Abstract
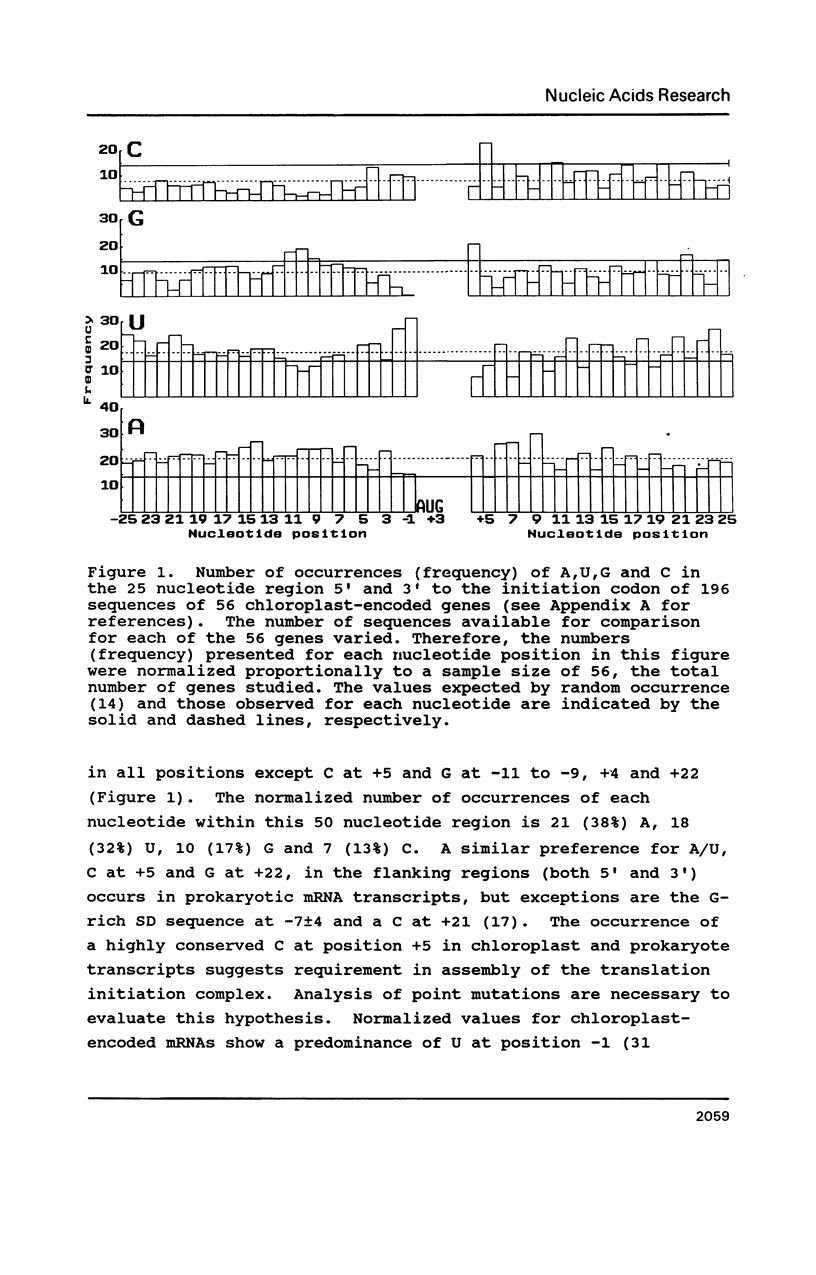
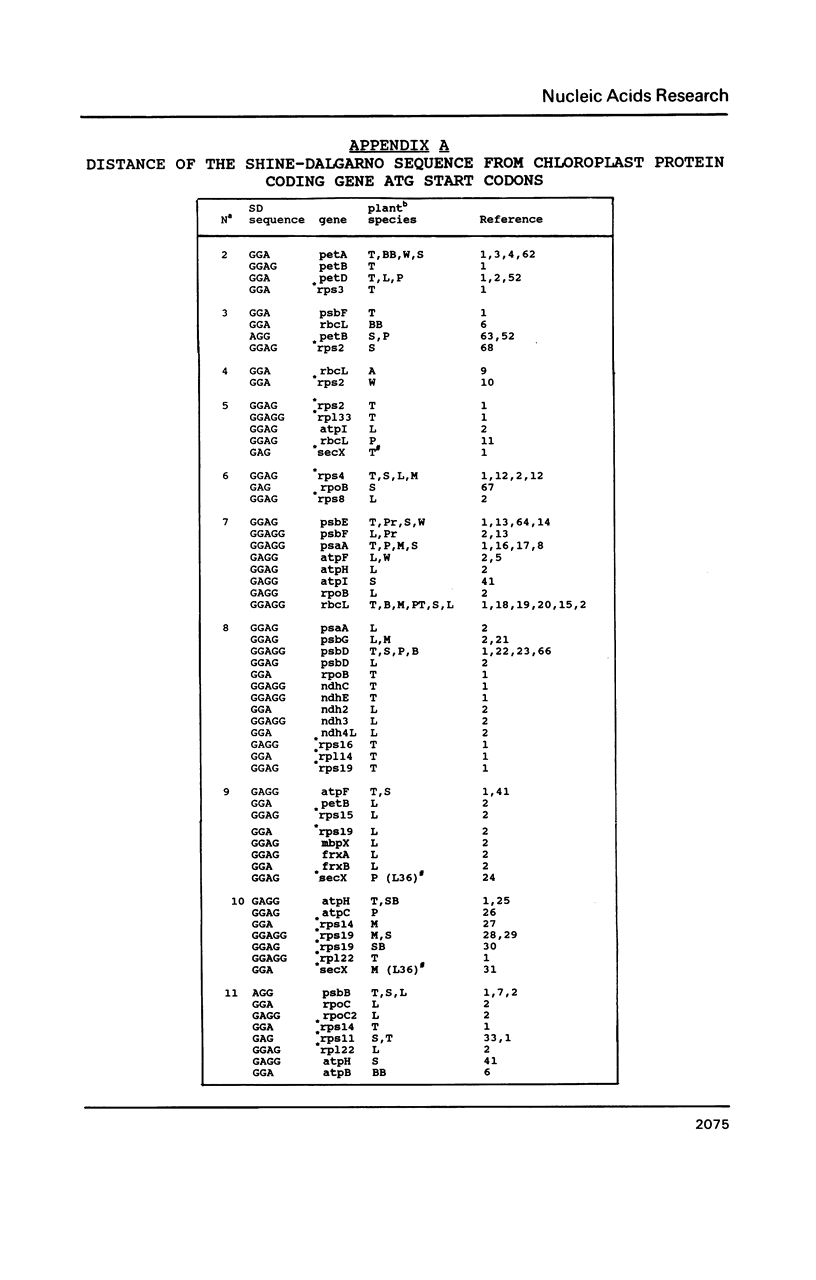
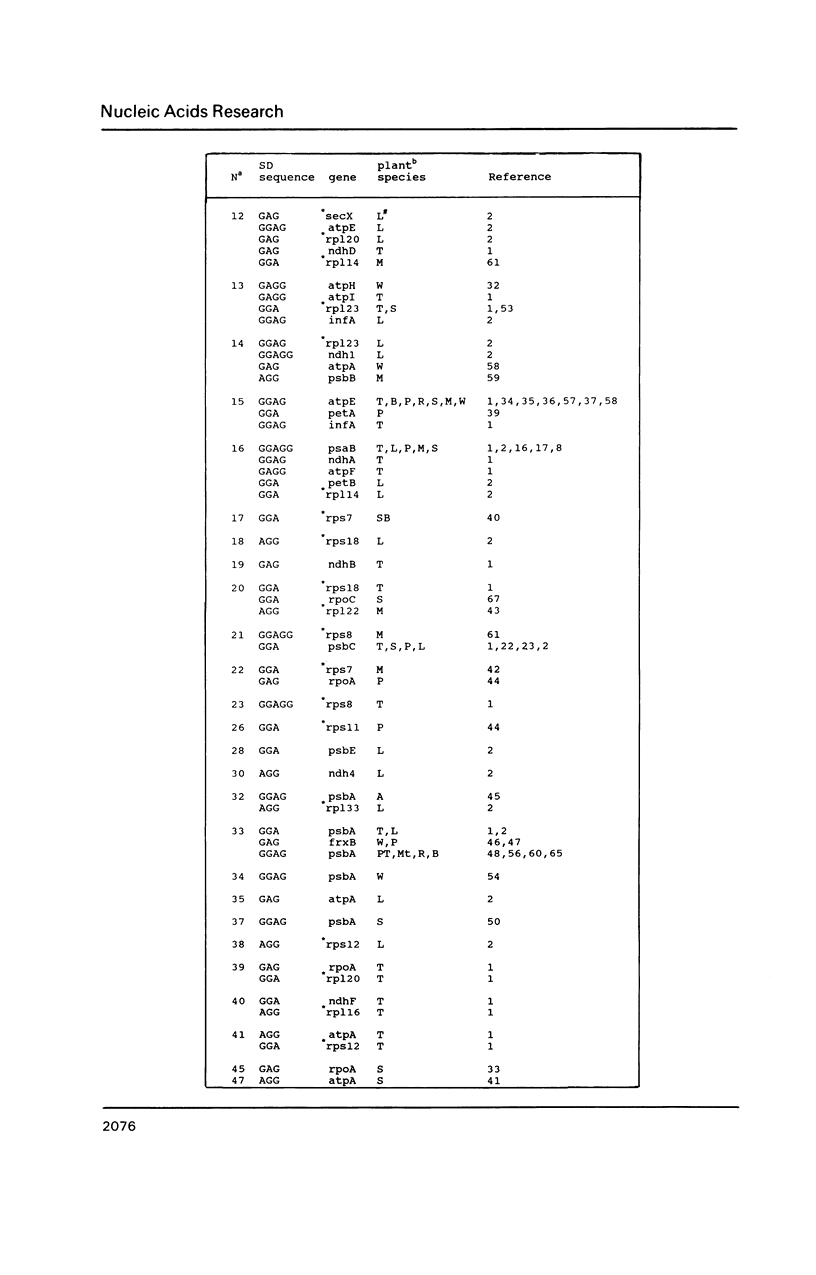
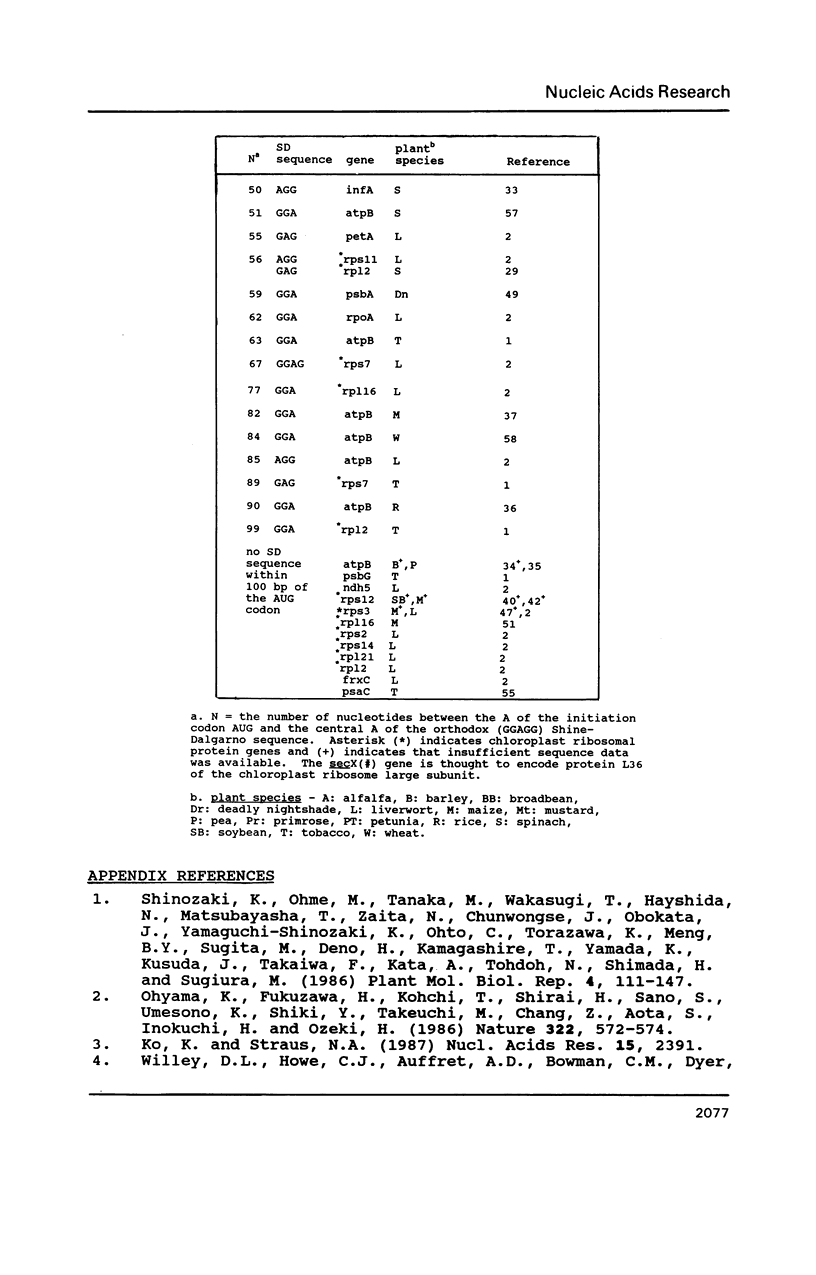
A survey of 196 protein-coding chloroplast DNA sequences demonstrated the preference for AUG and UAA codons for initiation and termination of translation, respectively. As in prokaryotes at every nucleotide position from -25 to +25 (AUG is +1 to +3) and for 25 nucleotides 5' and 3' to the termination codon an A or U is predominant, except for C at +5 and G at +22. A Shine-Dalgarno (SD) sequence (GGAGG or tri- or tetranucleotide variant) was found within 100 bp 5' to the AUG codon in 92% of the genes. In 40% of these cases, the location of the SD sequence was similar to that of the consensus for prokaryotes (-12 to -7 5' to AUG), presumed to be optimal for translation initiation. A SD sequence could not be located in 6% of the chloroplast sequences. We propose that mRNA secondary structures may be required for the relocation of a distal SD sequences to within the optimal region (-12 to -7) for initiation of translation. We further suggest that termination at UGA codons in chloroplast genes may occur by a mechanism, involving 16S rRNA secondary structure, which has been proposed for UGA termination in E. coli.
Full text
PDF







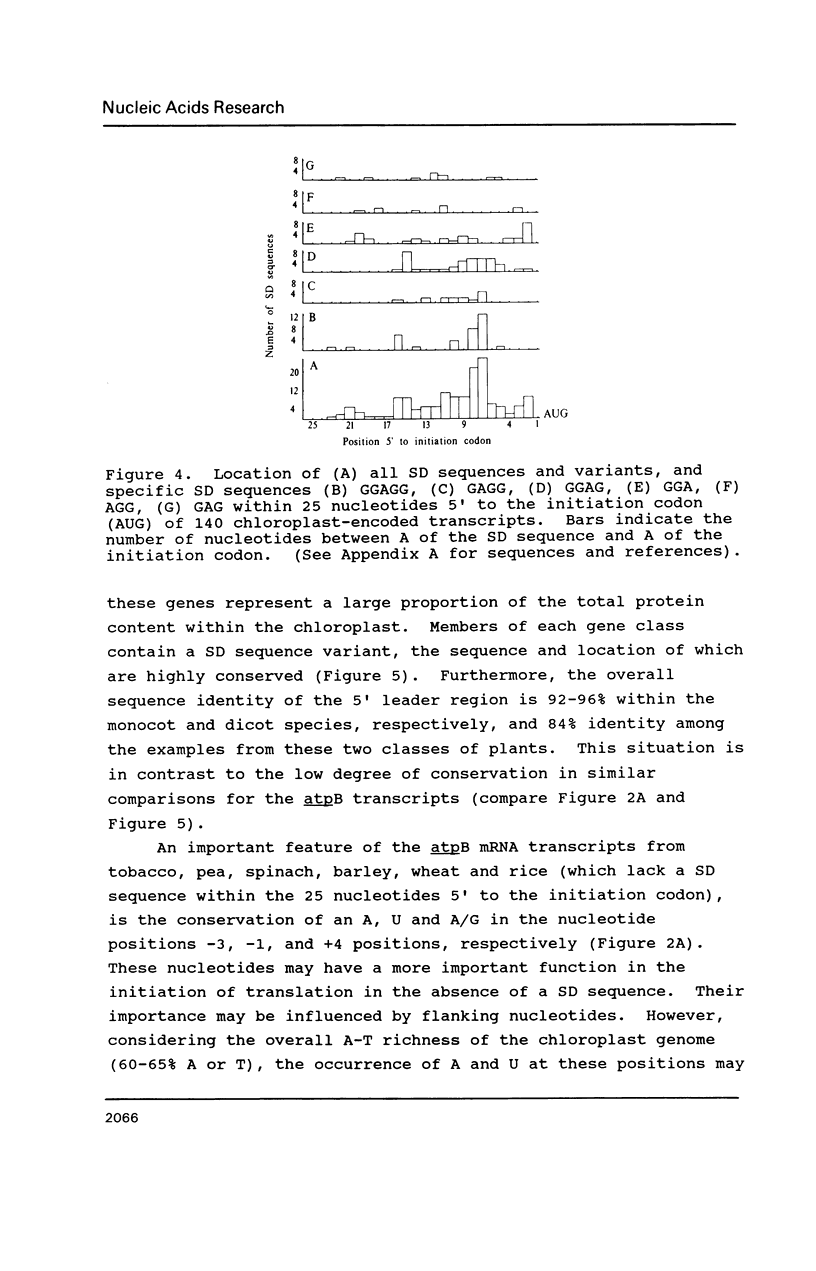


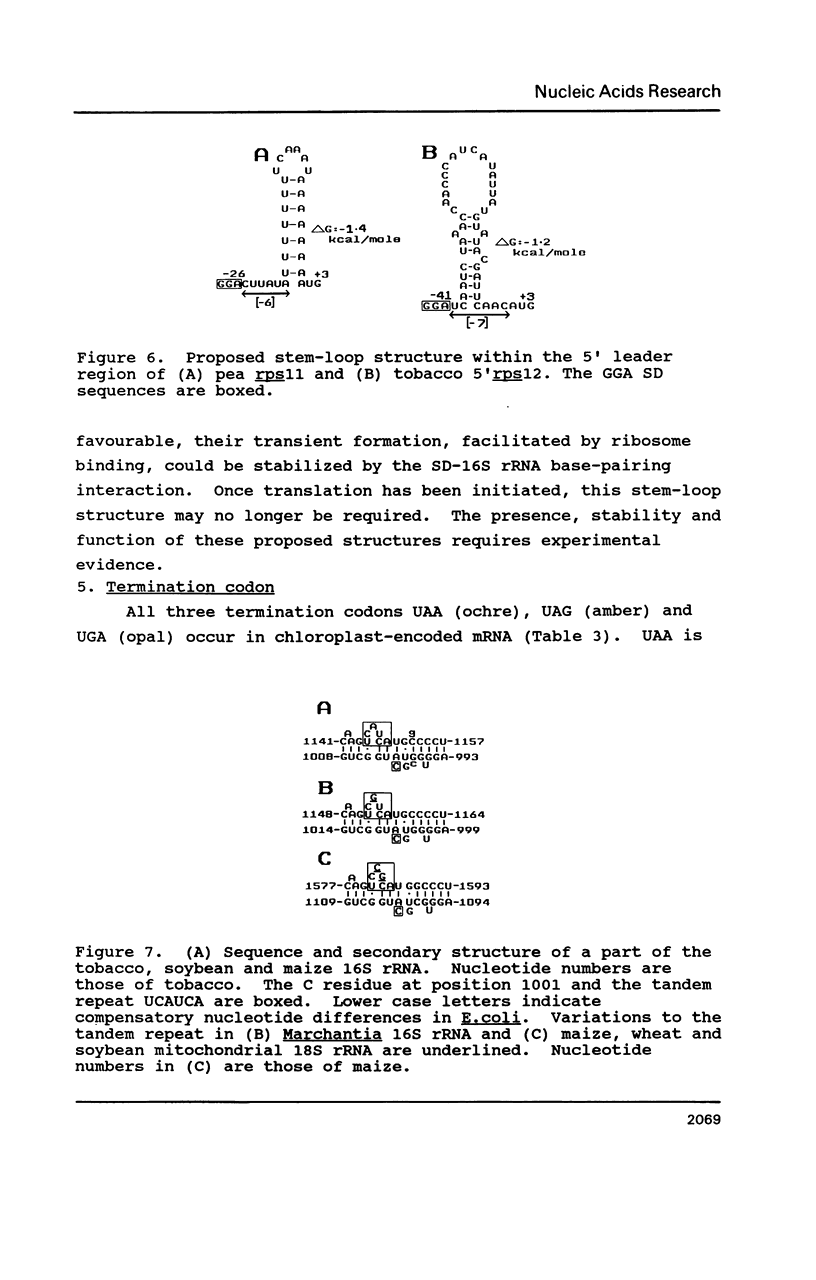
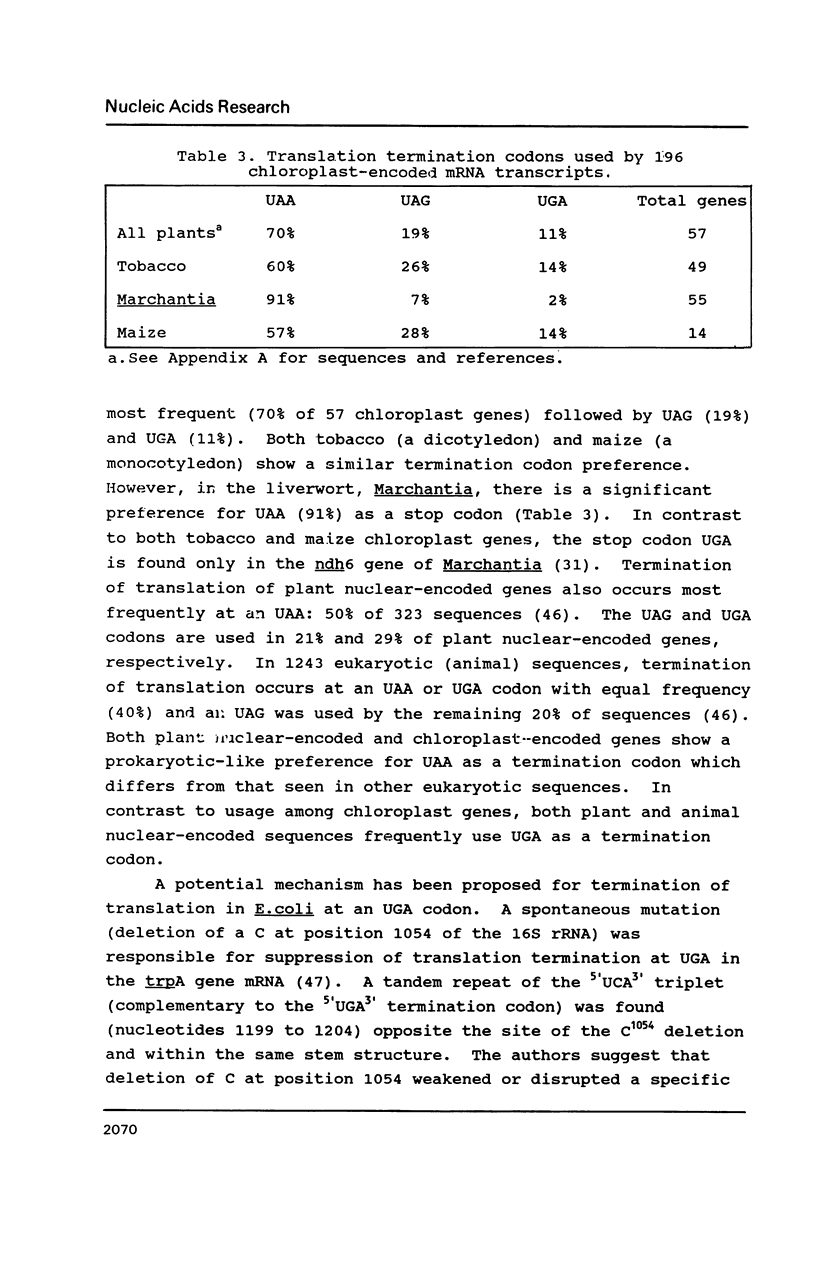







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich J., Cherney B., Merlin E., Christopherson L. A., Williams C. Sequence of the chloroplast-encoded psbA gene for the QB polypeptide of petunia. Nucleic Acids Res. 1986 Dec 9;14(23):9536–9536. doi: 10.1093/nar/14.23.9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich J., Cherney B., Merlin E., Palmer J. Sequence of the rbcL gene for the large subunit of ribulose bisphosphate carboxylase-oxygenase from alfalfa. Nucleic Acids Res. 1986 Dec 9;14(23):9535–9535. doi: 10.1093/nar/14.23.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich J., Cherney B., Merlin E., Palmer J. Sequence of the rbcL gene for the large subunit of ribulose bisphosphate carboxylase-oxygenase from petunia. Nucleic Acids Res. 1986 Dec 9;14(23):9534–9534. doi: 10.1093/nar/14.23.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich J., Cherney B., Merlin E. Sequence of the chloroplast-encoded psbA gene for the QB polypeptide of alfalfa. Nucleic Acids Res. 1986 Dec 9;14(23):9537–9537. doi: 10.1093/nar/14.23.