Abstract
Oxytocin (Oxt) and the Oxt receptor (Oxtr) are implicated in the onset of maternal behavior in a variety of species. Recently, we developed two Oxtr knockout lines: a total body knockout (Oxtr−/−) and a conditional Oxtr knockout (OxtrFB/FB) in which the Oxtr is lacking only in regions of the forebrain, allowing knockout females to potentially nurse and care for their biological offspring. In the current study, we assessed maternal behavior of postpartum OxtrFB/FB females toward their own pups and maternal behavior of virgin Oxtr−/− females toward foster pups and compared knockouts of both lines to wildtype (Oxtr+/+) littermates. We found that both Oxtr−/− and OxtrFB/FB females appear to have largely normal maternal behaviors. However, with first litters, approximately 40% of the OxtrFB/FB knockout dams experienced high pup mortality, compared to fewer than 10% of the Oxtr+/+ dams. We then went on to test whether or not this phenotype occurred in subsequent litters or when the dams were exposed to an environmental disturbance. We found that regardless of the degree of external disturbance, OxtrFB/FB females lost more pups on their first and second litters compared to wildtype females. Possible reasons for higher pup mortality in OxtrFB/FB females are discussed.
Keywords: conditional knockout, stress, maternal behavior, aggression
Across species, females undergo fundamental changes in behavior during pregnancy and in the postpartum period. After parturition, females display a new repertoire of behaviors collectively referred to as maternal behavior. In rodents, maternal behavior consists primarily of four readily observed behaviors: nest building, nursing and/or crouching over pups, retrieving pups to the nest, and body/genital licking of pups (Lonstein & Fleming, 2001; Rosenblatt, 1975; Rosenblatt, Mayer, & Giordano, 1988). Like many other behaviors, hormones can facilitate maternal behavior and, in particular, oxytocin (Oxt) is convincingly implicated (Pedersen, Ascer, Monroe, & Prange, 1982; Pedersen & Boccia, 2003; Pedersen, Vadlamudi, Boccia, & Amico, 2006; Ross & Young, 2009).
Oxt is a nonapeptide hormone best known for its role in female reproduction, particularly parturition and lactation. Both its central and peripheral actions are transduced by a single isoform of the Oxt receptor (Oxtr). One well-known role of Oxt is the neural regulation of maternal behavior. Oxt immunoreactivity increases in the female rat brain during pregnancy and in the early postpartum period in regions like the ventral septum (Landgraf, Neumann, Russell, & Pittman, 1992), the paraventricular nucleus (PVN), and the supraoptic nucleus (SON) of the hypothalamus (Caldwell, Greer, Johnson, Prange, & Pedersen, 1987; Landgraf et al., 1992; Mezey & Kiss, 1991), although its regulation at this time appears to be estrogen-dependent (for a recent review of Oxt-estradiol interactions, see Cameron et al., 2008). Oxt expression also increases in the postpartum period within the SON and PVN of several species (see Leng, Meddle, & Douglas, 2008), including rats (Lightman & Young, 1987), voles (Wang, Liu, Young, & Iusel, 2000), rabbits (Caba, Silver, Gonzalez-Mariscal, Jimenez, & Beyer, 1996), and sheep (Broad, Kendrick, Sirinathsinghji, Keverne, 1993).
It has been proposed that the increases in Oxt expression prior to and following parturition facilitate the onset and maintenance of maternal behavior, particularly in rats. Intracerebroventricular (i.c.v.) Oxt administration to virgin female rats increases the display of all aspects of maternal behavior but only in the presence of high endogenous estradiol levels (Pedersen & Prange, 1979). Similarly, ovariectomized female rats primed with estradiol and progesterone have reduced maternal behavior up to 24 hours after i.c.v. anti-Oxt antiserum administration (Pedersen, Caldwell, Johnson, & Prange, 1985). Oxt may be particularly important to the grooming and nursing aspects of maternal behavior as i.c.v. infusion of a selective Oxt antagonist significantly increases self-grooming and the frequency of prone posture over pups, rather than the upright posture that facilitates nursing (Pedersen & Boccia, 2003). However, some studies fail to find a faciliatory effect of Oxt on maternal behavior (Bolwerk & Swanson, 1984; Rubin, Menneti, & Bridges, 1983) or indicate that Oxt can only affect maternal behavior when associated with other systems, such as olfaction (Wamboldt & Insel, 1987).
Expression of the Oxtr also increases significantly throughout pregnancy in various hypothalamic regions, particularly the SON, PVN, medial preoptic area (mPOA), and the bed nucleus of the stria terminalis (BNST; Bealer, Lipschitz, Ramoz, & Crowley, 2006; Meddle, Bishop, Gkoumassi, Van Leeuwen, & Douglas, 2007), as well as the amygdala and olfactory bulbs (Meddle et al., 2007), although questions have been raised regarding the specificity of this Oxtr antibody (Yoshida et al., 2009). With Oxt, Oxtr expression is thought to aid in the onset and maintenance of maternal behavior. Evidence supporting this assumption is found in several species. In rats, dams displaying naturally high levels of pup licking, grooming, and arched-back nursing (i.e., High LG-ABN) have higher levels of Oxtr in the BNST, mPOA, and lateral septum than do mothers with low levels of these behaviors (i.e., Low LG-ABN; Champagne, Diorio, Sharma, & Meaney, 2001; Francis, Champagne, & Meaney, 2000). I.c.v. administration of an Oxtr antagonist effectively turns High LG-ABN mothers into Low LG-ABN mothers (Champagne et al., 2001). In female prairie voles, which readily express “spontaneous” maternal behavior, the Oxtr is more highly expressed in the nucleus accumbens compared to species that fail to show “spontaneous” maternal behavior, such as rats, mice, and meadow voles (Olazabal & Young, 2005; Olazabal & Young 2006a). The difference in Oxtr expression within the nucleus accumbens has been found to be physiologically relevant in prairie voles, as administration of an Oxtr antagonist into this area completely abolishes displays of maternal behavior (Olazabal & Young 2006b).
