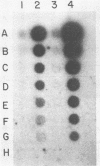
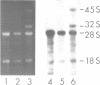
Abstract
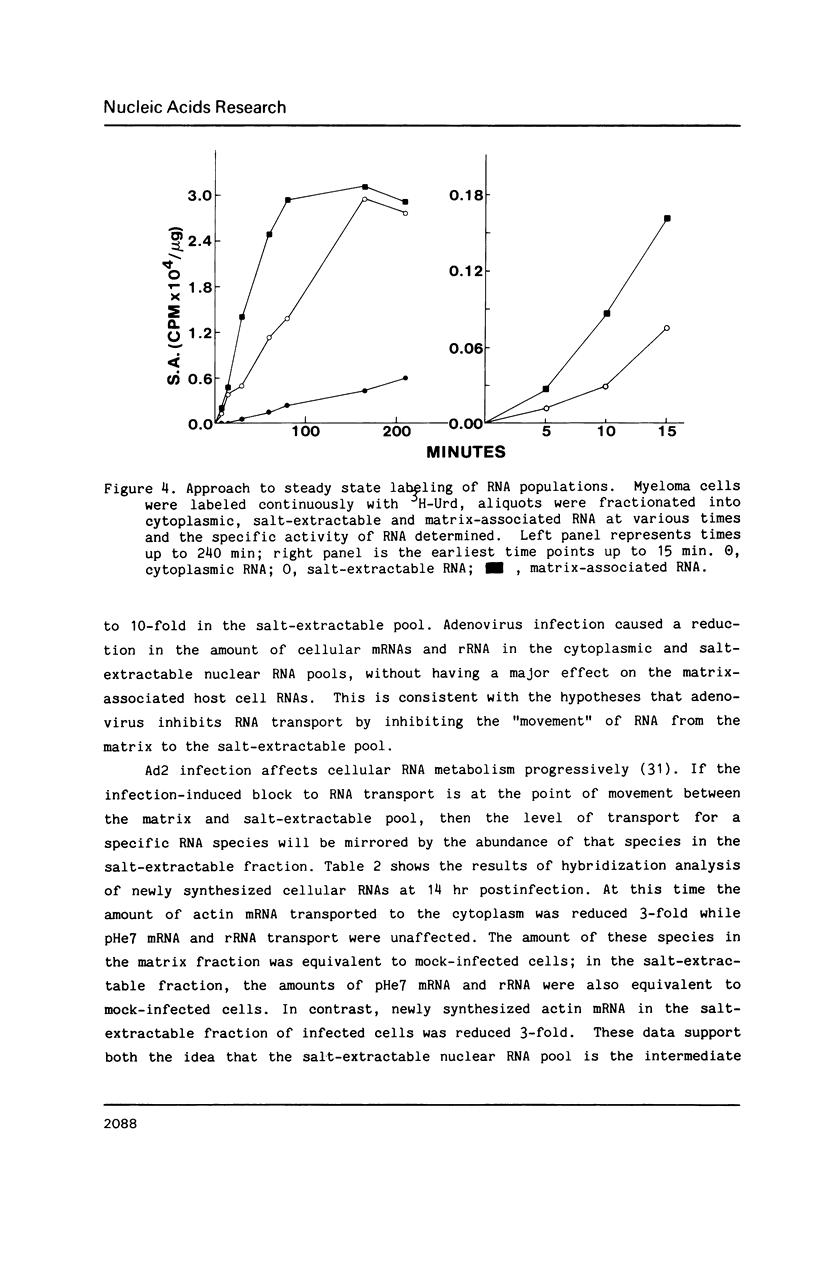
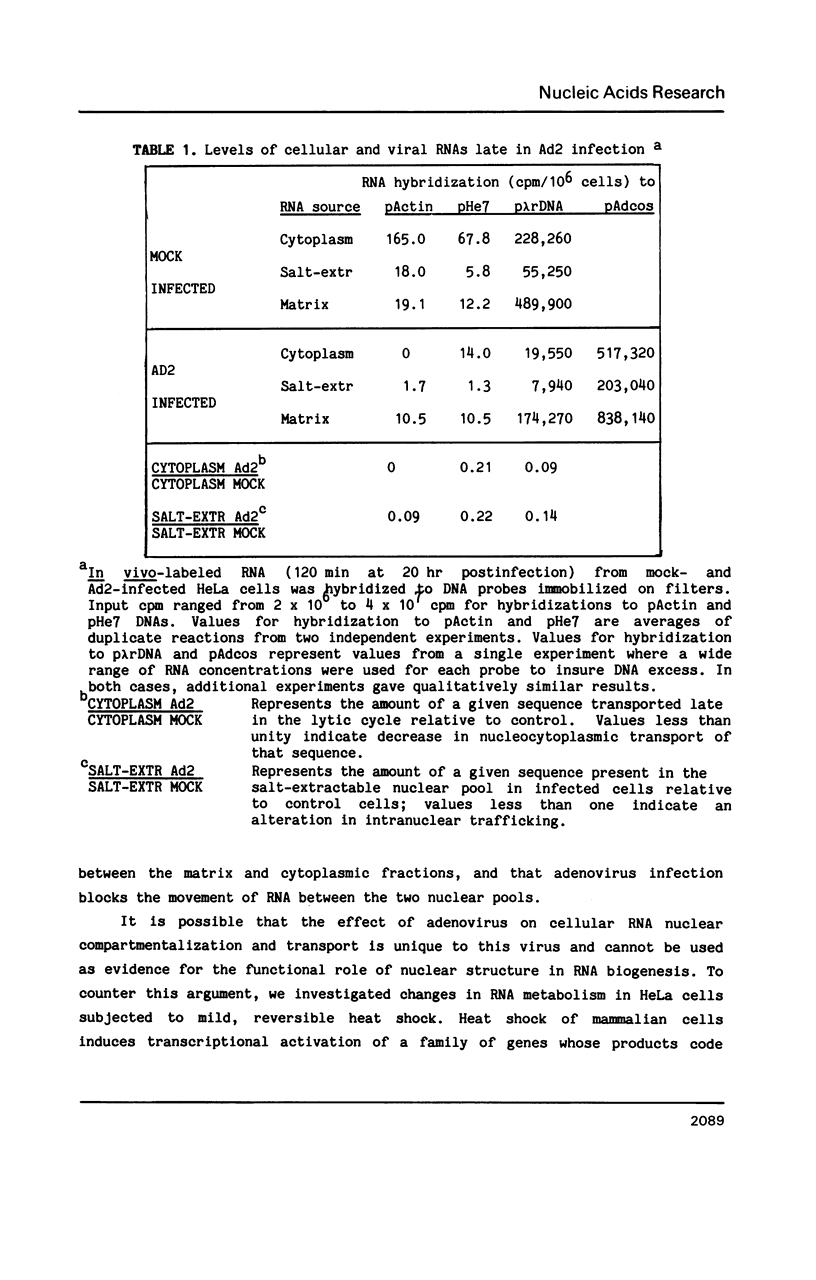
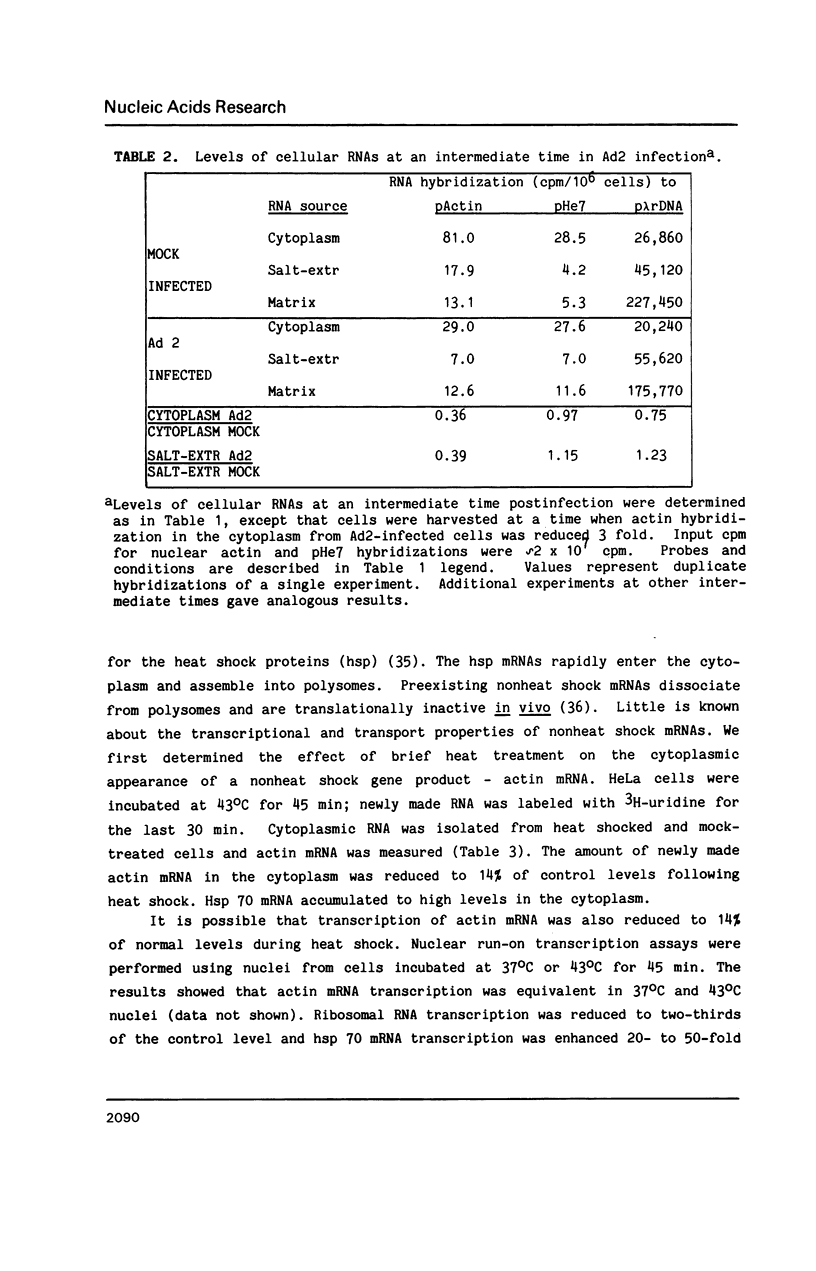
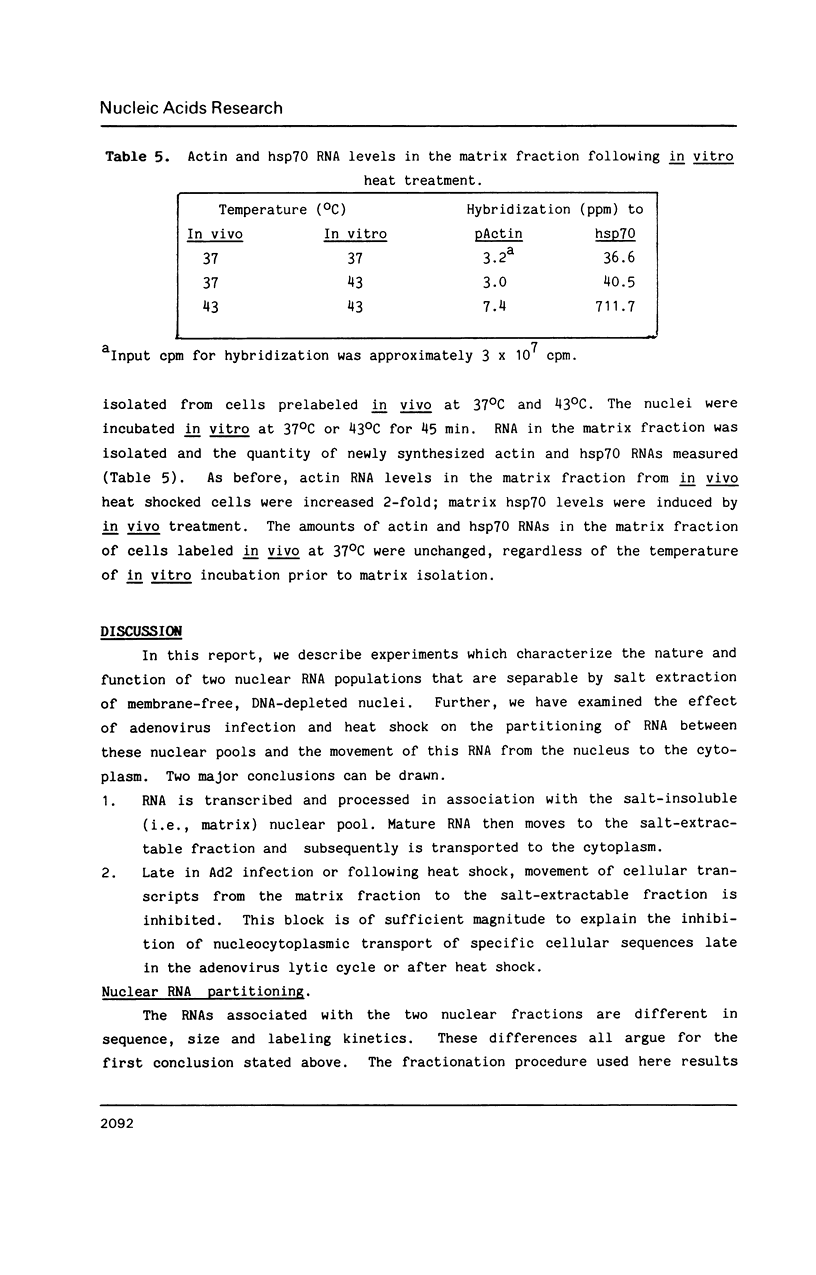
Nuclear RNA compartmentalization has received little attention as an area where regulation of gene expression could occur. RNA transcription and processing occur in association with the nuclear matrix, a salt-insoluble proteinaceous network that fills the nuclear space and is contiguous with the peripheral lamina and pore complexes. Described here are experiments that determine the fate of nuclear RNA after it has completed these matrix-associated maturation steps. Continuous label experiments indicate that after nuclear RNA is processed it changes its state of attachment in the nucleus so that it is now removed from the nucleus in the high salt extraction step of matrix isolation. It is this salt-extractable RNA that will be transported to the cytoplasm. Late in adenovirus infection and following heat shock, when transport of cellular RNA is decreased, cellular transcripts do not make the transition from the matrix-associated to the salt-extractable nuclear pool. The implication of these data for the regulation of gene expression is discussed.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Sedat J. W. Three-dimensional architecture of a polytene nucleus. Nature. 1983 Apr 21;302(5910):676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- Babich A., Feldman L. T., Nevins J. R., Darnell J. E., Jr, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol Cell Biol. 1983 Jul;3(7):1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S., Darnell J. E., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985 