Abstract
COQ10 deletion in Saccharomyces cerevisiae elicits a defect in mitochondrial respiration correctable by addition of coenzyme Q2. Rescue of respiration by Q2 is a characteristic of mutants blocked in coenzyme Q6 synthesis. Unlike Q6 deficient mutants, mitochondria of the coq10 null mutant have wild-type concentrations of Q6. The physiological significance of earlier observations that purified Coq10p contains bound Q6 was examined in the present study by testing the in vivo effect of over-expression of Coq10p on respiration. Mitochondria with elevated levels of Coq10p display reduced respiration in the bc1 span of the electron transport chain, which can be restored with exogenous Q2. This suggests that in vivo binding of Q6 by excess Coq10p reduces the pool of this redox carrier available for its normal function in providing electrons to the bc1 complex. This is confirmed by observing that extra Coq8p relieves the inhibitory effect of excess Coq10p. Coq8p is a putative kinase, and a high copy suppressor of the coq10 null mutant. As shown here, when over-produced in coq mutants, Coq8p counteracts turnover of Coq3p and Coq4p subunits of the Q-biosynthetic complex. This can account for the observed rescue by COQ8 of the respiratory defect in strains over-producing Coq10p.
Introduction
Coenzyme Q (ubiquinone) is an essential electron carrier of the mitochondrial respiratory chain. Its main function is to transfer electrons from the NADH- and succinate-coenzyme Q reductases to the bc1 complex [1]. Biosynthesis of coenzyme Q in eukaryotes occurs in mitochondria. The benzene ring of coenzyme Q has a polyprenyl side chain with 6 isoprenoid units (Q6) in Saccharomyces cerevisiae and 10 units (Q10) in humans [2]. Nine yeast nuclear genes (COQ1–9) have been shown to be involved in Q6 synthesis starting with the conjugation of the polyprenyl chain with 4-hydroxybenzoate (4-HB) [3–10]. Recent evidence indicates that para-aminobenzoic acid (pABA) is an alternative Q6 precursor capable of competing with 4-HB for the prenylation reaction catalyzed by Coq2p [11, 12]. Accordingly, mitochondrial ferredoxin [13], and ferredoxin reductase [14] are also required for Q6 synthesis from pABA [11, 12]. COQ gene products are located in the mitochondrial inner membrane [15]. Yeast mutants harboring deletions in coq3-coq9 genes accumulate the intermediate 3-hexaprenyl-4-hydroxy benzoic acid (HHB) and their respiratory deficiency is corrected by coenzyme Q2 addition to mitochondria and partially by Q6 to whole cells [10, 16, 17]. These common features are consistent with conversion of HHB to Q6 by a multi-subunit Q biosynthetic complex composed of Coq3p-Coq7p and Coq9p [5, 15, 18, 19]; it has also been suggested that three other genes Coq2p, Coq8p and Co10p are part of another complex [18]. Coq2p is the transferase that adds the polyprenyl side chain to HB [6] while Coq8p has been proposed to be a protein kinase that regulates the pathway by phosphorylating Coq3p [18, 20].
Unlike the Q biosynthetic mutants, coq10 null mutant have wild type level of Q6 but like the former, its respiratory deficiency is rescued by Q2 and Q6 [21]. Over-expression of COQ8 also partially suppresses the respiratory defect of coq10 null mutants, probably as a result of having two times more Q6 in mitochondria [21]. Recently we shown that coq10 mutants are responsive to antimycin, indicating an active Q-cycle [22] however, they did not respond to myxothiazol and are unable to transfers electrons through cytochrome c, suggesting that Coq10p might function in the delivery of Q6 to its proper site in the bc1 complex [21,22].
Coq10p is a member of the START domain super family [21–25]. This class of proteins has been shown to bind lipophilic compounds such as cholesterol [26]. When over-expressed in yeast, purified Coq10p contains Q6 [21, 23]. The amount of bound Q6, however, is considerably less than the protein on a molar basis, rendering the physiological significance of the bound Q6 questionable. To address this question we studied binding of Q6 by Coq10p in vivo by measuring the effect of over-expression of the protein on NADH oxidase and NADH cytochrome c reductase activity. Indeed, Coq10p overexpression was previously observed to inhibit the growth of Schizosaccharomyces pombe. [24]. We present evidences here that high levels of Coq10p compete for the large pool of Q6 in mitochondria of respiratory competent yeast, thereby preventing it from functioning in electron transport. We also show that the adverse effect of Coq10p on respiration can be reversed in vitro by addition of Q2 to mitochondria and in vivo by over-expressing COQ8 to raise the mitochondrial concentration of Q6. These results indicate that Coq10p binds Q6 in vivo and that this property is essential for a normal functioning of the electron transport chain.