Studies using Oxt and Oxtr knockout (KO) mice (Oxt−/− and Oxtr−/−, respectively) have also provided insight into the roles of Oxt and the Oxtr in the neural regulation of maternal behavior. Early studies in two independently derived lines of Oxt−/− mice found that females have normal parturition, an inability to milk eject, and unaltered maternal behavior (Nishimori et al., 1996; Young et al., 1996). A more recent study also found normal maternal behavior in both postpartum and virgin Oxt−/− females (Takayanagi et al., 2005). However, Pedersen et al. (2006) revealed moderate maternal behavior deficits in virgin Oxt−/− females, including a decrease in pup licking and impaired pup retrievals by virgin Oxt−/− females compared with wildtype (Oxt+/+) females. A limitation exists with this study in that the Oxt+/+ and Oxt−/− females used were not littermates and not obtained from genetically identical mothers. Possible differences in genetic background and intrauterine environment could contribute to the reported differences in maternal behavior. For example, Oxt−/− males derived from homozygous parents have higher levels of aggression than Oxt−/− males derived from heterozygous parents (Takayanagi et al., 2005; Winslow et al., 2000).
Initial studies in postpartum Oxtr−/− females have also reported deficits in maternal behavior compared with wildtype females, as measured by longer latencies to retrieve pups and reduced crouching over the retrieved pups (Takayanagi et al., 2005). Recently, we developed two lines from conditional Oxtr KO mice, a total knockout (Oxtr−/−) and an Oxtr knockout (OxtrFB/FB) in which the Oxtr is absent or reduced in most regions of the forebrain (Lee, Caldwell, Macbeth, Toln, & Young, 2008), sparing peripheral receptors. OxtrFB/FB females can eject milk (as verified by the presence of milk spots in their offsprings’ abdomens), thus allowing their offspring to survive and maternal behavior to be assessed. In the current study, we first observed maternal behavior in post-partum OxtrFB/FB and wildtype (Oxtr+/+) littermates, as well as maternal responsiveness of virgin Oxtr−/− females to foster pups. We then examined the likelihood of Oxtr+/+ and OxtrFB/FB dams to care for their pups following the application of an external stressor.
Methods
Animals and Housing
All subjects were littermates obtained from approximately eight breeders per line; equivalent numbers of wildtype and knockout subjects were taken from each breeder (as suggested in Crusio, Goldowitz, Holmes, & Wolfer, 2009). Females were group housed (two to five animals per cage) upon weaning at 21–28 days old in single-sex cages until at least 8 weeks old. Unless noted otherwise below, all females were virgins prior to testing. All animals were maintained under a 12:12 light–dark cycle (lights on at 0300h) with food and water available ad libitum. All procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines for the care and use of animals.
The development and genotyping of the Oxtr−/− and OxtrFB/FB females was as described previously (Lee et al., 2008; Macbeth, Lee, Edds, & Young, 2009). Briefly, we crossed the L7ag13 transgenic line (C57BL/6J genetic background) that expresses Cre recombinase under the control of the Camk2a promoter (Dragatsis & Zeitlin, 2000; Zakharenko et al., 2003) with Oxtr+/flox or Oxtrflox/flox mice. Oxtrflox/flox male mice were crossed with female Oxtr+/flox mice that contained one transgenic allele expressing Cre recombinase (Oxtr+/flox,cre or Oxtr+/FB). The offspring thus had the following genotypes: (a) Oxtr+/flox, (b) Oxtrflox/flox, (c) OxtrCre, +/flox, and (d) OxtrCre,Flox/flox. The first two are considered wildtypes, the third a heterozygous forebrain inactivation, and the fourth a forebrain-specific Oxtr knockout (OxtrFB/FB). Whole-body Oxtr knockout mice were generated by breeding male OxtrCre, +/flox with female Oxtrflox/flox mice, leading to heterozygous progeny (Oxtr+/−). These mice were crossed to obtain homozygous total Oxtr knockout (Oxtr−/−) females. As both the transgenic lines expressing Flp or Cre recombinase were on a C57BL/6J background, the resulting Oxtr−/− and OxtrFB/FB mice studied here were approximately 88% and 81% C57BL/6J, respectively (the remainder being 129/S). All OxtrFB/FB mice are heterozygous for the Cre recombinase transgene.
Experiment 1: Maternal Behavior in OxtrFB/FB and Oxtr−/− Females
OxtrFB/FB females
Oxtr+/+ (n = 9) and OxtrFB/FB (n = 10) females were used to assess maternal behavior. The average age of the dams at the time of their first litter was 12.9 ± 0.8 weeks for Oxtr+/+ and 13.7 ± 1.0 weeks for OxtrFB/FB females. Subject females were singly housed in a new, clean cage and mated to a C57BL/6J male (Jackson Laboratories). Mating was confirmed by the detection of a sperm plug, and males were removed approximately one week after mating. Females remained singly housed throughout parturition and during testing. Gestational length and litter sizes did not significantly differ between genotypes for either experiment. Upon completion of this experiment, all mothers and pups were euthanized.
