Abstract
Advanced-stage primary cutaneous T-cell lymphoma has an unfavorable prognosis and low survival rates. Aggressive treatment with chemotherapy is not curative and causes considerable side effects. The combination of bexarotene and denileukin diftitox is associated with an acceptable safety profile and a likely synergistic effect because bexarotene is capable of modulating expression of IL-2 receptor and enhance the susceptibility of T-cell leukemia cells to denileukin diftitox. In the case reported here, the response to this combined treatment was satisfactory and well tolerated. The patient showed a complete regression of pruritus, restlessness, and insomnia. Skin lesions improved partially, and lymphadenopathy was reduced and finally disappeared completely.
Key words: Cutaneous T-cell lymphoma, Mycosis fungoides, Bexarotene, Denileukin diftitox
Case Report
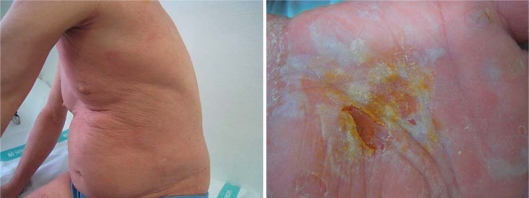
A 55-year-old man was diagnosed with a 10-year history of stage-IIIA erythrodermic advanced-stage primary cutaneous T-cell lymphoma (CTCL; T4N0M0). Initially, he was treated with topical corticosteroid cream, psoralen plus UVA phototherapy and IFN-a. The patient did not respond to this therapy and was started on a course of bexarotene monotherapy at a dose of 300 mg/m2/day, achieving a marked improvement of his skin lesions. Only few side effects (hypertriglyceridemia, hypercholesterolemia, and hyperthyroidism) which were clinically manageable occurred. After 4 years of bexarotene treatment during which clinical response was maintained, the patient experienced a relapse with skin infiltration of 90% of the total body surface (fig. 1, left), with palmoplantar keratoderma (fig. 1, right) and palpable axillary and inguinal lymph nodes. The patient was restless and nervous, had generalized pruritus, and was unable to sleep.
Fig. 1.
The patient presented skin infiltration of 90% of the total body surface (left), with palmoplantar keratoderma (right).
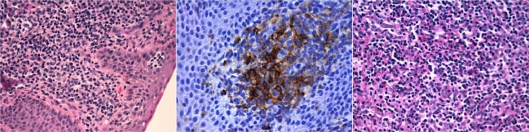
A skin biopsy confirmed the diagnosis of CTCL without large-cell transformation (fig. 2, left). In addition, 20% of the atypical lymphocytes were positive for CD25 expression by immunohistochemistry (fig. 2, center). The histological examination of the lymph node biopsy showed extensive infiltration (fig. 2, right). A complete blood count revealed the absence of circulating Sézary cells, computed tomography indicated no visceral involvement, and bone marrow biopsy revealed no abnormalities.
Fig. 2.
Skin biopsy showing CTCL without large-cell transformation (left, HE, ×200) and CD25 expression (center, HE, ×400), and lymph node biopsy revealing extensive infiltration of atypical lymphocytes (right, HE, ×200).
Due to the advanced stage of the lymphoma and the appearance of new lesions, denileukin diftitox was added to bexarotene therapy. The treatment schedule with intravenous denileukin diftitox was fixed at 18 mg/kg/day for the first 3 days of each 21-day cycle for a total of 4 cycles, with previous administration of steroid medication (dexamethasone 8 mg). Additionally, antihistamine (dexchlorpheniramine maleate 5 mg) and antipyretics (paracetamol 1 g) were administered. After infusion, the treatment was completed with adequate intravenous saline hydration in order to reduce the incidence of vascular leak syndrome.
Denileukin diftitox treatment was well tolerated, and no notable infusion-related effects occurred. Side effects of bexarotene, such as dyslipidemia and central hypothyroidism, were controlled with oral medication.
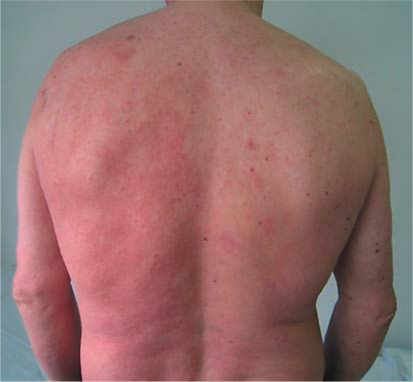
The response to this combined treatment was satisfactory. The patient showed a complete regression of pruritus, restlessness, and insomnia. Skin lesions improved partially (fig. 3), and lymphadenopathy was reduced and finally disappeared. Then, denileukin diftitox administration was stopped, and only bexarotene treatment was maintained at 300 mg/m2/day.
