.e. based on measurements). Due to these uncertainties commissioning of a Monte Carlo-based proton beam treatment head might require fine-tuning to ensure accurate dose distributions in the patient. Moreover, the commissioning process can still be difficult even if these parameters are precisely known since the integration of temporal geometries in the Monte Carlo model must precisely match the time-dependent geometrical set-up in the treatment system if identical beam current modulation functions are used. To help expedite the commissioning process we have presented a methodology to improve the accuracy of the SOBP dose distribution by optimizing the Monte Carlo beam current modulation function. Since the corrections would typically be small (as in our case), the change in the underlying beam characteristics (e.g. proton energy distribution) is insignificant for patient dose calculations. This study was based on the treatment head design by IBA (Louvain-la-Neuve, Belgium) at the Francis H. Burr Proton Therapy Center, nevertheless the basic elements for SOBP proton beam therapy are similar in most facilities.
Acknowledgements
The authors would like to thank Partners Research Computing group at Massachusetts General Hospital for all of their assistance with computing resources. This work was supported by Award Number P01CA21239 ("Proton Therapy Research") and R01CA140735 ("PBeam: Fast and Easy Monte Carlo System for Proton Therapy") from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- Andreo P. On the clinical spatial resolution achievable with protons and heavier charged particle radiotherapy beams. Phys. Med. Biol. 2009;54:N205–N215. doi: 10.1088/0031-9155/54/11/N01. [DOI] [PubMed] [Google Scholar]
- Ahlen SP. Theoretical and experimental aspects of the energy loss of relativistic heavily ionizing particles. Rev. Modern Phys. 1980;52:121–173. [Google Scholar]
- Engelsman M, Lu H-M, Herrup D, Bussiere M, Kooy HM. Commissioning a passive-scattering proton therapy nozzle for accurate SOBP delivery. Med. Phys. 2009;36:2172–2180. doi: 10.1118/1.3121489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitein M. A technique for calculating the influence of thin inhomogeneities on charged particle beams. Med. Phys. 1978;5:258–264. doi: 10.1118/1.594507. [DOI] [PubMed] [Google Scholar]
- Gottschalk B. 2010 http://huhepl.harvard.edu/~gottschalk.
- Hong L, Goitein M, Bucciolini M, Comiskey R, Gottschalk B, Rosenthal S, Serago C, Urie M. A pencil beam algorithm for proton dose calculations. Phys. Med. Biol. 1996;41:1305–1329. doi: 10.1088/0031-9155/41/8/005. [DOI] [PubMed] [Google Scholar]
- Lu H-M, Kooy H. Optimization of current modulation function for proton spread-out Bragg peak fields. Med. Phys. 2006;33(5):1281–1287. doi: 10.1118/1.2188072. [DOI] [PubMed] [Google Scholar]
- Lu H-M, Brett R, Engelsmen M, Slopsema R, Kooy H, Flanz J. Sensitivities in the production of spread-out Bragg peak dose distributions by passive scattering with beam current modulation. Med. Phys. 2007;34(10):3844–3854. doi: 10.1118/1.2776255. [DOI] [PubMed] [Google Scholar]
- Lu H-M. A point dose method for in vivo range verification in proton therapy. Phys. Med. Biol. 2008;53:N414–N422. doi: 10.1088/0031-9155/53/23/N01. [DOI] [PubMed] [Google Scholar]
- Newhauser W, Myers K, Rosenthal S, Smith A. Proton beam dosimetry for radiosurgery: implementation of ICRU Report 59 at the Harvard Cyclotron Laboratory. Phys. Med. Biol. 2002;47:1369–1389. doi: 10.1088/0031-9155/47/8/310. [DOI] [PubMed] [Google Scholar]
- Newhauser W, Kock N, Hummel S, Ziegler M, Titt U. Monte Carlo simulations of a nozzle for the treatment of ocular tumours with high-energy proton beams. Phys. Med. Biol. 2005;50:5229–5249. doi: 10.1088/0031-9155/50/22/002. [DOI] [PubMed] [Google Scholar]
- Paganetti H, Jiang H, Lee S-Y, Kooy HM. Accurate Monte Carlo simulations for nozzle design, commissioning, and quality assurance for a proton radiation therapy facility. Med. Phys. 2004;31(7):2107–2118. doi: 10.1118/1.1762792. [DOI] [PubMed] [Google Scholar]
- Paganetti H. Monte Carlo calculations for absolute dosimetry to determine machine outputs for proton therapy fields. Phys. Med. Biol. 2006;51:2801–2812. doi: 10.1088/0031-9155/51/11/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganetti H, Parodi K, Slopsema R, Engelsman M. Clinical implementation of full Monte Carlo dose calculation in proton beam therapy. Phys. Med. Biol. 2008;53:1–29. doi: 10.1088/0031-9155/53/17/023. [DOI] [PubMed] [Google Scholar]
- Sheikh-Bagheri D, Rogers DWO. Sensitivity of megavoltage photon beam Monte Carlo simulations to electron beam and other parameters. Med. Phys. 2002;29:379–390. doi: 10.1118/1.1446109. [DOI] [PubMed] [Google Scholar]
- Sisterson JM, Urie MM, Koehler AM, Goitein M. Distal penetration of proton beams: the effects of the air gaps between compensation bolus and patient. Phys. Med. Biol. 1998;34:1309–1315. doi: 10.1088/0031-9155/34/9/016. [DOI] [PubMed] [Google Scholar]
- Titt U, Zheng Y, Vassiliev ON, Newhauser WD. Monte Carlo investigation of collimator scatter of proton-therapy beams produced using the passive scattering method. Phys. Med. Biol. 2008;53:484–504. doi: 10.1088/0031-9155/53/2/014. [DOI] [PubMed] [Google Scholar]
- Urie M, Goitein M, Holley WR, Chen GTY. Degradation of the Bragg peak due to inhomogeneities. Phys. Med. Biol. 1986;31:1–15. doi: 10.1088/0031-9155/31/1/001. [DOI] [PubMed] [Google Scholar]
- Urie M, Sisterson JM, Koehler AM, Goitein M, Zoesman J. Proton beam penumbra: effects of separation between patient and beam modifying devices. Med. Phys. 1986;13:734–741. doi: 10.1118/1.595974. [DOI] [PubMed] [Google Scholar]
- Urie M, Goitein M, Wagner M. Compensating for heterogeneities in proton radiation therapy. Phys. Med. Biol. 1983;29:553–566. doi: 10.1088/0031-9155/29/5/008. [DOI] [PubMed] [Google Scholar]
- Zacharatou Jarlskog C, et al. Physics settings for using the Geant4 toolkit in proton therapy. IEEE Transactions in Nuclear Science. 2008;53:270–278. [Google Scholar]