Materials and methods
Yeast strains and growth media
The genotypes and sources of the yeast strains used in this study are listed in Table I. The compositions of YPD, YPEG and minimal glucose medium have been described elsewhere [21].
Table 1.
Genotypes and Sources of Saccharomyces cerevisiae Strains
| Strain | Genotype | Source |
|---|---|---|
| W303-1A | MATa ade2-1, trp1-1, his3-115, leu2-3,112 ura3-1ρ+, canR | a |
| aW303ΔCOQ1 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq1::LEU2 | [5] |
| aW303ΔCOQ2 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq2::HIS3 | [6] |
| aW303ΔCOQ3 | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq3::LEU2 | [7] |
| aW303ΔCOQ4 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq4::TRP1 | [8] |
| aW303ΔCOQ5 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq5::HIS3 | [9] |
| aW303ΔCOQ9 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq9::URA3 | [10] |
| aW303ΔCOQ10 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq10::HIS3 | [21] |
| aW303ΔCOQ2, ΔCOQ3 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq2::HIS3 coq3::LEU2 | this study |
| aW303ΔCOQ2, ΔCOQ4 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq2::HIS3 coq4::TRP1 | this study |
| aW303ΔCOQ2, ΔCOQ10 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 coq2::HIS3 coq10::HIS3 | this study |
| aW303ΔBCS1 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 bcs1::HIS3 | [27] |
Dr. R. Rothstein, Department of Human Genetics, Columbia University, New York, NY
Plasmid and strains constructions for COQ10 expression
COQ10 was amplified from pCOQ10/ST3 [21] with the primers 5′-ggcagatctatataatggttttgataa-taaggccc and 5′-gggaagcttcgg-agagccttctttagaag. The 626 bp fragment was digested with BglII and HindIII and fused to the GAL10, GPD1, or TEF1 promoters in YIp351-GAL, YIp352-GPD1, and YIp352-TEF1, respectively. The later two promoters were transferred from p4XXprom [28] to YIp351 or YIp352 [29]. The resultant pCOQ10/ST24, pCOQ10/ST38 and pCOQ10/ST39 plasmids containing, respectively, the GAL10-COQ10, TEF1-COQ10 and GPD1-COQ10 fusions, were linearized and integrated at the chromosomal LEU2 or URA3 locus in the strain aW303ΔCOQ10 by the one-step gene insertion method [30]. Transformants containing GAL10-COQ10 were also transformed with pMA210, a high copy plasmid containing GAL4 [31].
Plasmid and strains constructions for COQ8 expression
COQ8 was amplified from pCOQ8/T5 [21] with the primers 5′-ggcagatctatggttacaaatatggtgaa and 5′ ggcctgcagagcggggaagtattttaaac. The 2514 bp fragment was digested with BglII and PstI and fused to the TEF1 promoter. The resultant pTEF1-COQ8 was integrated at the URA3 locus in the strain aW303ΔCOQ8 [30]. A hybrid gene expressing a C-terminally HA tagged COQ8 was constructed after PCR amplification with the primers: 5′ ggggaattccgttacaaatatggtgaaatt, 5′ ggcactagttcaagcgtagtctgggacgtcgt-atgggtaaac-tttataggcaaaaat. The resultant fragment was digested with BamHI and SpeI and replaced into pCOQ8/T5, pICOQ8/T5, pTEF1-COQ8 plasmids.
O2 consumption
Oxygen consumption in mitochondria and spheroplasts was monitored on a computer-interfaced Clark-type electrode at 30°C with 1 μmol of NADH as substrate in the presence of 400 μg/ml mitochondrial protein and 0.002% digitonin. Cytochrome c oxidase was blocked with 1M KCN in the NADH-cytochrome c reductase assay [32].