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aota S., Gojobori T., Ishibashi F., Maruyama T., Ikemura T. Codon usage tabulated from the GenBank Genetic Sequence Data. Nucleic Acids Res. 1988;16 (Suppl):r315–r402. doi: 10.1093/nar/16.suppl.r315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird C. R., Koller B., Auffret A. D., Huttly A. K., Howe C. J., Dyer T. A., Gray J. C. The wheat chloroplast gene for CF(0) subunit I of ATP synthase contains a large intron. EMBO J. 1985 Jun;4(6):1381–1388. doi: 10.1002/j.1460-2075.1985.tb03790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Berlani R., Coruzzi G., Li M., Macino G., Nobrega F. G., Nobrega M. P., Thalenfeld B. E., Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudraa M., Perrin P. CpG and TpA frequencies in the plant system. Nucleic Acids Res. 1987 Jul 24;15(14):5729–5737. doi: 10.1093/nar/15.14.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. J., Levings C. S. Nucleotide Sequence of the F(1)-ATPase alpha Subunit Gene from Maize Mitochondria. Plant Physiol. 1985 Oct;79(2):571–577. doi: 10.1104/pp.79.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo N., Seyer P., Tyagi A., Herrmann R. G. Cytochrome b-559 genes from Oenothera hookeri and Nicotiana tabacum show a remarkably high degree of conservation as compared to spinach. The enigma of cytochrome b-559: highly conserved genes and proteins but no known function. Curr Genet. 1986;10(8):619–624. doi: 10.1007/BF00418129. [DOI] [PubMed] [Google Scholar]
- Chao S., Sederoff R., Levings C. S., 3rd Nucleotide sequence and evolution of the 18S ribosomal RNA gene in maize mitochondria. Nucleic Acids Res. 1984 Aug 24;12(16):6629–6644. doi: 10.1093/nar/12.16.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E., Phillips A. L., Huttly A. K., Gray J. C. A sixth subunit of ATP synthase, an F(0) component, is encoded in the pea chloroplast genome. EMBO J. 1986 Feb;5(2):217–222. doi: 10.1002/j.1460-2075.1986.tb04201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988 Jan;7(1):245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Buzash-Pollert E., Studier F. W. Mutations of bacteriophage T7 that affect initiation of synthesis of the gene 0.3 protein. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2741–2745. doi: 10.1073/pnas.75.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn P. P., Gray J. C. Nucleotide sequence of the frxB gene in wheat chloroplast DNA. Nucleic Acids Res. 1988 Jan 11;16(1):348–348. doi: 10.1093/nar/16.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt H., Lührmann R. Recognition by initiator transfer ribonucleic acid of a uridine 5' adjacent to the AUG codon: different conformational states of formylatable methionine-accepting transfer ribonucleic acid at the ribosomal peptidyl site. Biochemistry. 1981 Apr 14;20(8):2075–2080. doi: 10.1021/bi00511a002. [DOI] [PubMed] [Google Scholar]