Oct;5(10):2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Abulafia R., Aloni Y. SV40 virions and viral RNA metabolism are associated with cellular substructures. EMBO J. 1982;1(10):1225–1231. doi: 10.1002/j.1460-2075.1982.tb00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Bond U. Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 1988 Nov;7(11):3509–3518. doi: 10.1002/j.1460-2075.1988.tb03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Castiglia C. L., Flint S. J. Effects of adenovirus infection on rRNA synthesis and maturation in HeLa cells. Mol Cell Biol. 1983 Apr;3(4):662–671. doi: 10.1128/mcb.3.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E. M., Nordstrom J. L., Tsai M. J., O'Malley B. W. Ribonucleic acid precursors are associated with the chick oviduct nuclear matrix. Biochemistry. 1982 Sep 28;21(20):4945–4953. doi: 10.1021/bi00263a018. [DOI] [PubMed] [Google Scholar]
- Fetherston J., Werner E., Patterson R. Processing of the external transcribed spacer of murine rRNA and site of action of actinomycin D. Nucleic Acids Res. 1984 Sep 25;12(18):7187–7198. doi: 10.1093/nar/12.18.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Beltz G. A., Linzer D. I. Synthesis and processing of simian virus 40-specific RNA in adenovirus-infected, simian virus 40-transformed human cells. J Mol Biol. 1983 Jun 25;167(2):335–359. doi: 10.1016/s0022-2836(83)80339-8. [DOI] [PubMed] [Google Scholar]
- Franke W. W. Nuclear lamins and cytoplasmic intermediate filament proteins: a growing multigene family. Cell. 1987 Jan 16;48(1):3–4. doi: 10.1016/0092-8674(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Cutt J. R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985 Oct;56(1):250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlan G., Eckert W. A., Kaffenberger W., Wunderlich F. Isolation and characterization of an RNA-containing nuclear matrix from Tetrahymena macronuclei. Biochemistry. 