Fig. 3.
After combined treatment with bexarotene and denileukin diftitox, the skin lesions improved partially.
After 6 months, the patient showed a deterioration of facial skin lesions, with no increase in lymphadenopathy, systemic symptoms, or extracutaneous involvement. Currently, the patient receives treatment with extracorporeal photopheresis and radiotherapy.
Discussion
Bexarotene is a retinoid which is specifically selective for retinoid X receptors. Its efficacy for the clinical treatment of CTCL in the initial and advanced stages has been proven previously [1, 2]. Dyslipidemia, hypothyroidism, and leukopenia are possible adverse drug reactions that may occur in connection with bexarotene [1, 2].
Denileukin diftitox is a relatively novel engineered fusion protein obtained from the combination of interleukin-2 and the enzymatically active domains of diphtheria toxin [3, 4, 5]. The therapeutic targets of the protein are neoplastic cells that express interleukin-2 receptors (IL2R). Although its binding affinity is higher for heterotrimeric proteins of the receptors composed of α/p55/CD25, β/p75/CD122, and γ/p64/CD132 chains, it also has an effect on intermediate affinity receptors with β and γ chains. The expression of different subunits of IL2R is dynamically regulated by specific cytokines and genes associated with cellular activation [3, 4, 5]. Denileukin diftitox has been used for the treatment of several hematologic neoplasms [6, 7], and some clinical trials have identified its utility in the treatment of mycosis fungoides and other CTCLs [8, 9, 10, 11]. The most common adverse reactions include constitutional and gastrointestinal symptoms, acute hypersensitivity reactions which can usually be reduced by systemic corticosteroid premedication [12, 13], and vascular leak syndrome (hypotension, edema, and hypoalbuminemia) [6, 7, 8, 9, 10, 11, 12, 13]. Furthermore, denileukin diftitox has been used as monotherapy of mycosis fungoides. Olsen et al. [8] obtained an objective response rate of 30% (10% complete response), with a mean duration of 6.9 months, using dose levels of 45 or 90 μg/kg/cycle. In a more recent study using the same dosage procedure [9], a response rate of 51% was observed (14% complete response). Moreover, in 3 of the 6 patients with Sézary syndrome included in this clinical trial skin lesions and lymph nodes improved.
The combination of bexarotene and denileukin diftitox was based on in vitro and in vivo studies. These studies have shown that bexarotene positively regulated both p55 and p75 subunits of IL2R and enhanced the susceptibility of neoplasm cells to denileukin diftitox action [13, 14].
A phase-I clinical trial was published including 14 patients with mycosis fungoides at different stages (IA-IVB) and combining both drugs: 75-300 mg/day oral bexarotene and 54 μg/kg/cycle intravenous denileukin diftitox were administered, using systemic corticosteroid premedication [13]. In this study, an overall response rate of 67% (33% complete responses) was obtained, which is clearly better than the rates observed in monotherapy. Regarding adverse reactions, no significant increase in toxicity was observed. Although a higher percentage of lymphopenia was observed, it was not possible to establish a relationship to the lymphoma, bexarotene, or the combination of both drugs. Moreover, the authors observed that the positive regulation of CD25 expression was achieved with relatively low levels of bexarotene of at least 150 mg/day.
Kerl et al. [15] reported a case involving significant regression of a nasal-type extranodal natural killer/T-cell lymphoma treated with both bexarotene (150 mg/day) and denileukin diftitox (90 μg/kg/cycle). In this case, lesions improved after the first cycle of treatment, but the disease progressed when treatment was stopped after a period of 5 cycles [15].
Our patient, who was diagnosed with stage-IIIA erythrodermic CTCL, was treated with oral bexarotene (300 mg/day) after not responding to psoralen plus UVA phototherapy and IFN-a therapy, and good control of the disease was achieved. After 4 years of bexarotene monotherapy, skin lesions reappeared, covering 90% of the body surface, and lymph nodes were involved as well. Another biopsy of the skin lesions confirmed the CTCL diagnosis without large-cell transformation and showed a high CD25 expression. At this point, denileukin diftitox was added to the monotherapy (18 μg/kg/day × 3 days/cycle × 4 cycles). The combination of bexarotene and denileukin diftitox was well tolerated. Pruritus and insomnia were reduced, lymphadenopathy diminished, and skin lesions improved.