Miscellaneous procedures
Total mitochondrial proteins were separated by polyacrylamide gel electrophoresis in the buffer system of Laemmli [33] and Western blots were treated with antibodies against Coq10p [21] followed by a second reaction with anti-rabbit IgG conjugated to a horseradish peroxidase (Sigma). The antibody-antigen complexes were visualized by the SuperSignal chemiluminescent substrate kit (Pierce). Densitometric traces of the x-ray films were performed using 1DscanEX software (Scananlytics)
Results
COQ10 over-expression impaired mitochondrial respiration
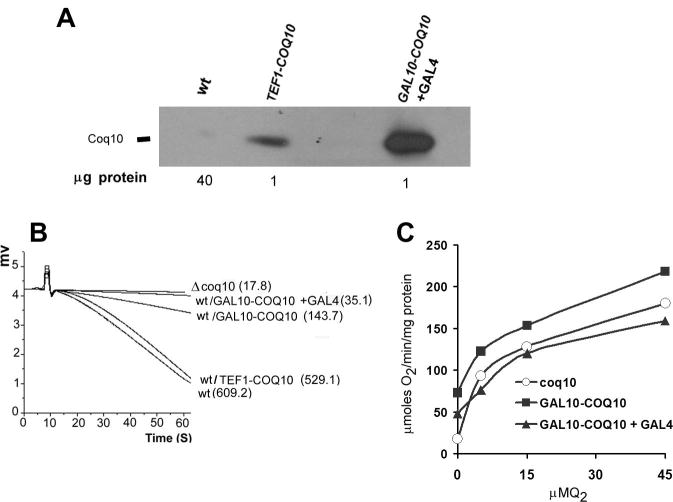
The effect of Coq10p over-production on growth and respiration was studied in strains of yeast harboring chromosomally integrated copies of COQ10 fused to the GAL10, TEF1 or GPD1 promoters. All the strains displayed elevated concentrations of Coq10p (shown for GAL10 and TEF1 fusions in Fig 1A). The highest level of Coq10p, seen in the transformant with the GAL10-COQ10 fusion, was further increased when co-transformed with a plasmid containing GAL4 [31] (Fig 1A). Based on the amount of mitochondrial protein used in the Western analysis and the mean of densitometric traces of the x-ray films we estimate up to 300 fold increase of Coq10p in the strain transformed with GAL10-COQ10 + GAL4 and 100 fold increase for the strain transformed with TEF1-COQ10 fusion (Fig. 1A)
Fig. 1.
NADH oxidase activity in wild type yeast over-producing Coq10p. A) Mitochondria of W303-1A (WT), and W303-1A transformed with pCOQ10/ST38 (TEF1-COQ10), and pCOQ10/ST24 plus pMA210 (GAL10-COQ10+GAL4) were separated by SDS-PAGE on a 12% polyacrylamide gel. Because of the large variation in the levels of Coq10p in these strains, different amounts of proteins were loaded in each lane as indicated on the bottom of the panel. B) Mitochondria (400 μg protein) of the coq10 null mutant W303ΔCOQ10 (Δcoq10) and of the strains used in A) were assayed for NADH-oxidase with a Clark electrode as described in the Materials and methods section. The numbers in parentheses are nmoles of O2 consumed/min/mg protein. C) NADH oxidase activity of mitochondria from the Δcoq10 null mutant and of the wild type W303-1A over-producing Coq10p from GAL10-COQ10 and GAL10-COQ10 + GAL4 measured in the presence of different concentrations of Q2 in the assay.
Purified Coq10p has been shown to contain Q6, albeit in amounts considerably less than stoichiometric with the protein [21, 23]. We reasoned that if binding of Q6 by Coq10p is part of its normal function, at elevated concentrations Coq10p may sequester enough of Q6 to affect respiration. This prediction was borne out when COQ10 was over-expressed from the GAL10 promoter, which diminished the NADH oxidase activity to approximately 20% of the wild type after correction for the rate in the mutant (Fig. 1B). An almost complete loss of NADH oxidase activity was observed when expression of COQ10 was further increased by co-transformation of the wild type strain with GAL10-COQ10 in combination with pMA210, a plasmid containing GAL4 [31]. In contrast, the NADH oxidase activities of wild type transformed with the GPD1-COQ10, and the TEF1-COQ10 constructs were not altered (Fig. 1 shown for TEF1-COQ10 fusion). Even though these promoters also increase the mitochondrial concentration of Coq10p by a factor of 100, as estimated in figure 1A, this was still below the threshold needed for inhibition.
Restoration of NADH and succinate oxidase activity by Q2 is a hallmark of coenzyme Q deficient mutants. Addition of Q2 was previously found to also restore the NADH oxidase and NADH-cytochrome c reductase activity of mitochondria from the coq10 null mutant [21]. To assess if the respiratory defect induced by high levels of Coq10p could be similarly reversed, NADH oxidase activity was measured as a function of Q2 in the assay. These activity measurements confirmed that mitochondria of the coq10 null mutant and of wild type cells over-expressing Coq10p from the GAL10 promoter show the same response to Q2 concentration (Fig. 1C).
Like NADH oxidase, NADH cytochrome c reductase activity was also diminished in strains expressing COQ10 from the GAL10 promoter and was corrected by the addition of exogenous Q2 to mitochondria (Fig 2B). COQ2 codes for p-hydroxybenzoate: polyprenyl transferase that catalyzes the second step of coenzyme Q biosynthesis [6]. The NADH-cytochrome c reductase activity of mitochondria from the coq2 null mutant lacking Q6, was activated by Q2, as had been reported previously [6]. As expected, no activation of NADH cytochrome c reductase activity was observed in the bcs1 mutant in which the Rieske iron-sulfur protein fails to be incorporated into the bc1 complex [27].