Day of parturition (first day pups observed in the cage by 0900 hours) was designated postnatal day (PND) 0. Dams were tested for maternal behavior in their home cages beginning on PND 1 (first day where the pups had the presence of a milk spot) and continued through PND 3. On PND 1-PND 3, maternal behavior was videotaped for three 10-min observation sessions during “lights on” (light phase) at 900, 1100, and 1300 hours and one 20-min observation session during “lights off” (dark phase) at 1500 hours. Cages were moved to the testing room at least 30 minutes prior to testing and returned to the animal room immediately after each observation session. The observation session during the dark phase was videotaped under dim red light illumination with an infrared camera. From 900 to 1520 hours, the cages were not disturbed but remained stationary during videotaping. Following the last observation session at 1500 hours, pup retrieval by the dams was quantified by removal of pups from the dam for five minutes, during which time the female was videotaped. Pups were then scattered opposite of the dam in the home cage and the dam’s behavior was videotaped for an additional 10 minutes. Maternal aggression was tested on PND 4–6 at 1300 hours by removing the pups and immediately introducing an intruder Balb/c male to the home cage. The pups were removed to reduce their risk of injury; removal of pups has not been found to affect displays of maternal aggression (Svare, Betteridge, Katz, & Samuels, 1981).
Maternal behavior and aggression were later viewed and scored by an observer blind to genotype using Observer 5.0 (Noldus, Leesburg, VA). For the data collected on PND 1–3, dams were scored for 1) pup interactions (sniffing/licking and nursing pups); 2) nonsocial behaviors (resting alone, feeding, and moving around the cage); 3) nest building; and 4) self-grooming. Pup retrieval latencies were measured by determining the amount of time it took to the dam to retrieve the first pup (this was used because the number of pups varied among the dams). On PND 4–6, dams were scored for maternal aggression. Any dams failing to attack the intruder male in the first 5 minutes of testing were given a latency score of 300 seconds. If an attack occurred, the female’s behavior was scored for an additional 2 minutes. Behaviors measured included tail rattles, fleeing, attack behavior (lunge-bite), nonattack aggression (pushing), defensive behavior (upright and sideways defensive posturing), and nonsocial behavior (eating, sleeping, and climbing sides of the cage).
Maternal behaviors, with the exception of pup retrievals, were analyzed within each day using a two-way ANOVA with genotype and light phase as the main factors. For this analysis, the amount of time the dams engaged in all behaviors (maternal and nonmaternal) over the three light-phase observational sessions were first summed, and the percentage of time the females engaged in each behavior was determined. The percentage of time the females engaged in each behavior was then compared between the light and dark phases and between genotypes. Percentages were used, rather than durations, because of the difference in the amount of time the data were collected in the two different light conditions; that is, 30 minutes during “lights on” and 20 minutes during “lights off.” Pup retrieval latency was analyzed within each day using a one-way ANOVA. A p value of ≤0.05 was considered statistically significant. No statistical analyses were performed for measures of maternal aggression as too few animals attacked.
For this experiment, the number of pups surviving was also recorded. At the completion of the measures of maternal behavior and maternal aggression, females used in this experiment were mated a second time to determine whether the number of pups surviving differed across the two genotypes on a second litter.
Oxtr−/− females
Oxtr+/+ (n = 9) and Oxtr−/− (n = 8) virgin females (21 ± 0.8 weeks old) were used to assess maternal responsiveness toward foster pups. Foster pups were obtained from eight breeding pairs of C57BL/6J mice (Jackson Labs, Bar Harbor, Maine). Approximately one week prior to testing, subject females were singly housed in new, clean cages. The day pups were found in the C57BL/6J cage was designated PND 0; testing began on PND 1. Subject females were moved into the testing room at least 30 minutes prior to introduction of foster pups. Females were presented with four pups (two male, two female as determined by anogenital distance) from no more than two C57BL/6J breeders for a period of three days (PND 1–3). Only pups with observable milk spots were used as foster pups. Subject females were exposed to pups from the same breeder(s) each day. Testing occurred at approximately 1100 hours. Pups were scattered into the three corners of the cage not containing the nest; the females’ behavior was videotaped for a single 30-min period, after which pups were returned to their biological mothers. If subject females were observed to attack any foster pups, the session was immediately terminated and the injured pup euthanized. Maternal behaviors were scored as described for OxtrFB/FB females. Aggression was not measured in these virgin mice.
Experiment 2: Effects of Environmental Disturbances on Pup Mortality in OxtrFB/FB Females
Oxtr+/+ (n = 30) and OxtrFB/FB females (n = 30) were mated with C57BL/6J males as described above. The average age of the dams at the time their first litter was born was 17.6 ± 0.5 weeks for Oxtr+/+ and 19.9 ± 0.6 weeks for OxtrFB/FB females. Females from each genotype were randomly assigned to one of three disturbance groups: NONE, LOW, and HIGH (n = 10 of each genotype per group). For all three groups, the number of pups present on the day of parturition (PND 0: the day pups were present in the nest by 0900 hours) was counted. Females in the NONE group remained undisturbed in their cage until PND4, when a final pup count was taken. On PND 1–3, cages of females in the LOW and HIGH groups were removed from their shelves, and a pup count was taken at 0900 hours each day. Additionally, at 0900, 1100, and 1300 hours (the same times at which maternal behavior was assessed in Experiment 1), a cage disturbance was administered. For females in the LOW group, this disturbance consisted only of removing the lid of the cage and visually inspecting the inside of the cage. Pups were not moved in any way, and if the dam was blocking the pups for the pup count, she was gently moved off the nest to get an accurate count. For females in the HIGH group, all pups and nest material were scattered to the three previously empty corners of the cage, forcing the mother to rebuild the nest and retrieve all pups three times a day. A final pup count occurred at 0900 hours on PND 4.