In conclusion, the combination of bexarotene and denileukin diftitox does not seem to significantly increase the adverse reactions associated with monotherapy with each drug, and probably a synergistic therapeutic effect can be obtained due to the upregulation of IL2R. Nevertheless, randomized clinical trials would be necessary to confirm this hypothesis and to establish the most suitable dosing regimen for both drugs.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1.Duvic M, Martin AG, Kim Y, et al. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol. 2001;137:581–593. [PubMed] [Google Scholar]
- 2.Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for the treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 3.Bacha P, Williams DP, Waters C, Williams JM, Murphy JR, Strom TB. Interleukin 2 receptor-targeted cytotoxicity. Interleukin 2 receptor-mediated action of a diphtheria toxin-related interleukin 2 fusion protein. J Exp Med. 1988;167:612–622. doi: 10.1084/jem.167.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters CA, Snider CE, Itoh K, et al. DAB486IL-2 (IL-2 toxin) selectively inactivates high-affinity IL-2 receptor-bearing human peripheral blood mononuclear cells. Ann NY Acad Sci. 1991;636:403–405. doi: 10.1111/j.1749-6632.1991.tb33479.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams DP, Snider CE, Strom TB, Murphy JR. Structure/function analysis of interleukin-2-toxin (DAB486-IL-2). Fragment B sequences required for the delivery of fragment A to the cytosol of target cells. J Biol Chem. 1990;265:11885–11889. [PubMed] [Google Scholar]
- 6.Le Maistre CF, Saleh MN, Kuzel TM, et al. Phase I trial of a ligand fusion-protein (DAB389IL-2) in lymphomas expressing the receptor for interleukin-2. Blood. 1998;91:399–405. [PubMed] [Google Scholar]
- 7.Wong BY, Gregory SA, Dang NH. Denileukin diftitox as novel targeted therapy for lymphoid malignancies. Cancer Invest. 2007;25:495–501. doi: 10.1080/07357900701360096. [DOI] [PubMed] [Google Scholar]
- 8.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 9.Chin KM, Foss FM. Biologic correlates of response and survival in patients with cutaneous T-cell lymphoma treated with denileukin diftitox. Clin Lymphoma Myeloma. 2006;7:199–204. doi: 10.3816/CLM.2006.n.059. [DOI] [PubMed] [Google Scholar]
- 10.Hathaway T, Subtil A, Kuo P, Foss F. Efficacy of denileukin diftitox in subcutaneous panniculitis-like T-cell lymphoma. Clin Lymphoma Myeloma. 2007;7:541–545. doi: 10.3816/clm.2007.n.040. [DOI] [PubMed] [Google Scholar]
- 11.Assaf C. Denileukin diftitox therapy for patients with tumour-stage mycosis fungoides. Dermatol Clin. 2008;(suppl 1):21–22. [PubMed] [Google Scholar]
- 12.Foss FM, Bacha P, Osann KE, Demierre MF, Bell T, Kuzel T. Biological correlates of acute hypersensitivity events with DAB(389)IL-2 (denileukin diftitox, ONTAK) in cutaneous T-cell lymphoma: decreased frequency and severity with steroid premedication. Clin Lymphoma. 2001;1:298–302. doi: 10.3816/clm.2001.n.005. [DOI] [PubMed] [Google Scholar]
- 13.Foss F, Demierre MF, DiVenuti G. A phase-I trial of bexarotene and denileukin diftitox in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood. 2005;106:454–457. doi: 10.1182/blood-2004-11-4570. [DOI] [PubMed] [Google Scholar]
- 14.DiVenuti GM, Foss FM. Phase I dose escalation study of Targretin and Ontak in hematologic malignancies: upregulation of IL2R expression by low dose Targretin. Blood. 2001;98:601a. [Google Scholar]
- 15.Kerl K, Prins C, Cerroni L, French LE. Regression of extranodal natural killer/T-cell lymphoma, nasal type with denileukin diftitox (Ontak) and bexarotene (Targretin): report of a case. Br J Dermatol. 2006;154:988–991. doi: 10.1111/j.1365-2133.2006.07151.x. [DOI] [PubMed] [Google Scholar]