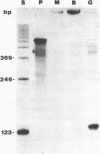
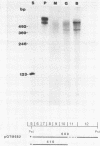
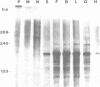
Abstract
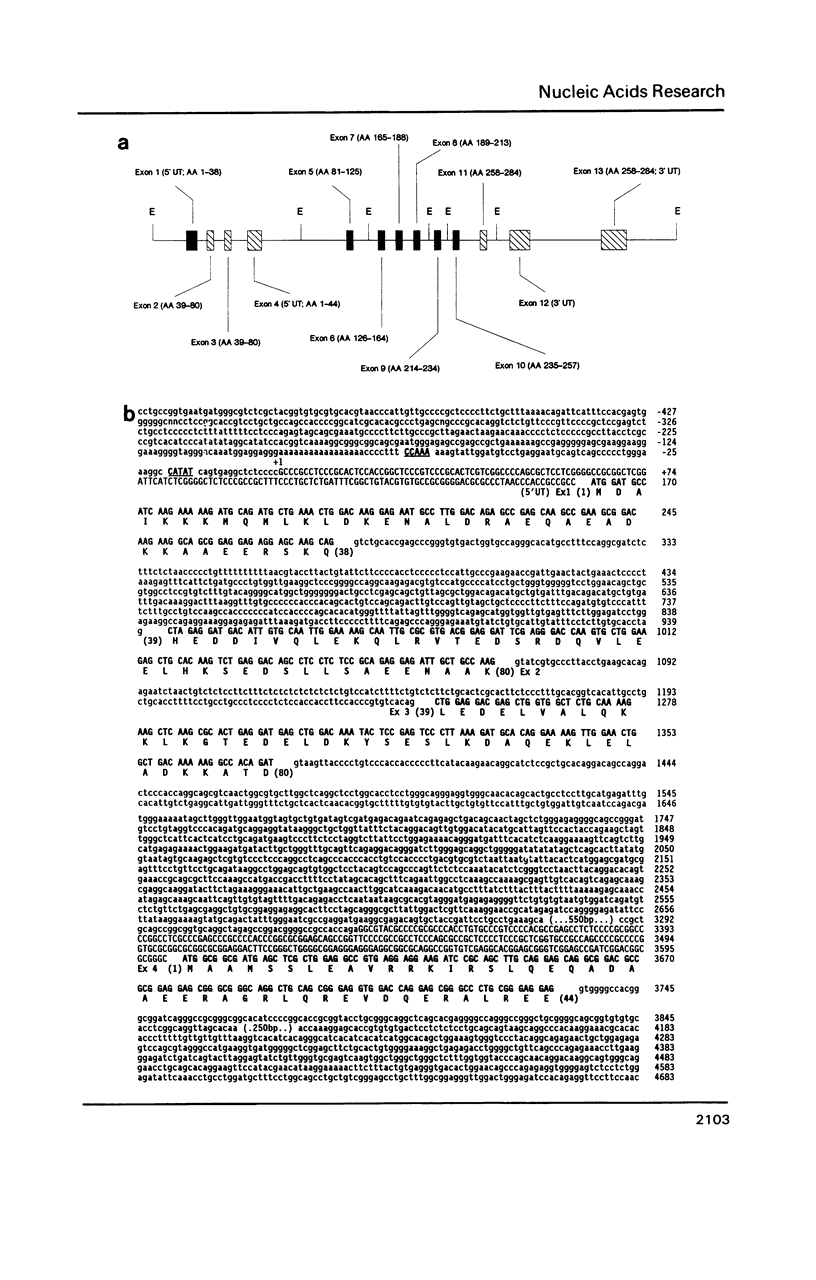
Sequence analysis of overlapping fragments from a quail genomic library has revealed a tropomyosin gene consisting of 13 exons spaced over about 18 kilobase pairs of DNA. Skeletal muscle and smooth muscle transcripts share the same 5' untranslated sequence and may initiate from the same promoter. However, the regions encoding amino acids 39-80 and 258-284 are specific to each muscle type. The two sets of exons encoding these regions undergo mutually exclusive alternative splicing in a tissue-specific manner as determined by Northern blots and S1-nuclease protection. Similarly, the 3' ends of the transcripts are different in skeletal muscle and smooth muscle, and each contains two polyadenylation signals which appear to be utilized in vivo. The avian alpha-tropomyosin gene is not expressed in cardiac muscle. The sequence of the gene shows great homology with other muscle-specific tropomyosins and includes a region homologous to the amino terminus of nonmuscle tropomyosins.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Basi G. S., Boardman M., Storti R. V. Alternative splicing of a Drosophila tropomyosin gene generates muscle tropomyosin isoforms with different carboxy-terminal ends. Mol Cell Biol. 1984 Dec;4(12):2828–2836. doi: 10.1128/mcb.4.12.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautch V. L., Storti R. V. Identification of a cytoplasmic tropomyosin gene linked to two muscle tropomyosin genes in Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7123–7127. doi: 10.1073/pnas.80.23.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Broschat K. O., Burgess D. R. Low Mr tropomyosin isoforms from chicken brain and intestinal epithelium have distinct actin-binding properties. J Biol Chem. 1986 Oct 5;261(28):13350–13359. [PubMed] [Google Scholar]
- Chacko S., Conti M. A., Adelstein R. S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):129–133. doi: 10.1073/pnas.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Bell G., Tischer E., DeNoto F. M., Ullrich A., Pictet R., Rutter W. J., Goodman H. M. Isolation and characterization of a cloned rat insulin gene. Cell. 1979 Oct;18(2):533–543. doi: 10.1016/0092-8674(79)90070-9. [DOI] [PubMed] [Google Scholar]
- Covington M., Fleenor D., Devlin R. B. Analysis of xanthine dehydrogenase mRNA levels in mutants affecting the expression of the rosy locus. Nucleic Acids Res. 1984 Jun 11;12(11):4559–4573. doi: 10.1093/nar/12.11.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Chemical and immunochemical characteristics of tropomyosins from striated and smooth muscle. Biochem J. 1974 Jul;141(1):43–49. doi: 10.1042/bj1410043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Haskell S. G. Use of a cDNA library for the study of mRNA changes during muscle differentiation. Dev Biol. 1983 Feb;95(2):476–483. doi: 10.1016/0012-1606(83)90049-0. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Flach J., Lindquester G., Berish S., Hickman K., Devlin R. Analysis of tropomyosin cDNAs isolated from skeletal and smooth muscle mRNA. Nucleic Acids Res. 1986 Nov 25;14(22):9193–9211. doi: 10.1093/nar/14.22.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallauer P. L., Hastings K. E., Baldwin A. S., Pearson-White S., Merrifield P. A., Emerson C. P., Jr Closely related alpha-tropomyosin mRNAs in quail fibroblasts and skeletal muscle cells. J Biol Chem. 1987 Mar 15;262(8):3590–3596. [PubMed] [Google Scholar]
- Hayashi J., Ishimoda T., Hirabayashi T. On the heterogeneity and organ specificity of chicken tropomyosins. J Biochem. 1977 May;81(5):1487–1495. [PubMed] [Google Scholar]