In Experiment 1, no pups were found dead after PND 4, indicating the observed pup mortality (see Results) occurs shortly after parturition. Therefore, pups from litter 1 were euthanized by PND 7, and the dams were remated to novel males. Disturbances for the second litter were carried out in an identical manner as for the first litter. For their second litter, the average age of the dams was 23.8 ± 0.7 weeks for Oxtr+/+ females and 26.3 ± 0.8 weeks for OxtrFB/FB females. Litter 2 pups were euthanized by PND 7, and the dams mated a third time. As a further manipulation to assess the effects of stress on pup mortality, for the third litter we switched the NONE and HIGH groups (average age of the dams was 29.3 ± 0.9 weeks for Oxtr+/+ and 32.8 ± 1.0 weeks for OxtrFB/FB females). Average time between litters was approximately 6 weeks. Females who failed to become pregnant for any litter after two mating attempts were excluded from the study, accounting for the unequal group sizes across litters and groups (Figure 4, legend). Litter sizes did not significantly differ between the genotypes (Oxtr+/+: litter 1 = 7.62 ± 0.46; litter 2 = 9.03 ± 0.32; litter 3 = 8.88 ± 0.46. OxtrFB/FB: litter 1 = 7.61 ± 0.43; litter 2 = 8.07 ± 0.42; litter 3 = 7.48 ± 0.50). Upon completion of this experiment, all mothers and pups from litter 3 were euthanized.
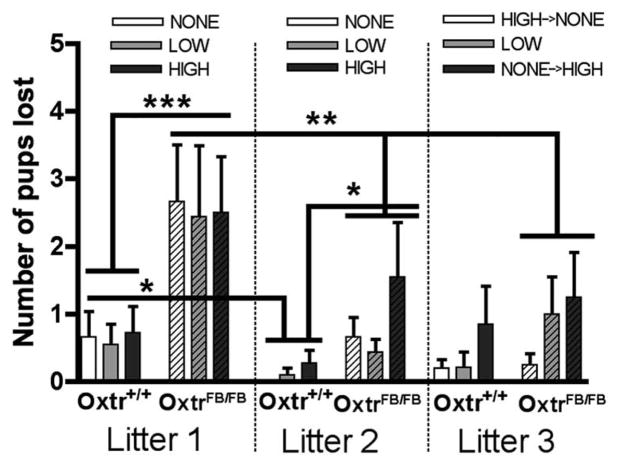
Figure 4.
Comparison of pup mortality in OxtrFB/FB females across three litters. Both Oxtr+/+ and OxtrFB/FB females lost significantly more pups on their first litter compared with their second litter, regardless of disturbance group. Only OxtrFB/FB females lost significantly more pups on their first litter as compared with their third litter. On both litter 1 (left) and litter 2 (middle), OxtrFB/FB females lost significantly more pups compared with Oxtr+/+ females, regardless of disturbance group. On litter 3, there were no significant differences in pup loss between the two genotypes, and switching the NONE and HIGH groups did not significantly affect pup mortality. Litter 1: Oxtr+/+: NONE = 9; LOW = 9; HIGH = 10; OxtrFB/FB: NONE = 9; LOW = 9; HIGH = 10. Litter 2: Oxtr+/+: NONE = 8, LOW = 10; HIGH = 10; OxtrFB/FB: NONE = 9; LOW = 9; HIGH = 9. Litter 3: Oxtr+/+: NONE = 10; LOW = 9; HIGH = 7; OxtrFB/FB: NONE = 8; LOW = 5; HIGH = 8. *p < .05; **p < .01; ***p < .001.
Two separate repeated measures three-way ANOVAs were carried out on the dependent variable (number of pups lost), with genotype, disturbance, and litter as the main factors. In the first ANOVA, only litters 1 and 2 were compared (disturbance groups remained identical), and in the second ANOVA litters 1 and 3 were compared to analyze the effect of switching NONE and HIGH groups on litter 3. Significant main effects and/or interactions were analyzed via independent-samples t tests. A p value of ≤0.05 was considered significant.
Results
Experiment 1
OxtrFB/FB females
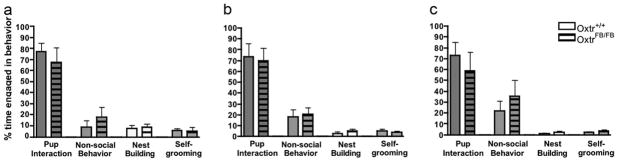
Four of 10 OxtrFB/FB dams showed total pup mortality by PND 1, with all pups found dead in the cages. In contrast, none of the nine Oxtr+/+ females exhibited pup mortality. Therefore, behavior from only six OxtrFB/FB dams was included in the final statistical analysis of maternal behavior. Of the OxtrFB/FB dams that displayed maternal care, there were no statistical differences between genotypes in any of the behaviors measured during the light phase of the circadian cycle on PND 1–3 (Figures 1a, b, c). Latency to retrieve the first pup back to the nest also did not differ between Oxtr+/+ and OxtrFB/FB dams (within 20s; data not shown). Furthermore, 100% of dams of both genotypes retrieved all pups back to the nest on all three test days. Maternal behavior of OxtrFB/FB dams was also assessed during the dark cycle; for all three days, there was decreased percent of time engaged in nest building and nonsocial behavior and increased sniffing/licking of pups in the dark phase compared to the light phase (data not shown). During the maternal aggression task, only three out of nine Oxtr+/+ females and only one out of six OxtrFB/FB females displayed any aggression (data not shown), indicating low levels of maternal aggression regardless of genotype.
Figure 1.
Maternal behavior of postpartum Oxtr+/+ and OxtrFB/FB females toward biological offspring. No statistically significant genotypic differences in maternal behavior were observed on (a) Day 1, (b) Day 2, or (c) Day 3.