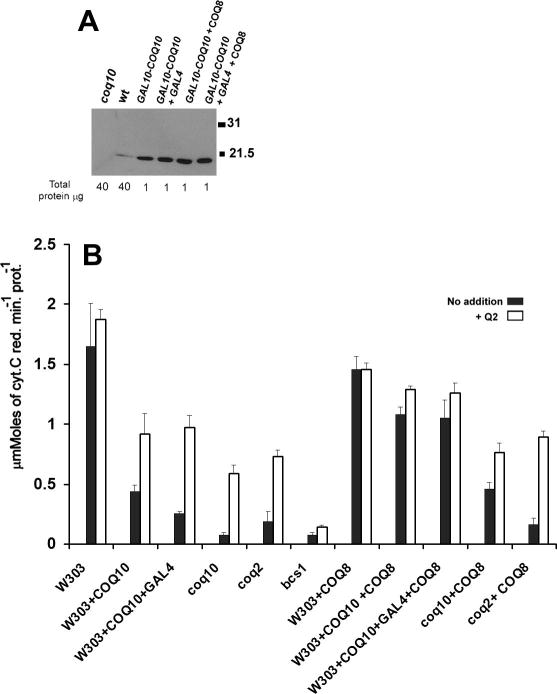
Fig. 2.
COQ8 is a high-copy suppressor of the respiratory defect of strains over- producing Coq10p. A) Immunodetection of Coq10p in mitochondria of W303-1A (WT), W303ΔCOQ10 (Δcoq10), and W303-1A with chromosomally integrated GAL10-COQ10, GAL10-COQ10 + GAL4, GAL10-COQ10 + pTEF1-COQ8 and GAL10-COQ10 + GAL4 + pTEF1-COQ8. The amounts of proteins used for the Western analysis is indicated at the bottom of the panel. B) Mitochondria of W303-1A (WT), W303-1A with chromosomally integrated GAL10-COQ10 and GAL10-COQ10 + GAL4), W303-1A with null mutations in COQ10 (Δcoq10), COQ2 (Δcoq2) and BCS1 (Δbcs1), and the same strains transformed with pTEF1-COQ8, were assayed for NADH cytochrome c reductase activity with and without 1μM Q2 in the assay.
Over-expression of COQ8 increases mitochondrial Q6 by a factor of 2 [21]. The higher concentration of Q6 has been invoked to explain the partial suppression of the respiratory defect of coq10 null mutants and coq9 point mutants by COQ8 [10, 21] (see also Fig. 2B). To see if the COQ8 over-expression could also counteract the respiratory inhibition imposed by excess Coq10p, NADH-cytochrome c reductase activity was measured in wild type cells containing the integrated GAL10-COQ10 constructs, and in the coq10 and the coq2 null mutants as positive and negative controls, respectively. The NADH-cytochrome c reductase activity was largely restored when the wild type strain with either GAL10-COQ10 alone or together with GAL4 were transformed with COQ8 on a high copy plasmid (Fig. 2B). The respiratory activities of the mitochondria from these cells were also increased by Q2.
