Abstract
Although studies exploring relationships between obesity and cognitive impairment in the elderly are conflicting, literature suggests that overweight and obesity may be protective against cognitive impairment and dementia in older women. We examine the associations between changes in weight and waist circumference with global and domain-specific cognitive function in a large, well-defined cohort of 2283 older, post-menopausal women (age 65-79) prospectively followed through the Women's Health Initiative (WHI) Study of Cognitive Aging (WHISCA). We assessed the associations between changes in weight and waist circumference collected up to 5 years prior to WHISCA enrollment and mean levels of global and domain-specific cognitive performance across an average of 5.4 years of subsequent follow-up. There was a lack of associations between weight and cognition in women who remained stable or gained weight. The only significant relationships observed were in association with weight loss (p≤0.05), most likely signaling incipient disease. Moreover, cognition was not related to changes in waist circumference. Relationships were largely independent of initial BMI, self-reported caloric intake or dieting. The lack of associations between weight gain and cognition in women is consistent with the existent literature.
INTRODUCTION
With a rapidly growing elderly population, the prevalence of dementia is expected to increase exponentially (1). Risk for dementia may be even greater when coupled with obesity (2). Mounting evidence suggests that mid-life obesity is associated with a long-term increased risk of dementia (3-7) and cognitive decline (8-10) in later life. Moreover, correlates of mid- and later-life obesity, such as diabetes, hypertension, and cardiovascular disease, are also associated with cognitive impairment and dementia (11-17).
Studies exploring relationships between obesity, dementia and cognitive impairment in the elderly are conflicting. Literature suggests that overweight, obesity, and central adiposity may be protective against cognitive impairment and dementia in older women (18-23), and that low body mass index (BMI) may be associated with poorer cognition (24) and greater cognitive decline (25). Conversely, it is common for weight loss to precede the dementia diagnosis (26-29), perhaps as a result of pre-clinical pathophysiological changes (30). Further characterization of these relationships is clearly needed.
Women's Health Initiative (WHI) and its ancillary offer an unprecedented opportunity to examine relationships between changes in weight and waist circumference (WC) with cognition in a large, well-defined cohort of older women. We ask: 1) Is weight change associated with subsequent mean cognitive performance?, 2) Are similar relationships found for changes in waist circumference?, and 3) Do relationships vary depending on initial BMI or self-reported levels of caloric intake or dieting?
METHODS AND PROCEDURES
Participants
This is a cohort study of 2283 prospectively-followed women from the Women's Health Initiative Study of Cognitive Aging (WHISCA) (31), an ancillary study to the WHI placebo-controlled, randomized clinical trials of hormone therapy in postmenopausal women. On average, 3 years post WHI enrollment, women from 14 of 40 original WHI centers were invited to participate in WHISCA if they were English speaking and had not been classified as having probable dementia, with 67% of the women participating in these clinics enrolled (32, 33). At WHI enrollment, the women were 65 to 79 years of age. Sample characteristics at the time of WHI enrollment stratified by change in percent weight and waist circumference that occurred prior to WHISCA enrollment are described in Table 1. All participants provided written informed consent. Studies were approved by the National Institutes of Health and Institutional Review Boards of participating institutions. Participant selection and detailed study designs have been previously published (31, 34).
Table 1.
Characteristics of WHISCA women at WHI enrollment.