A Fisher’s exact test comparing pup mortality between the genotypes had a two-tailed p value of 0.087. These data suggested that offspring from OxtrFB/FB dams might have increased mortality. To test this, we mated the same females a second time and only monitored the females for pup death from PND0-4. Unlike with their first litter, we found no genotypic differences in pup mortality (1/7 and 1/10 of Oxtr+/+ and OxtrFB/FB dams, respectively).
Oxtr−/− females
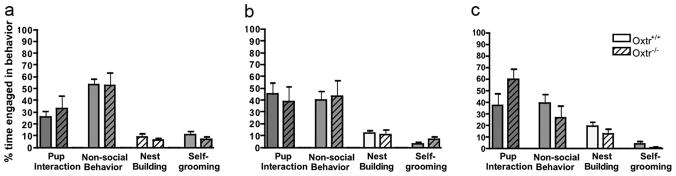
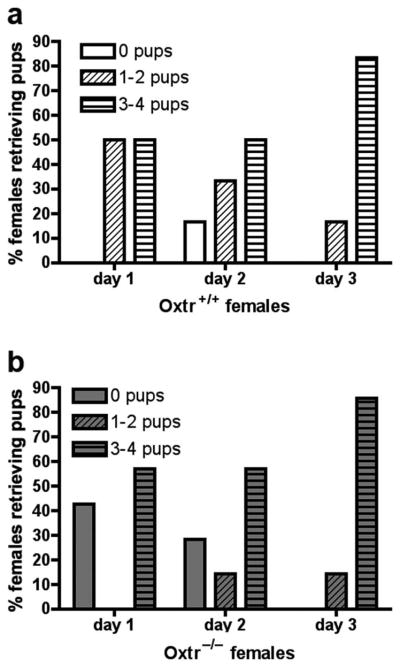
Data from three Oxtr+/+ females and one Oxtr−/− female were excluded because they attacked a foster pup on the first day of testing. Therefore, maternal behavior was assessed from six Oxtr+/+ females and seven Oxtr−/− females. No genotypic differences in maternal care of foster pups were observed over the three test days (Figures 2a, b, c). Additionally, Oxtr−/− females did not differ from Oxtr+/+ females in latency to retrieve the first pup on any of the three testing days (data not shown). However, on Day 1, only 40% of Oxtr−/− females retrieved at least two foster pups, compared to 100% of the Oxtr+/+ females. By Day 3, over 80% of females from both genotypes retrieved all four foster pups (see Figure 3).
Figure 2.
Maternal behavior of virgin Oxtr+/+ and Oxtr−/− females toward foster pups. No statistically significant genotypic differences in maternal behavior were observed on (a) Day 1, (b) Day 2, or (c) Day 3.
Figure 3.
Pup retrievals by virgin Oxtr+/+ and Oxtr−/− females. 100% of Oxtr+/+ females (a) retrieved at least one pup to the nest on Day 1 of testing. In comparison, over 40% of Oxtr−/− females (b) failed to retrieve any pups to the nest on Day 1. However, by Day 3 both genotypes had identical levels of pup retrieval, with 100% retrieving at least one pup and over 80% retrieving 3–4 pups.
Experiment 2
In this experiment, we assessed whether or not application of an external stress (cage disturbance) influences pup mortality in OxtrFB/FB dams. Comparison of the data from litters 1 and 2 via three-way ANOVA (genotype × litter × disturbance) revealed significant main effects of genotype, F1,101 = 17.61, p < .001, and litter, F1,101 = 11.83, p < .001. There were no significant interactions. Analyses of the main effects via t test are shown in Figure 4. Regardless of disturbance group, all dams lost more pups on litter 1 (Figure 4, left) compared to litter 2 (Figure 4, middle; p < .05 for Oxtr+/+ dams and p < .01 for OxtrFB/FB dams). Additionally, regardless of disturbance group, OxtrFB/FB dams lost significantly more pups on both litters 1 and 2 compared to Oxtr+/+ dams (p < .001 for litter 1 and p < .05 for litter 2).
Comparison of the data from litters 1 and 3 via three-way ANOVA (genotype × litter × disturbance) revealed a significant genotype × litter interaction, F1,92 = 4.60, p < .05. Analyses via t tests are shown in Figure 4. Oxtr+/+ dams did not significantly differ in number of pups lost from litter 1 to litter 3 (p > .10). In contrast, regardless of disturbance group, OxtrFB/FB dams lost significantly more pups on litter 1 (Figure 4, left) than litter 3 (Figure 4, right; p < .01). There were no genotypic differences in pup loss between Oxtr+/+ and OxtrFB/FB dams on litter 3 (p > .10).
Discussion
In the current paper, we used a forebrain knockout of the Oxtr gene, as well as a total Oxtr knockout to assess the role of the Oxtr in female maternal behavior. We had hypothesized that lack of the Oxtr in the entire brain, and specifically in the forebrain, would result in impaired maternal behavior as compared to WT littermates. Contrary to our hypothesis, we found that OxtrFB/FB females did not significantly differ from Oxtr+/+ females on our measures of maternal behavior, including retrieval latencies, at least in those that did not lose their pups (see below). The normal maternal behavior in the remaining OxtrFB/FB females is impressive in the face of the altered pattern of expression with absence in some brain regions and maintained expression to varying degrees in others, such as the medial amygdala, olfactory bulbs, and neocortex (Lee et al., 2008). This altered Oxtr expression pattern, developing after weaning in OxtrFB/FB females, could allow for the continued maintenance of maternal behavior, including maternal aggression, as Oxt activity in the olfactory bulbs and amygdala is implicated in maternal behavior and aggression, respectively (Bosch, Meddle, Beiderbeck, Douglas, & Neumann, 2005; Brennan & Keverne, 1997; Fleming & Rosenblatt, 1974; Lubin, Elliot, Black, & Johns, 2003; Nephew, Bridges, Lovelock, & Byrnes, 2009). However, it should be noted that in a more natural, socially competitive environment, female Oxt−/− mice have high levels of infanticide and display high levels of aggression toward intruders (Ragnauth et al., 2005).