- Efimov V. A., Andreeva A. V., Reverdatto S. V., Chakhmakhcheva O. G. Nucleotide sequence of the barley chloroplast psbD gene for the D2 protein of photosystem II. Nucleic Acids Res. 1988 Jun 24;16(12):5686–5686. doi: 10.1093/nar/16.12.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov V. A., Andreeva A. V., Reverdatto S. V., Jung R., Chakhmakhcheva O. G. Nucleotide sequence of the barley chloroplast psbA gene for the QB protein of photosystem II. Nucleic Acids Res. 1988 Jun 24;16(12):5685–5685. doi: 10.1093/nar/16.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L. E., Kück U., Bogorad L. Two partially homologous adjacent light-inducible maize chloroplast genes encoding polypeptides of the P700 chlorophyll a-protein complex of photosystem I. J Biol Chem. 1985 Feb 10;260(3):1413–1421. [PubMed] [Google Scholar]
- Ganoza M. C., Kofoid E. C., Marlière P., Louis B. G. Potential secondary structure at translation-initiation sites. Nucleic Acids Res. 1987 Jan 12;15(1):345–360. doi: 10.1093/nar/15.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoza M. C., Sullivan P., Cunningham C., Hader P., Kofoid E. C., Neilson T. Effect of bases contiguous to AUG on translation initiation. J Biol Chem. 1982 Jul 25;257(14):8228–8232. [PubMed] [Google Scholar]
- Giese K., Subramanian A. R., Larrinua I. M., Bogorad L. Nucleotide sequence, promoter analysis, and linkage mapping of the unusually organized operon encoding ribosomal proteins S7 and S12 in maize chloroplast. J Biol Chem. 1987 Nov 5;262(31):15251–15255. [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Edelman M., Hallick R. B. Chloroplast-coded atrazine resistance in Solanum nigrum: psbA loci from susceptible and resistant biotypes are isogenic except for a single codon change. Nucleic Acids Res. 1984 Dec 21;12(24):9489–9496. doi: 10.1093/nar/12.24.9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. Robert bews kerr. Can Med Assoc J. 1984 Jan 15;130(2):194–194. [PMC free article] [PubMed] [Google Scholar]
- Gruissem W., Zurawski G. Analysis of promoter regions for the spinach chloroplast rbcL, atpB and psbA genes. EMBO J. 1985 Dec 16;4(13A):3375–3383. doi: 10.1002/j.1460-2075.1985.tb04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Hayashida N., Matsubayashi T., Shinozaki K., Sugiura M., Inoue K., Hiyama T. The gene for the 9 kd polypeptide, a possible apoprotein for the iron-sulfur centers A and B of the photosystem I complex, in tobacco chloroplast DNA. Curr Genet. 1987;12(4):247–250. doi: 10.1007/BF00435285. [DOI] [PubMed] [Google Scholar]