1979 May 1;18(9):1782–1788. doi: 10.1021/bi00576a023. [DOI] [PubMed] [Google Scholar]
- Hutchison N., Weintraub H. Localization of DNAase I-sensitive sequences to specific regions of interphase nuclei. Cell. 1985 Dec;43(2 Pt 1):471–482. doi: 10.1016/0092-8674(85)90177-1. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Coffey D. S., Shaper J. H. Considerations in the isolation of rat liver nuclear matrix, nuclear envelope, and pore complex lamina. Exp Cell Res. 1981 Mar;132(1):105–123. doi: 10.1016/0014-4827(81)90088-4. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., van Eekelen C. A., Reinders R. J., Berns A. J., van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with the host nuclear matrix during splicing. J Mol Biol. 1982 Jan 5;154(1):103–119. doi: 10.1016/0022-2836(82)90420-x. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H., Maxwell F., Hahn W. E. Removal of RNase activity from DNase by affinity chromatography on agarose coupled aminophenylphosphoryl-uridine-2' (3')-phosphate. Nucleic Acids Res. 1977 Jan;4(1):241–246. doi: 10.1093/nar/4.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Setyono B., Greenberg J. R., Pederson T. Structure of nuclear ribonucleoprotein: identification of proteins in contact with poly(A)+ heterogeneous nuclear RNA in living HeLa cells. J Cell Biol. 1981 Aug;90(2):380–384. doi: 10.1083/jcb.90.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M., Whalen A. M., Smith D. E., Fisher P. A. Heat shock-induced changes in the structural stability of proteinaceous karyoskeletal elements in vitro and morphological effects in situ. J Cell Biol. 1987 Sep;105(3):1087–1098. doi: 10.1083/jcb.105.