Previous studies have indicated subtle deficits in maternal behavior, specifically longer retrieval latencies, in both total Oxtr and Oxt KO lines (Takayanagi et al., 2005; Pedersen et al., 2006, respectively). We also found deficits in pup retrieval but only on the first presentation of pups. Even about half of the virgin wild-types left some pups scattered about the cage initially. This may indicate that our Oxtr−/− females may have a greater initial aversion to pups that is overcome by repeated exposure. It is interesting to note that by day three, both virgin genotypes’ patterns of behaviors begin to resemble that of postpartum wildtype mice, with trends toward increased pup interactions and decreased non-social behaviors. While we did not assess maternal behavior for longer than minutes across a day, similar short durations have been used in previous studies (Keller, Pawluski, Brock, Douhard, & Bakker, 2010; Olazabal & Young 2006a; Olazabal & Young 2006b). It may be useful to have a more prolonged measure of time spent in contact with pups, although this becomes difficult when using foster pups. Based upon the methods used in this study, we find it remarkable just how robust basic maternal behaviors appear to be despite the absence of the Oxtr.
Given that Oxt cannot be involved in the expression of maternal behavior in Oxtr−/− and OxtrFB/FB (at least where it is absent in the latter) females, other neural mechanisms must compensate for the loss of Oxt action. One possibility is the vasopressin (Avp) system. Avp is closely related to Oxt and regulates many of the same behaviors as Oxt. In terms of parental care, Avp has primarily been studied for its importance in rodent paternal behavior (see Caldwell, Lee, Macbeth, & Young, 2008, for review). However, recent studies indicate that Avp does contribute to maternal behavior, predominantly through actions at the Avp 1a receptor (Bosch & Neumann, 2010; Bosch, Pfortsch, Beiderbeck, Landgraf, & Neumann, 2010; Nephew & Bridges, 2008a; Nephew & Bridges, 2008b). Obviously, maternal behavior is critical for species propagation so it would be logical for the species to have evolved as much redundancy as possible for essential functions.
In Experiment 1, we found that in OxtrFB/FB dams whose pups survive, there are no impairments in maternal behavior. However, we did find a unique pup mortality phenotype in which offspring from 40% of OxtrFB/FB dams died by PND 4. This phenotype was present only in OxtrFB/FB dams and only with their first litter. As these animals had been disturbed daily from PND 1–3 to test for maternal behavior, we hypothesized that OxtrFB/FB dams were unusually susceptible to an environmental disturbance, which resulted in increased pup mortality.
To test this hypothesis, in Experiment 2, the home cage environment of Oxtr+/+ and OxtrFB/FB females was disturbed from PND 1–3. We found no significant overall effect of type of disturbance type (NONE, LOW, or HIGH) on pup mortality. Both nondisturbed (NONE) and disturbed (LOW and HIGH) OxtrFB/FB dams lost similar numbers of pups from litters 1 and 2, indicating that the stress applied in this experiment did not influence the pup mortality phenotype. However, OxtrFB/FB dams lost significantly more pups than Oxtr+/+ dams from each of the first two litters, confirming the pup mortality phenotype in OxtrFB/FB dams observed in Experiment 1. By their third litter, OxtrFB/FB dams no longer lost a significantly greater number of pups than Oxtr+/+ dams and switching the NONE and HIGH disturbance groups did not alter pup death in either genotype. The discrepancy between Experiment 2 (in which OxtrFB/FB dams still lost more pups than Oxtr+/+ dams from litters 2), and Experiment 1 (in which OxtrFB/FB dams did not lose more pups than Oxtr+/+ dams from litter 2) may indicate that disturbing the cages in Experiment 2 has a subtle effect on pup loss.
The results of this study indicate that the pup mortality phenotype in OxtrFB/FB dams is not significantly facilitated by disturbances to their environment and the underlying cause of the pup death remains unknown. In this study, none of the offspring were homozygous, as all females were mated to C57BL/6 males, so Oxtr+/+ offspring were WT, and OxtrFB/FB offspring were heterozygotes. We have not previously observed increased pup deaths of heterozygotes from wildtype or heterozygous dams in development of the line. However, there may be a fundamental difference in the offspring of Oxtr+/+ and OxtrFB/FB females. For example, ultrasonic vocalizations in both Oxt−/− and Oxtr−/− male infant pups are decreased compared with wildtype littermates (Winslow et al., 2000; Takayanagi et al., 2005, respectively). It is possible that the heterozygous offspring of OxtrFB/FB dams have impaired vocalizations. It does not appear that the dams are attacking their pups, as the pups are whole when found dead in the cage. Both Oxtr+/+ and OxtrFB/FB mothers clean their pups and engage in placentophagia as would be expected. The pups do not appear to die from starvation, as milk spots are noted in their stomachs. However, given that Oxt plays a causal role in prolactin release and subsequent milk ejection (Kennett et al., 2009; Bertram et al., 2010; Tabak et al., 2010), the possibility remains that OxtrFB/FB mothers’ milk supply, while intact, may be diminished compared to Oxtr+/+ mothers. If this is the case, lactation would seem to improve in subsequent litters.