- Hildebrand M., Hallick R. B., Passavant C. W., Bourque D. P. Trans-splicing in chloroplasts: the rps 12 loci of Nicotiana tabacum. Proc Natl Acad Sci U S A. 1988 Jan;85(2):372–376. doi: 10.1073/pnas.85.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Structure of the spinach chloroplast genes for the D2 and 44 kd reaction-centre proteins of photosystem II and for tRNASer (UGA). Nucleic Acids Res. 1984 Dec 11;12(23):8819–8834. doi: 10.1093/nar/12.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C. J., Auffret A. D., Doherty A., Bowman C. M., Dyer T. A., Gray J. C. Location and nucleotide sequence of the gene for the proton-translocating subunit of wheat chloroplast ATP synthase. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6903–6907. doi: 10.1073/pnas.79.22.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G. S., Holton T. A., Whitfield P. R., Bottomley W. Spinach chloroplast rpoBC genes encode three subunits of the chloroplast RNA polymerase. J Mol Biol. 1988 Apr 20;200(4):639–654. doi: 10.1016/0022-2836(88)90477-9. [DOI] [PubMed] [Google Scholar]
- Hudson G. S., Mason J. G., Holton T. A., Koller B., Cox G. B., Whitfeld P. R., Bottomley W. A gene cluster in the spinach and pea chloroplast genomes encoding one CF1 and three CF0 subunits of the H+-ATP synthase complex and the ribosomal protein S2. J Mol Biol. 1987 Jul 20;196(2):283–298. doi: 10.1016/0022-2836(87)90690-5. [DOI] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K., Straus N. A. Sequence of the apocytochrome f gene encoded by the Vicia faba chloroplast genome. Nucleic Acids Res. 1987 Mar 11;15(5):2391–2391. doi: 10.1093/nar/15.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B., Fromm H., Galun E., Edelman M. Evidence for in vivo trans splicing of pre-mRNAs in tobacco chloroplasts. Cell. 1987 Jan 16;48(1):111–119. doi: 10.1016/0092-8674(87)90361-8. [DOI] [PubMed] [Google Scholar]
- Krebbers E. T., Larrinua I. M., McIntosh L., Bogorad L. The maize chloroplast genes for the beta and epsilon subunits of the photosynthetic coupling factor CF1 are fused. Nucleic Acids Res. 1982 Aug 25;10(16):4985–5002. doi: 10.1093/nar/10.16.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G. DNA sequence requirements for the accurate transcription of a protein-coding plastid gene in a plastid in vitro system from mustard (Sinapis alba L.). EMBO J. 1984 Aug;3(8):1697–1704. doi: 10.1002/j.1460-2075.1984.tb02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G., Langridge U. Structure of the chloroplast gene for the precursor of the Mr 32,000 photosystem II protein from mustard (Sinapis alba L.). Nucleic Acids Res. 1984 Jan 25;12(2):945–958. doi: 10.1093/nar/12.