- Helfman D. M., Cheley S., Kuismanen E., Finn L. A., Yamawaki-Kataoka Y. Nonmuscle and muscle tropomyosin isoforms are expressed from a single gene by alternative RNA splicing and polyadenylation. Mol Cell Biol. 1986 Nov;6(11):3582–3595. doi: 10.1128/mcb.6.11.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Mahaffey J. W., Coutu M. D., Fyrberg E. A. Organization of contractile protein genes within the 88F subdivision of the D. melanogaster third chromosome. Cell. 1984 Jun;37(2):469–481. doi: 10.1016/0092-8674(84)90377-5. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Growth-dependent expression of dihydrofolate reductase mRNA from modular cDNA genes. Mol Cell Biol. 1983 Sep;3(9):1598–1608. doi: 10.1128/mcb.3.9.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S. Y., Sanders C., Smillie L. B. Amino acid sequence of chicken gizzard gamma-tropomyosin. J Biol Chem. 1985 Jun 25;260(12):7257–7263. [PubMed] [Google Scholar]
- Lewis W. G., Cote G. P., Mak A. S., Smillie L. B. Amino acid sequence of equine platelet tropomyosin. Correlation with interaction properties. FEBS Lett. 1983 Jun 13;156(2):269–273. doi: 10.1016/0014-5793(83)80511-0. [DOI] [PubMed] [Google Scholar]
- Lewis W. G., Smillie L. B. The amino acid sequence of rabbit cardiac tropomyosin. J Biol Chem. 1980 Jul 25;255(14):6854–6859. [PubMed] [Google Scholar]
- MacLeod A. R. Distinct alpha-tropomyosin mRNA sequences in chicken skeletal muscle. Eur J Biochem. 1982 Aug;126(2):293–297. doi: 10.1111/j.1432-1033.1982.tb06778.x. [DOI] [PubMed] [Google Scholar]
- MacLeod A. R., Houlker C., Reinach F. C., Smillie L. B., Talbot K., Modi G., Walsh F. S. A muscle-type tropomyosin in human fibroblasts: evidence for expression by an alternative RNA splicing mechanism. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7835–7839. doi: 10.1073/pnas.82.23.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod A. R., Houlker C., Reinach F. C., Talbot K. The mRNA and RNA-copy pseudogenes encoding TM30nm, a human cytoskeletal tropomyosin. Nucleic Acids Res. 1986 Nov 11;14(21):8413–8426. doi: 10.1093/nar/14.21.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V. The regulation of contractile activity in muscle. Biochem Soc Trans. 1979 Aug;7(4):593–617. doi: 10.1042/bst0070593. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., MacLeod A. R. Tissue-specific expression of the human tropomyosin gene involved in the generation of the trk oncogene. Nature. 1986 Aug 14;322(6080):648–650. doi: 10.1038/322648a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Opazo N., Nadal-Ginard B. Alpha-tropomyosin gene organization. Alternative splicing of duplicated isotype-specific exons accounts for the production of smooth and striated muscle isoforms. J Biol Chem. 1987 Apr 5;262(10):4755–4765. [PubMed] [Google Scholar]
- Ruiz-Opazo N., Weinberger J., Nadal-Ginard B. Comparison of alpha-tropomyosin sequences from smooth and striated muscle. Nature. 1985 May 2;315(6014):67–70. doi: 10.1038/315067a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura M., Saborío J. L. Separation of the mRNAs coding for different forms of chick embryo gizzard tropomyosin. J Biol Chem. 1982 Aug 10;257(15):8867–8871. [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Myosin-linked calcium regulation in vertebrate smooth muscle. J Mol Biol. 1976 Mar 25;102(1):75–92. doi: 10.1016/0022-2836(76)90074-7. [DOI] [PubMed] [Google Scholar]
- Stone D., Smillie L. B. The amino acid sequence of rabbit skeletal alpha-tropomyosin. The NH2-terminal half and complete sequence. J Biol Chem. 1978 Feb 25;253(4):1137–1148. [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Wieczorek D. F., Smith C. W., Nadal-Ginard B. The rat alpha-tropomyosin gene generates a minimum of six different mRNAs coding for striated, smooth, and nonmuscle isoforms by alternative splicing. Mol Cell Biol. 1988 Feb;8(2):679–694. doi: 10.1128/mcb.8.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]