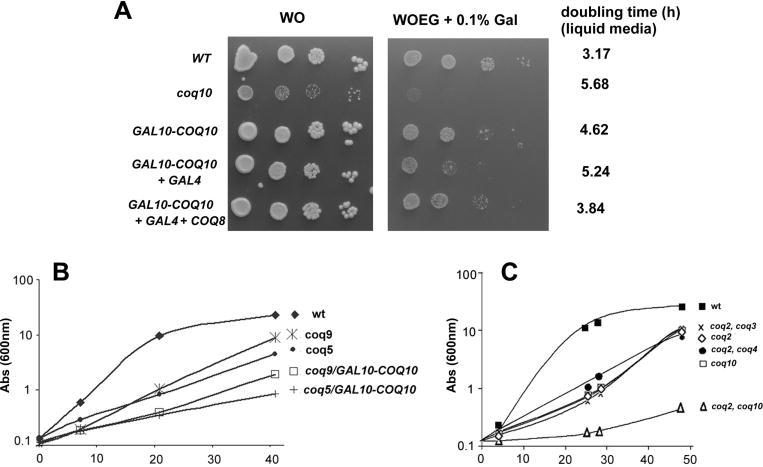
The deleterious effect of high levels of Coq10p on respiration was confirmed by the growth phenotypes of the different strains harboring COQ10 under the control of strong promoters. Supplementation of the minimal medium WOEG with 0.1% galactose inhibited growth that correlated with the increase in the mitochondrial concentration of Coq10p (Fig. 1A and Fig. 2). The most severe impairment of growth on non-fermentable substrates was seen in the wild type co-transformed with the GAL10-COQ10 and GAL4, which correlated with the Coq10p over-production. Growth of this strain was comparable to the coq10 null mutant (Fig. 3A). COQ8 over-expression improved growth of the wild type with the GAL10-COQ10 fusion alone or together with GAL4. Transformants with the GPD1-COQ10 and TEF1-COQ10 fusions (not shown) grew as well as the parental wild type on minimal glucose and WOEG supplemented with 0.1% galactose. The growth properties are completely consistent with the results obtained with the measurements of NADH-oxidase and NADH-cytochrome c reductase. Moreover, we also check the effect of COQ10 over expression on different coq mutants. Q6 supplementation partially rescues respiratory growth, in liquid media, of all coenzyme Q mutants [10, 16, 17, 21]. To test the effect of the GAL10-COQ10 fusion on the Q6–dependent rescue of respiration, coq5 or coq9 mutants were transformed with the plasmid containing the GAL10-COQ10 fusion. Following growth in rich galactose, growth of the transformants were measured in glycerol/ethanol medium supplemented with 5 μM Q6. Rescue by Q6 was diminished in both mutants harboring the plasmid with the GAL10-COQ10 fusion confirming the respiratory toxicity of Coq10p excess. (Fig. 3B). On the other hand total depletion of Coq10p exacerbates the mutant phenotype of coenzyme Q biosynthesis mutants Single and double coq2 mutants in which the second mutation was also in a gene involved in Q6 biosynthesis (coq3 or coq4) had similar generation time in rich glycerol/ethanol medium supplemented with 15 μM Q6 (Fig. 3C). In contrast, the generation time of a coq2/coq10 double mutant in such media was 2 times longer.
Fig. 3.
Growth of strains over-producing Coq10p and Coq8p on ethanol/glycerol with limiting amounts of galactose. W303-1A (WT), the coq10 null mutant (Δcoq10), and W303-1A expressing Coq10p from GAL10-COQ10 alone or GAL10-COQ10 plus GAL4 without and with COQ8 over expression achieved with pTEF1-COQ8 construct (+ COQ8) were diluted serially and spotted on minimal glucose (WO) and glycerol/ethanol (WOEG) media containing 0.1% of galactose. The plates were incubated for 2 days at 30°. Alternatively growth in liquid WOEG plus 0.1% galactose media was monitored and the doubling time of each strain calculated as indicated at the right of the panel. B) Over expression of COQ10 impair the respiratory growth of coq mutants supplemented with Q6. Growth curve on YEPG (rich glycerol plus ethanol) supplemented with 5μM Q6 of the parental respiratory competent strain W303, the null mutants coq5 and coq9, and the two mutants transformed with the GAL10-COQ10 construct (coq5/GAL10-COQ10 and coq9/GAL10-COQ10). C) Rescue of single and double mutants by exogenous CoQ6. The respiratory competent parental strain W303-1A (WT), the Δcoq2 and Δcoq10 null mutants and the double null mutants Δcoq2 Δcoq3, Δcoq2 Δcoq4 and Δcoq2 Δcoq10 were grown in liquid YPEG supplemented with 15 μM of Q6. Growth was monitored by measuring absorbance at 600 nm during 50 hours. Samples were periodically checked for contaminants. The growth curves shown are representative of three independent experiments.