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Secondary structure of bacteriophage f2 ribonucleic acid and the initiation of in vitro protein biosynthesis. J Mol Biol. 1970 Jun 28;50(3):689–702. doi: 10.1016/0022-2836(70)90093-8. [DOI] [PubMed] [Google Scholar]
- Markmann-Mulisch U., Subramanian A. R. Nucleotide sequence and linkage map position of the genes for ribosomal proteins L14 and S8 in the maize chloroplast genome. Eur J Biochem. 1988 Jan 4;170(3):507–514. doi: 10.1111/j.1432-1033.1988.tb13728.x. [DOI] [PubMed] [Google Scholar]
- Markmann-Mulisch U., von Knoblauch K., Lehmann A., Subramanian A. R. Nucleotide sequence and linkage map position of the secX gene in maize chloroplast and evidence that it encodes a protein belonging to the 50S ribosomal subunit. Biochem Int. 1987 Nov;15(5):1057–1067. [PubMed] [Google Scholar]
- Maruyama T., Gojobori T., Aota S., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1986;14 (Suppl):r151–r197. doi: 10.1093/nar/14.suppl.r151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin W. E., Larrinua I. M. The sequence of the first exon and part of the intron of the maize plastid encoded rpl 16 locus. Nucleic Acids Res. 1987 Jul 24;15(14):5896–5896. doi: 10.1093/nar/15.14.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin W. E., Larrinua I. M. The sequence of the maize plastid encoded rpl 22 locus. Nucleic Acids Res. 1987 May 26;15(10):4356–4356. doi: 10.1093/nar/15.10.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin W. E., Larrinua I. M. The sequence of the maize plastid encoded rps3 locus. Nucleic Acids Res. 1987 Jun 11;15(11):4689–4689. doi: 10.1093/nar/15.11.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin W. E., Larrinua I. M. The sequence of the maize rps19 locus and of the inverted repeat/unique region junctions. Nucleic Acids Res. 1987 May 11;15(9):3932–3932. doi: 10.1093/nar/15.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin W. E., Larrinua I. M. The sequence of the maize rps19 locus and of the inverted repeat/unique region junctions. Nucleic Acids Res. 1987 May 11;15(9):3932–3932. doi: 10.1093/nar/15.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters D. S., Christensen A., Young E. T., Stormo G., Gold L. Translational regulation of expression of the bacteriophage T4 lysozyme gene. Nucleic Acids Res. 1986 Jul 25;14(14):5813–5826. doi: 10.1093/nar/14.14.