| Variable | N | Percent Change in Weight Mean (SE) |
|---|---|---|
| Age–yrs (Missing=0) | ||
| 65-69 | 1062 | 0.53 (0.18) |
| 70-74 | 845 | -0.20 (0.20) |
| 75+ | 376 | -0.59 (0.30) |
| P-value | P=0.002 | |
| Education (Missing=7) | ||
| < High school | 118 | 0.23 (0.55) |
| High school/GED | 488 | 0.05 (0.27) |
| > High school < 4 yr college | 936 | 0.34 (0.19) |
| ≥ 4 yr college | 734 | -0.31 (0.22) |
| p-value | P=0.17 | |
| Ethnicity (Missing=4) | ||
| American Indian/Alaskan native | 6 | -6.04 (2.39) |
| Asian/Pacific Islander | 26 | 1.17 (1.15) |
| Black/African-American | 142 | 0.11 (0.50) |
| Hispanic/Latino | 29 | -0.43 (1.09) |
| White, non-Hispanic | 2053 | 0.08 (0.13) |
| Other | 23 | -1.51 (1.22) |
| p-value | P=0.09 | |
| Smoking status (Missing=26) | ||
| Never | 1247 | -0.35 (0.19) |
| Former | 886 | 0.38 (0.19) |
| Current | 124 | 2.50 (0.53) |
| p-value | P<0.0001 | |
| Alcohol intake (Missing=2) | ||
| None | 1011 | 0.00 (0.19) |
| < 1 per day | 989 | 0.20 (0.19) |
| ≥1 per day | 281 | -0.15 (0.3) |
| p-value | P=0.61 | |
| Waist Hip Ratio (Missing=8) | ||
| < 0.80 | 802 | 0.43 (0.21) |
| ≥ 0.80 | 1473 | -0.11 (0.15) |
| p-value | P=0.04 | |
| Hypertension (Missing=0) | ||
| No | 1190 | 0.35 (0.17) |
| Yes | 1093 | -0.23 (0.18) |
| p-value | P=0.02 | |
| Prior CVD (Missing=0) | ||
| No | 2070 | 0.03 (0.13) |
| Yes | 213 | 0.49 (0.40) |
| p-value | P=0.27 | |
| Diabetes (Missing=2) | ||
| No | 2109 | 0.10 (0.13) |
| Yes | 172 | -0.31 (0.46) |
| p-value | P=0.39 | |
| Depressive symptoms (missing=124) | ||
| No | 2159 | 0.04 (0.14) |
| Yes | 386 | 0.14 (0.30) |
| p-value | P=0.77 | |
| Intervention assignment | ||
| CEE-Alone placebo | 450 | -0.15 (0.27) |
| CEE | 430 | -0.44 (0.28) |
| CEE+MPA Placebo | 681 | 0.46 (0.22) |
| CEE+MPA | 722 | 0.13 (0.22) |
| p-value | P=0.07 | |
Anthropometric Measurements
Trained and certified staff obtained anthropometric measurements at each WHI visit. Weight to the nearest 0.1 kg and height to the nearest 0.1 cm were recorded annually and used to calculate BMI, defined as weight in kilograms divided by the square of height in meters. Waist circumference at the natural waist or narrowest torso part and maximal hip circumference were measured to the nearest 0.1 cm. Waist circumference was measured on all women at baseline and year 1, and on a random sample of 25% of women at years 3 and 6. Caloric intake was assessed at WHI baseline using the Food Frequency Questionnaire (FFQ) (35). Dieting history was based on self-report.
Cognitive Assessments
Detailed cognitive assessments occurred during each WHISCA visit. Data in this report include cognitive assessments collected up until April, 2007. Tests were chosen based on their sensitivity to age- and hormone-related changes (32). Test battery and protocol details have been published previously (31). Table 2 lists the individual tests and cognitive domain grouping. Individual test scores were converted to z-scores, expressed as deviations from the cohort-wide mean at WHISCA enrollment divided by their standard deviations.
Table 2.