Coq8p over-production increases the steady state level of Coq3 and Coq4 proteins in coq selected mutants
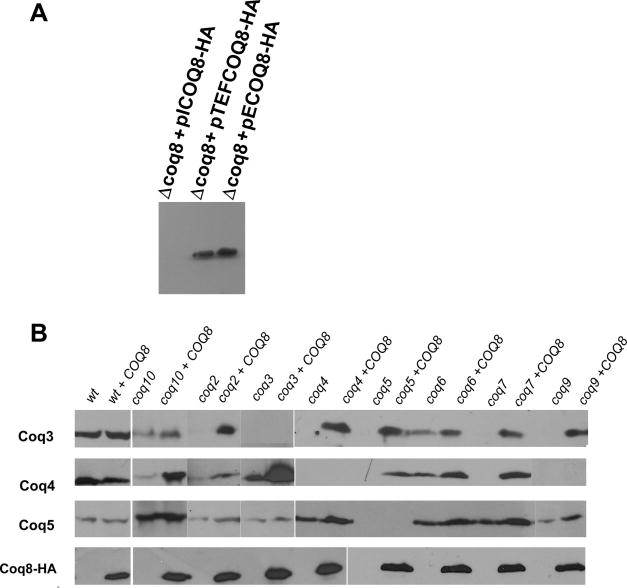
Coq3p-Coq7p and Coq9p are part of the Q synthesis complex [9, 19, 20]. Although coq8 mutants have the same phenotype as the other coq mutants, Coq8p is not associated with the Q synthesis complex. Coq8p has been implicated in activation of the O-methylase Coq3p [18]. When over-expressed, COQ8 suppresses not only coq10 [21] but also coq9 mutants by raising the mitochondrial concentration of Q6 [10]. To gain a better understanding of how Coq8p affects mitochondrial Q6 levels, we analyzed the effect of Coq8p over-expression on several subunits of the Q synthesis complex in wild type and in different coq mutants. The abundance of Coq8p was compared in a coq8 mutant in which the protein was expressed with a C-terminal HA tag. The respiratory defect of the coq8 mutant was complemented with all fusion constructs indicating that the tag did not affect the function of the protein (not shown). The results of Westerns reveal a strong signal in mitochondria isolated from strains harboring the multi-copy plasmid (pECOQ8-HA), or the TEF1-COQ8-HA fusion (pTEF1-COQ8-HA) (Fig. 4A). However we could not detect any signal for the single-copy transformant (pICOQ8-HA), perhaps because of its low expression.
Fig. 4.
Effect of Coq8p over-production in the steady state level of Coq3p, Coq4p Coq5p and Coq8p. A) Immunodetection of HA-tagged Coq8p from the modified gene on a high-copy plasmid (pECOQ8-HA) and the integrants: pICOQ8-HA and pTCOQ8-HA (TEF1-COQ8-HA fusion). B) Immunodetection of Coq3p, Coq4p and Coq5 in the W303-1A (WT) and in the indicated coq null mutants without and with the TEF1-COQ8-HA fusion. Mitochondrial proteins were separated by SDS-PAGE on 12% polyacrylamide gels and western blots were reacted with a rat monoclonal antibody against the HA tag and rabbit polyclonal antibodies against Coq3p, Coq4p and Coq5p. Antigens were visualized with the SuperSignal chemiluminescent substrate kit (Pierce Chemical Co.) after a secondary reaction with peroxidase-conjugated anti-rat and anti-rabbit IgG (Sigma).
In agreement with published data [15], coq mutants display severe reductions in Coq3p and Coq4p but not Coq5p (Fig. 4B). With the exception of the coq9 mutant, both proteins are restored to different degrees when the mutants are transformed with pTEF1-COQ8-HA indicating that Coq8p probably stabilizes these components of the Q-biosynthesis complex (Fig. 4B). Curiously in the coq9 mutant, extra COQ8 did not stabilize Coq4p. The increased stability of Coq3p and Coq4p helps to explain how COQ8 over-expression increases the mitochondrial concentration of Q6, which was previously invoked to be responsible for rescue of respiration in the coq10 mutant [21].
Discussion
The yeast COQ10 gene codes for a mitochondrial inner membrane protein that is essential for respiration. Unlike coq1–9 mutants that fail to synthesize Q6 [3–10], yeast coq10 mutants [21, 25] have normal amounts of Q6 but are defective in reducing cytochrome c. The respiratory block can be completely restored in isolated mitochondria by Q2, a more diffusible substrate of the bc1 complex than Q6 [16, 18].
Coq10p is a homolog of Caulobacter crescentus reading frame CC1736 [23]. This bacterial protein is a member of the START superfamily implicated in the delivery of polycyclic compounds [26], which are thought to bind to a hydrophobic tunnel that is a characteristic structural feature of this protein family. This tunnel also appears to be essential for Coq10p function [25]. Coq10p was proposed to be a coenzyme Q binding protein based on the presence of Q6 in a preparation purified from an over-expressing strain of yeast. In view of the very low amount of bound Q6 (0.035 moles/mol protein) there was the question of whether Coq10p binds Q6 under in vivo conditions.