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon E., Kao T. H., Wu R. Sequence of the chloroplast-encoded atpB-atpE-trnM gene clusters from rice. Nucleic Acids Res. 1987 May 26;15(10):4358–4359. doi: 10.1093/nar/15.10.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J., Herrmann R. G. Nucleotide sequence of the gene for the P680 chlorophyll alpha apoprotein of the photosystem II reaction center from spinach. Nucleic Acids Res. 1984 Mar 26;12(6):2837–2850. doi: 10.1093/nar/12.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgola E. J., Hijazi K. A., Göringer H. U., Dahlberg A. E. Mutant 16S ribosomal RNA: a codon-specific translational suppressor. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4162–4165. doi: 10.1073/pnas.85.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V. Operators and promoters in the OR region of phage 434. Nucleic Acids Res. 1979 Apr;6(4):1495–1508. doi: 10.1093/nar/6.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Purton S., Gray J. C. Nucleotide sequence of the gene for ribosomal protein L36 in pea chloroplast DNA. Nucleic Acids Res. 1987 Nov 11;15(21):9080–9080. doi: 10.1093/nar/15.21.9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton S., Gray J. C. Nucleotide sequence of the gene for ribosomal protein S11 in pea chloroplast DNA. Nucleic Acids Res. 1987 Feb 25;15(4):1873–1873. doi: 10.1093/nar/15.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton S., Gray J. C. Nucleotide sequence of the gene for ribosomal protein S11 in pea chloroplast DNA. Nucleic Acids Res. 1987 Feb 25;15(4):1873–1873. doi: 10.1093/nar/15.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdot C., Fayat G., Dessen P., Springer M., Plumbridge J. A., Grunberg-Manago M., Blanquet S. Sequence of a 1.26-kb DNA fragment containing the structural gene for E.coli initiation factor IF3: presence of an AUU initiator codon. EMBO J. 1982;1(3):311–315. doi: 10.1002/j.1460-2075.1982.tb01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M., Manderschied U., Kyriatsoulis A., Brinckmann U., Gassen H. G. Tetranucleotides as effectors for the binding of initiator tRNA to Escherichia coli ribosomes. Eur J Biochem. 1980 Aug;109(1):291–299. doi: 10.1111/j.1432-1033.1980.tb04794.x. [DOI] [PubMed] [Google Scholar]