The pup mortality phenotype is difficult to quantify. The pups did not die in the same manner across both experiments. In Experiment 1, all dead pups were found in the cage by PND 1. In Experiment 2, most pups died by PND 1, yet some were found stillborn, and others did not die until PND 2–3. Lastly, the pup mortality phenotype is not universal across all OxtrFB/FB dams. In Experiment 1, only 40% of OxtrFB/FB dams were observed to have the phenotype. A slightly higher percentage (50–60%) was observed to have the phenotype on the first litter in Experiment 2, but still not 100%. Oxtr+/+ and OxtrFB/FB females from both experiments were tested in the same animal facility, were obtained from like breeding pairs, and all came from litters containing both male and female offspring, indicating that testing environment, parenting, and/or early life experience likely did not play a role in pup mortality differences across the two experiments. It is possible that some unobserved part of the delivery process is impaired with their first litter, inducing pup mortality, which is avoided in subsequent litters. For example, stress, induced by fear of peripartum events and usually dampened by the actions of Oxt in the amygdala (Hansen & Ferreira, 1986), could be elevated in the OxtrFB/FB females, leading to greater corticosterone exposure and untoward consequences (Rangon et al., 2007; Zahwa, Yorty, & Bonneau, 2008). Perhaps prior experience produces a less stressful second pregnancy, parturition, and postpartum, leading to reduced pup mortality.
Oxt is heavily involved in the regulation of affiliative behaviors in rodents, with Oxt administration increasing social interactions, partner preference, and parental behavior (reviewed in Lee, Macbeth, Pagani, & Young, 2009). As discussed above, Oxt is particularly involved in the onset and maintenance of maternal behavior. Pups are initially anxiety provoking when encountered by females (Fleming, Cheung, Myhal, & Kessler, 1989; Fleming & Luebke, 1981); as Oxt aids in decreasing anxiety in the postpartum period, it may aid in forming proper pup attachment. Oxt administration inhibits infanticide normally observed in virgin and pregnant wild mice (McCarthy, 1990; McCarthy, Bare, & vom Saal, 1986). In women, higher levels of Oxt during the third trimester correlate with self-reported feelings of “bonding” with the fetus (Levine, Zagoory-Sharon, Feldman, & Weller, 2007), and in the postpartum period plasma Oxt levels are correlated with maternal bonding behaviors, such as infant-directed gaze, vocalizations, and affectionate touch (Feldman, Weller, Zagoory-Sharon, & Levine, 2007). Recent work also indicates that a less efficient variant of the Oxtr gene in human mothers is correlated with lower levels of maternal responsiveness toward their toddlers (Bakermans-Kranenburg and van Ijzendoorn, 2008). The unique Oxtr expression pattern present in our OxtrFB/FB females may result in impaired pup attachment, resulting in the pup mortality phenotype. It does result in an altered ability for social recognition (Macbeth et al., 2009). Yet the attachment failure is not complete, as not all OxtrFB/FB dams exhibit the phenotype, and they are able to overcome the deficit, as by litter 3 we observed no genotype differences in pup mortality. Future studies are planned to assess whether nonmaternal behavior differences exist between Oxtr+/+ and OxtrFB/FB females in the postpartum period (such as altered depressive-like behaviors and responses to stress and/or rewarding stimuli) that could indirectly influence the pup mortality phenotype.
Acknowledgments
We thank James Heath for technical assistance and Selen Tolu and Anna Brownstein for assistance with behavioral testing and scoring. This research was supported in part by the NIMH Intramural Research Program (Z01-MH-002498-21).
Contributor Information
Abbe H. Macbeth, Section on Neural Gene Expression, National Institute of Mental Health, National Institutes of Health
Jennifer E. Stepp, Section on Neural Gene Expression, National Institute of Mental Health, National Institutes of Health
Heon-Jin Lee, School of Dentistry, Kyungpook National University.
W. Scott Young, 3rd, Section on Neural Gene Expression, National Institute of Mental Health, National Institutes of Health.
Heather K. Caldwell, Laboratory of Neuroendocrinology and Behavior, Department of Biological Sciences and the School of Biomedical Sciences, Kent State University
References
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bealer SL, Lipschitz DL, Ramoz G, Crowley WR. Oxytocin receptor binding in the hypothalamus during gestation in rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2006;291:R53–R58. doi: 10.1152/ajpregu.00766.2005. [DOI] [PubMed] [Google Scholar]
- Bolwerk EL, Swanson HH. Does oxytocin play a role in the onset of maternal behaviour in the rat? Journal of Endocrinology. 1984;101:353–357. doi: 10.1677/joe.0.1010353. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: Link to anxiety. Journal of Neuroscience. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. European Journal of Neuroscience. 2010;31:883–891. doi: 10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. Journal of Neuroendocrinology. 2010;22:420–429. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Progress in Neurobiology. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Broad KD, Kendrick KM, Sirinathsinghji DJ, Keverne EB. Changes in oxytocin immunoreactivity and mRNA expression in the sheep brain during pregnancy, parturition and lactation and in response to oestrogen and progesterone. Journal of Neuroendocrinology. 1993;5:435–444. doi: 10.1111/j.1365-2826.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Caba M, Silver R, Gonzalez-Mariscal G, Jimenez A, Beyer C. Oxytocin and vasopressin immunoreactivity in rabbit hypothalamus during estrus, late pregnancy, and postpartum. Brain Research. 1996;720:7–16. doi: 10.1016/0006-8993(96)00036-4. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: Behavioral roles of an “original” neuropeptide. Progress in Neurobiology. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JD, Greer ER, Johnson MF, Prange AJ, Jr, Pedersen CA. Oxytocin and vasopressin immunoreactivity in hypothalamic and extrahypothalamic sites in late pregnant and postpartum rats. Neuroendocrinology. 1987;46:39–47. doi: 10.1159/000124794. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. Journal of Neuroendocrinology. 2008;20:795–801. doi: 10.1111/j.1365-2826.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences, USA of the United States of America. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes, Brain and Behavior. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Dragatsis I, Zeitlin S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26:133–135. doi: 10.1002/(sici)1526-968x(200002)26:2<133::aid-gene10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Cheung U, Myhal N, Kessler Z. Effects of maternal hormones on ‘timidity’ and attraction to pup-related odors in female rats. Physiology and Behavior. 1989;46:449–453. doi: 10.1016/0031-9384(89)90019-x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: Emotionality differences between nulliparous and parturient females. Physiology and Behavior. 1981;27:863–868. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. Journal of Comparative Physiology, A Sensory, Neural, and Behavioral Physiology. 1974;86:221–232. doi: 10.1037/h0035937. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Hansen S, Ferreira A. Food intake, aggression, and fear behavior in the mother rat: Control by neural systems concerned with milk ejection and maternal behavior. Behavioral Neuroscience. 1986;100:64–70. doi: 10.1037//0735-7044.100.1.64. [DOI] [PubMed] [Google Scholar]