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Sollner-Webb B. Transcription of mouse rRNA genes by RNA polymerase I: in vitro and in vivo initiation and processing sites. Cell. 1981 Nov;27(1 Pt 2):165–174. doi: 10.1016/0092-8674(81)90370-6. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Huang C. Y., Pogo A. O. Rat liver nuclear skeleton and ribonucleoprotein complexes containing HnRNA. J Cell Biol. 1978 Mar;76(3):675–691. doi: 10.1083/jcb.76.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Moore M., Schaack J., Baim S. B., Morimoto R. I., Shenk T. Induced heat shock mRNAs escape the nucleocytoplasmic transport block in adenovirus-infected HeLa cells. Mol Cell Biol. 1987 Dec;7(12):4505–4512. doi: 10.1128/mcb.7.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe S. H. Ribonucleoprotein structure of adenovirus nuclear RNA probed by nuclease digestion. J Mol Biol. 1982 Dec 15;162(3):585–606. doi: 10.1016/0022-2836(82)90390-4. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B., Coffey D. S. A fixed site of DNA replication in eucaryotic cells. Cell. 1980 Feb;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Patterson R., Werner E., Fetherston J. RNA metabolism in nuclei: selective transport of kappa exons from myeloma nuclei and adenoviral transcripts from infected HeLa nuclei. Nucleic Acids Res. 1986 May 27;14(10):4159–4169. doi: 10.1093/nar/14.10.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Ross D. A., Yen R. W., Chae C. B. Association of globin ribonucleic acid and its precursors with the chicken erythroblast nuclear matrix. Biochemistry. 1982 Feb 16;21(4):764–771. doi: 10.1021/bi00533a029. [DOI] [PubMed] [Google Scholar]