- Schneider T. D., Stormo G. D., Gold L., Ehrenfeucht A. Information content of binding sites on nucleotide sequences. J Mol Biol. 1986 Apr 5;188(3):415–431. doi: 10.1016/0022-2836(86)90165-8. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijben-Müller G., Hallick R. B., Alt J., Westhoff P., Herrmann R. G. Spinach plastid genes coding for initiation factor IF-1, ribosomal protein S11 and RNA polymerase alpha-subunit. Nucleic Acids Res. 1986 Jan 24;14(2):1029–1044. doi: 10.1093/nar/14.2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Singer B. S., Gold L. A mutation that confers temperature sensitivity on the translation of rIIB in bacteriophage T4. J Mol Biol. 1976 May 25;103(3):627–646. doi: 10.1016/0022-2836(76)90221-7. [DOI] [PubMed] [Google Scholar]
- Smiley B. L., Lupski J. R., Svec P. S., McMacken R., Godson G. N. Sequences of the Escherichia coli dnaG primase gene and regulation of its expression. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4550–4554. doi: 10.1073/pnas.79.15.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. F., Schnare M. N., Gray M. W. Pronounced structural similarities between the small subunit ribosomal RNA genes of wheat mitochondria and Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):493–497. doi: 10.1073/pnas.81.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann A., Roux E., von Allmen J. M., Stutz E. The soybean chloroplast genome: complete sequence of the rps19 gene, including flanking parts containing exon 2 of rpl2 (upstream), but rpl22 (downstream). Nucleic Acids Res. 1988 Feb 11;16(3):1199–1199. doi: 10.1093/nar/16.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann A., Roux E., von Allmen J. M., Stutz E. The soybean chloroplast genome: complete sequence of the rps19 gene, including flanking parts containing exon 2 of rpl2 (upstream), but rpl22 (downstream). Nucleic Acids Res. 1988 Feb 11;16(3):1199–1199. doi: 10.1093/nar/16.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz A. A., Castroviejo M., Sayre R. T., Bogorad L. Protein PSII-G. An additional component of photosystem II identified through its plastid gene in maize. J Biol Chem. 1986 Feb 25;261(6):2485–2488. [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F., Massenet O., Dorne A. M., Briat J. F., Mache R. Expression of the rpl23, rpl2 and rps19 genes in spinach chloroplasts. Nucleic Acids Res. 1988 Mar 25;16(6):2461–2472. doi: 10.1093/nar/16.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff P., Herrmann R. G. Complex RNA maturation in chloroplasts. The psbB operon from spinach. Eur J Biochem. 1988 Feb 1;171(3):551–564. doi: 10.1111/j.1432-1033.1988.tb13824.x. [DOI] [PubMed] [Google Scholar]
- Willey D. L., Auffret A. D., Gray J. C. Structure and topology of cytochrome f in pea chloroplast membranes. Cell. 1984 Feb;36(2):555–562. doi: 10.1016/0092-8674(84)90248-4. [DOI] [PubMed] [Google Scholar]