- Keller M, Pawluski JL, Brock O, Douhard Q, Bakker J. The alpha-fetoprotein knock-out mouse model suggests that parental behavior is sexually differentiated under the influence of prenatal estradiol. Hormones and Behavior. 2010;57:434–440. doi: 10.1016/j.yhbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann I, Russell JA, Pittman QJ. Push-pull perfusion and microdialysis studies of central oxytocin and vasopressin release in freely moving rats during pregnancy, parturition, and lactation. Annals of the New York Academy of Sciences. 1992;652:326–339. doi: 10.1111/j.1749-6632.1992.tb34364.x. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: The great facilitator of life. Progress in Neurobiology. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Meddle SL, Douglas AJ. Oxytocin and the maternal brain. Current Opinion in Pharmacology. 2008;8:731–734. doi: 10.1016/j.coph.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: Individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., 3rd Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. Journal of Physiology. 1987;394:23–39. doi: 10.1113/jphysiol.1987.sp016858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Fleming AS. Parental behaviors in rats and mice. Current Protocols in Neuroscience. 2001;chap 8(Unit 8.15) doi: 10.1002/0471142301.ns0815s17. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behavioral Neuroscience. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Lee HJ, Edds J, Young WS., 3rd Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes, Brain and Behavior. 2009;8(5):558–567. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Oxytocin inhibits infanticide in female house mice (Mus domesticus) Hormones and Behavior. 1990;24:365–375. doi: 10.1016/0018-506x(90)90015-p. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Bare JE, vom Saal FS. Infanticide and parental behavior in wild female house mice: Effects of ovariectomy, adrenalectomy and administration of oxytocin and prostaglandin F2 alpha. Physiology and Behavior. 1986;36:17–23. doi: 10.1016/0031-9384(86)90066-1. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- Mezey E, Kiss JZ. Coexpression of vasopressin and oxytocin in hypothalamic supraoptic neurons of lactating rats. Endocrinology. 1991;129:1814–1820. doi: 10.1210/endo-129-4-1814. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiology and Behavior. 2008a;95:182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacology, Biochemistry and Behavior. 2008b;91:77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behavioral Neuroscience. 2009;123:949–957. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Variability in “spontaneous” maternal behavior is associated with anxiety-like behavior and affiliation in naive juvenile and adult female prairie voles (Microtus ochrogaster) Developmental Psychobiology. 2005;47:166–178. doi: 10.1002/dev.20077. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Hormones and Behavior. 2006a;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006b;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams’ oral grooming and upright posturing over pups. Physiology and Behavior. 2003;80:233–241. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Johnson MF, Fort SA, Prange AJ., Jr Oxytocin antiserum delays onset of ovarian steroid-induced maternal behavior. Neuropeptides. 1985;6:175–182. doi: 10.1016/0143-4179(85)90108-8. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain and Behavior. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, Pfaff DW. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes, Brain and Behavior. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Rangon CM, Fortes S, Lelievre V, Leroux P, Plaisant F, Joubert C, Gressens P. Chronic mild stress during gestation worsens neonatal brain lesions in mice. Journal of Neuroscience. 2007;27:7532–7540. doi: 10.1523/JNEUROSCI.5330-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS. Prepartum and postpartum regulation of maternal behaviour in the rat. Ciba Found Symp. 1975;33:17–37. doi: 10.1002/9780470720158.ch3. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psycho-neuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Menniti FS, Bridges RS. Intracerebroventricular administration of oxytocin and maternal behavior in rats after prolonged and acute steroid pretreatment. Hormones and Behavior. 1983;17:45–53. doi: 10.1016/0018-506x(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Svare B, Betteridge C, Katz D, Samuels O. Some situational and experiential determinants of maternal aggression in mice. Physiology and Behavior. 1981;26:253–258. doi: 10.1016/0031-9384(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16096–101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamboldt MZ, Insel TR. The ability of oxytocin to induce short latency maternal behavior is dependent on peripheral anosmia. Behavioral Neuroscience. 1987;101:439–441. doi: 10.1037//0735-7044.101.3.439. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Liu Y, Young LJ, Insel TR. Hypothalamic vasopressin gene expression increases in both males and females post-partum in a biparental rodent. Journal of Neuroendocrinology. 2000;12:111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Hormones and Behavior. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. Journal of Neuroscience. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, 3rd, Shepard E, Amico J, Hennighausen L, Wagner KU, LaMarca ME, Ginns EI. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. Journal of Neuroendocrinology. 1996;8:847–853. doi: 10.1046/j.1365-2826.1996.05266.x. [DOI] [PubMed] [Google Scholar]
- Zahwa H, Yorty JL, Bonneau RH. Elevated maternal corticosterone during lactation hinders the neonatal adaptive immune response to herpes simplex virus (HSV) infection. Brain, Behavior, and Immunity. 2008;22:339–353. doi: 10.1016/j.bbi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]