- Sadis S., Hickey E., Weber L. A. Effect of heat shock on RNA metabolism in HeLa cells. J Cell Physiol. 1988 Jun;135(3):377–386. doi: 10.1002/jcp.1041350304. [DOI] [PubMed] [Google Scholar]
- Schröder H. C., Trölltsch D., Friese U., Bachmann M., Müller W. E. Mature mRNA is selectively released from the nuclear matrix by an ATP/dATP-dependent mechanism sensitive to topoisomerase inhibitors. J Biol Chem. 1987 Jun 25;262(18):8917–8925. [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Smith H. C., Berezney R., Brewster J. M., Rekosh D. Properties of adenoviral DNA bound to the nuclear matrix. Biochemistry. 1985 Feb 26;24(5):1197–1202. doi: 10.1021/bi00326a022. [DOI] [PubMed] [Google Scholar]
- Stein R., Ziff E. B. HeLa cell beta-tubulin gene transcription is stimulated by adenovirus 5 in parallel with viral early genes by an E1a-dependent mechanism. Mol Cell Biol. 1984 Dec;4(12):2792–2801. doi: 10.1128/mcb.4.12.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorakis N. G., Morimoto R. I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder S. S., Berget S. M. Posttranscriptional control of DHFR gene expression during adenovirus 2 infection. J Virol. 1985 Apr;54(1):72–77. doi: 10.1128/jvi.54.1.72-77.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Zeitlin S., Parent A., Silverstein S., Efstratiadis A. Pre-mRNA splicing and the nuclear matrix. Mol Cell Biol. 1987 Jan;7(1):111–120. doi: 10.1128/mcb.7.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z. H., Nickerson J. A., Krochmalnic G., Penman S. Alterations in nuclear matrix structure after adenovirus infection. J Virol. 1987 Apr;61(4):1007–1018. doi: 10.1128/jvi.61.4.1007-1018.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]