- Wintz H., Skunca M., Pillay D. T. Nucleotide sequence and transcription analysis of the gene coding for subunit III of soybean chloroplast proton-translocating ATPase. Gene. 1987;59(1):47–53. doi: 10.1016/0378-1119(87)90265-4. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Junctions of the large single copy region and the inverted repeats in Spinacia oleracea and Nicotiana debneyi chloroplast DNA: sequence of the genes for tRNAHis and the ribosomal proteins S19 and L2. Nucleic Acids Res. 1984 Aug 24;12(16):6547–6558. doi: 10.1093/nar/12.16.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Junctions of the large single copy region and the inverted repeats in Spinacia oleracea and Nicotiana debneyi chloroplast DNA: sequence of the genes for tRNAHis and the ribosomal proteins S19 and L2. Nucleic Acids Res. 1984 Aug 24;12(16):6547–6558. doi: 10.1093/nar/12.16.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Sequence of the genes for the beta and epsilon subunits of ATP synthase from pea chloroplasts. Nucleic Acids Res. 1986 May 12;14(9):3974–3974. doi: 10.1093/nar/14.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Structures of the genes for the beta and epsilon subunits of spinach chloroplast ATPase indicate a dicistronic mRNA and an overlapping translation stop/start signal. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6260–6264. doi: 10.1073/pnas.79.20.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Clegg M. T., Brown A. H. The Nature of Nucleotide Sequence Divergence between Barley and Maize Chloroplast DNA. Genetics. 1984 Apr;106(4):735–749. doi: 10.1093/genetics/106.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Clegg M. T. The barley chloroplast DNA atpBE, trnM2, and trnV1 loci. Nucleic Acids Res. 1984 Mar 12;12(5):2549–2559. doi: 10.1093/nar/12.5.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Whitfeld P. R., Bottomley W. Sequence of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from pea chloroplasts. Nucleic Acids Res. 1986 May 12;14(9):3975–3975. doi: 10.1093/nar/14.9.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Allmen J. M., Stutz E. Complete sequence of 'divided' rps12 (r-protein S12) and rps7 (r-protein S7) gene in soybean chloroplast DNA. Nucleic Acids Res. 1987 Mar 11;15(5):2387–2387. doi: 10.1093/nar/15.5.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Allmen J. M., Stutz E. The soybean chloroplast genome: nucleotide sequence of a region containing tRNA-Val (GAC) and 16S rRNA gene. Nucleic Acids Res. 1988 Feb 11;16(3):1200–1200. doi: 10.1093/nar/16.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]