In the present study this question was examined by measuring respiration in cells expressing different levels of Coq10p. Coq10p overexpression was previously observed to inhibit the growth of S. pombe [24]. Fusion of COQ10 to strong yeast promoters such as GAL10 raised the mitochondrial concentration of Coq10p by more than 300 fold. The over-expressing cells show a mild growth defect on minimal ethanol/glycerol media containing a low concentration of galactose for induction of the GAL10 promoter. Although the cells grew with a longer generation time, the full effect on growth was mitigated by the only partial activation of the GAL10-COQ10 fusion gene by the two main sources of carbon (glycerol and ethanol) in the growth medium. More direct evidence of a respiratory defect was obtained by enzymatic assays of NADH-oxidase, and NADH-cytochrome c reductase in isolated mitochondria of the over-producing cells grown on galactose. The deleterious effect of excess Coq10p on respiration is specific and related to a lower effective concentration of Q6 available for electron transport. This was evident from the ability of mitochondria obtained from the cells harboring the GAL10-COQ10 fusion gene to oxidize NADH when Q2 was added to the assays. Similarly, significant rescue of the respiratory defect was attained by over-expression of COQ8, which was previously shown to double the mitochondrial concentration of Q6. These results substantiate in vivo binding of the large mitochondrial pool of Q6 by Coq10p.
Coq8p has been proposed to be a protein kinase that targets the Coq3p O-methylase [18] and functions in some aspect of the organization of this and other components of the Q-biosynthetic complex [18–20]. Mutations in COQ genes lead to instability of most components the complex [15]. This raised the possibility that Coq8p over-expression may reduce turnover of some components of the complex and in this manner enhance synthesis of Q6. The mechanism by which Coq8p suppress the growth and respiratory defect of the coq10 null mutant as well the toxicity of Coq10p over-production, was studied by comparing the steady- state levels of Coq3p, Coq4p and Coq5p in wild type, in several coq mutant and in the same strains transformed with pTEF1-COQ8-HA fusion plasmid. These immunochemical analyses disclosed a marked difference in the concentrations of Coq3p and Coq4p in coq mutants transformed with COQ8-HA. Coq5p, which been previously shown to be stable in all the coq mutants, was also not affected in the strains used here.
HHB, an early intermediate in Q6 synthesis, accumulates in coq3-coq9 independent of where the mutational block is in the pathway [16]. This has been explained by the already mentioned high turnover of the Q-biosynthetic complex when any one of its components is mutated [15]. This circumstance has made it difficult to place some of the COQ gene products in the Q6 biosynthesis pathway. The ability of extra Coq8p to stabilize components such as Coq3p and Coq4p may offer a way out of this impasse by increasing the steady-state concentrations of precursors in mutants such as coq4 and coq9 for which a specific role on Q synthesis is still lacking.
Human patients containing mutations in genes involved in the coenzyme Q synthesis have been described in the last few years and the present study points out that an over abundance of Coq10p can be another cause of such disorders.
Highlights.
COQ10 deletion elicits a defect in mitochondrial respiration correctable by addition of coenzyme Q2, a synthetic diffusible ubiquinone.
The significance that purified Coq10p contains bound Q6 was examined by
testing over-expression of Coq10p on respiration.
Inhibition of CoQ function due to Coq10p excess strength our hypothesis of Coq10p function in CoQ delivery.
Respiratory deficiency caused by more Coq10p was specific and restored by Q2
in mitochondria or by Coq8p in cells.
Coq8p over production on other coq mutants revealed a surprisingly higher stability of other Coq proteins.
Acknowledgments
We thank Dr. Catherine F. Clarke, University of California for providing yeast strains and antibodies. This work was supported by grants and fellowships from Fundação de Amparo a Pesquisa de São Paulo (FAPESP – 2007/01092-5; 2006/03713-4), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 470058/2007-2) and Research Grant HL022174 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hatefi Y. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- 2.Gloor U, Wiss O. Experientia. 1958;14:410–411. doi: 10.1007/BF02160434. [DOI] [PubMed] [Google Scholar]
- 3.Tzagoloff A, Dieckmann CL. Microbiol Rev. 1990;54:211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran UC, Clarke CF. Mitochondrion. 2007;7S:S62–S71. doi: 10.1016/j.mito.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gin P, Clarke CF. J Biol Chem. 2005;280:2676–2681. doi: 10.1074/jbc.M411527200. [DOI] [PubMed] [Google Scholar]
- 6.Ashby MN, Kutsunai SY, Ackerman SH, Tzagoloff A, Edwards PA. J Biol Chem. 1992;267:4128–4136. [PubMed] [Google Scholar]
- 7.Do TQ, Schultz JR, Clarke CF. Proc Natl Acad Sci. 1996;93:7534–7539. doi: 10.1073/pnas.93.15.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu AY, Do TQ, Lee PT, Clarke CF. Biochim Biophys Acta. Vol. 1484. 2000. pp. 287–297. [DOI] [PubMed] [Google Scholar]
- 9.Barkovich RJ, Shtanko A, Shepherd JA, Lee PT, Myles DC, Tzagoloff A, Clarke CF. J Biol Chem. 1997;272:9182–9188. doi: 10.1074/jbc.272.14.9182. [DOI] [PubMed] [Google Scholar]
- 10.Johnson A, Gin P, Marbois BN, Hsieh EJ, Wu M, Barros MH, Clarke CF, Tzagoloff A. J Biol Chem. 2005;280:31397–31404. doi: 10.1074/jbc.M503277200. [DOI] [PubMed] [Google Scholar]
- 11.Pierrel F, Hamelin O, Douki T, Kieffer-Jaquinod S, Mühlenhoff U, Ozeir M, Lill R, Fontecave M. Chem Biol. 2010;17:449–459. doi: 10.1016/j.chembiol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Marbois B, Xie LX, Choi S, Hirano K, Hyman K, Clarke CF. J Biol Chem. 2010;285:27827–27838. doi: 10.1074/jbc.M110.151894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barros MH, Nobrega FG. Gene. 1999;233:197–203. doi: 10.1016/s0378-1119(99)00137-7. [DOI] [PubMed] [Google Scholar]
- 14.Manzella L, Barros MH, Nobrega FG. Yeast. 1998;14:839–846. doi: 10.1002/(SICI)1097-0061(19980630)14:9<839::AID-YEA283>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh EJ, Gin P, Gulmezian M, Tran UC, Saiki R, Marbois BN, Clarke CF. Arch Biochem Biophys. 2007;463:19–26. doi: 10.1016/j.abb.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poon WW, Marbois BN, Faull KF, Clarke CF. Arch Biochem Biophys. 1995;320:305–314. doi: 10.1016/0003-9861(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 17.Poon WW, Do TQ, Marbois BN, Clarke CF. Molec Aspects Med. 1997;18:121–127. doi: 10.1016/s0098-2997(97)00004-6. [DOI] [PubMed] [Google Scholar]
- 18.Tauche A, Krause-Buchholz U, Rödel G. FEMS Yeast Res. 2008;8:1263–1275. doi: 10.1111/j.1567-1364.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh EJ, Gin P, Gulmezian M, Tran UC, Saiki R, Marbois BN, Clarke CF. Arch Biochem Biophys. 2007;463:19–26. doi: 10.1016/j.abb.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagier-Tourenne C, Tazir M, López LC, Quinzii CM, Assoum M, Drouot N, Busso C, Makri S, Ali-Pacha L, Benhassine T, Anheim M, Lynch DR, Thibault C, Plewniak F, Bianchetti L, Tranchant C, Poch O, DiMauro S, Mandel JL, Barros MH, Hirano M, Koenig M. Am J Hum Genet. 2008;82:661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barros MH, Johnson A, Gin P, Marbois BN, Clarke CF, Tzagoloff A. J Biol Chem. 2005;280:42627–42635. doi: 10.1074/jbc.M510768200. [DOI] [PubMed] [Google Scholar]
- 22.Busso C, Tahara EB, Ogusucu R, Augusto O, Ferreira-Junior JR, Tzagoloff A, Kowaltowski AJ, Barros MH. Saccharomyces cerevisiae coq10 null mutants are responsive to antimycin A. FEBS J. 2010 doi: 10.1111/j.1742-4658.2010.07862.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Y, Goldsmith-Fischman S, Atreya HS, Acton T, Ma L, Xiao R, Honig B, Mantelione GT, Aravind L. Proteins. 2005;58:747–750. doi: 10.1002/prot.20365. [DOI] [PubMed] [Google Scholar]
- 24.Cui TZ, Kawamukai M. FEBS J. 2009;276:748–759. doi: 10.1111/j.1742-4658.2008.06821.x. [DOI] [PubMed] [Google Scholar]
- 25.Busso C, Bleicher L, Ferreira-Junior JR, Barros MH. FEBS Lett. 2010;584:1609–1614. doi: 10.1016/j.febslet.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Soccio RE, Adams RM, Romanowski MJ, Sehayek E, Burley SK, Breslow JL. Proc Natl Acad Sci U S A. 2002;99:6943–6948. doi: 10.1073/pnas.052143799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobrega FG, Nobrega MP, Tzagoloff A. EMBO J. 1992;11:3821–3829. doi: 10.1002/j.1460-2075.1992.tb05474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mumberg D, Müller R, Funk M. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 29.Hill JE, Myers AM, Koerner TJ, Tzagoloff A. Yeast. 1986;2:163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- 30.Rothstein RJ. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Ptashne M. Cell. 1987;48:847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- 32.Tzagoloff A, Akai A, Needleman RB, Zulch G. J Biol Chem. 1975;250:8236–8242. [PubMed] [Google Scholar]
- 33.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]