Components of the WHISCA cognitive battery.
| Domain | Tests | Mean (SD) of Tests (used in creating z-scores) |
|---|---|---|
| Global Cognition | 3MS score | 95.17 (4.35) |
| Verbal Knowledge | PMA total correct – 1/3 incorrect | 36.49 (9.80) |
| Verbal Fluency | Letter Fluency | 39.65 (12.48) |
| Category Fluency | 28.96 (6.27) | |
| Figural Memory | BVRT: total figures with errors | 7.14 (3.80)* |
| Verbal Memory | CVLT-A | 28.63 (6.38) |
| CVLT-A Long Delay | 9.12 (3.08) | |
| CVLT-A Short Delay | 8.37 (3.11) | |
| Attention and Working Memory | Digit Span Forward | 7.94 (2.05) |
| Digit Span Backward | 6.67 (2.01) | |
| Spatial Ability | CRT: Total correct – total incorrect | 55.52 (27.12) |
| Fine Motor Speed | Finger Tapping Test Total dominant + non-dominant hand | 37.44 (6.77) |
Abbreviations: 3MS=Modified Mini-Mental State; PMA=Primary Mental Abilities Vocabulary; BVRT=Benton Visual Retention Test; CVLT=California Verbal Learning Test; CRT=Card Rotation Test.
Higher scores reflect poorer performance
Covariates
Covariates include age, caloric intake, race/ethnicity, education, hypertension, smoking, history of stroke, heart disease, diabetes, alcohol intake, and WHI treatment assignment. The presence of depressive symptoms was defined as a score >0.009 (Burnam Index) (36). Potential moderators include baseline measures of BMI, WC, 3MS, self-reported dieting, caloric intake, and waist/hip ratio.
Statistical Analyses
Analyses were limited to women with less than ±20% change in weight (98%) or waist circumference (99%), to exclude possible errors or extreme conditions. Weight or waist circumference change is defined as the difference between the WHI baseline and last available measurement preceding WHISCA. Weight change was related to mean levels of global and domain-specific cognitive performance across an average of 5.4 years of WHISCA follow-up.
Associations between changes in weight or waist circumference and subsequent mean cognitive performance were modeled as follows: xij = β wi + αi + λ1 tij + λ2 tij2 + χik yik + εij , where xij is the test score of participant i at visit j, β is the regression coefficient linking percent weight changes to cognitive test scores, wi represents markers for weight change group (to test for differences among groups) prior to WHISCA enrollment for participant i, αi is an intercept term for participant i, tij is the time from WHISCA enrollment for participant i at visit j, λ1 and λ2 are regression coefficients to control for curvilinear learning effects, χik and yik parameterize the relationships between cognitive test scores and the remaining k covariates and εij denotes random errors. Because two different word lists for the CLVT test were used over time, a covariate term to distinguish these was included in models involving verbal memory scores. Maximum likelihood algorithms were used and an autocorrelation structure was adopted to express the longitudinal correlation of repeated measures. Models were fitted without and with adjustment for risk factors for dementia. Tests of interactions assessed whether relationships varied depending on baseline BMI, caloric intake, or self-reported dieting.
RESULTS
Anthropometric measurements collected through WHI follow-up and prior to WHISCA enrollment were available for 98% (2,256) of the sample. Table 1 examines the distribution of pre-WHISCA weight changes across subgroups defined by a range of risk factors for cognitive decline and dementia. Average weight at WHI enrollment was 74.1 kg with interquartile range 62.6 kg to 82.9 kg. Mean BMI was 28.6 kg/m2, with interquartile range 24.5 to 31.8 kg/m2, and the following distribution: 2.3% < 20.0 kg/m2, 27.3% from 20.0-24.9 kg/m2, 35.8% from 25.0-29.9 kg/m2, 21.6% from 30.0-34.9 kg/m2, and 13.0% ≥ 35.0 kg/m2. The percent weight change prior to WHISCA enrollment (follow-up weight minus baseline weight, divided by baseline weight and multiplied by 100) averaged 0.1%, with interquartile range -3.1% to 3.4%. The time span defining these changes ranged from 1.1 to 5.6 years, with mean 3.0 years and interquartile range of 2.5 to 3.5 years. Weight gains tended to occur in women who were younger, smokers at WHI baseline, had lower waist/hip ratios, or had no hypertension.
Table 2 provides the mean and standard deviations of the individual cognitive tests at the initial WHISCA visits, which were used to develop the standardized domain scores. These reflect an average of 5.4 assessments occurring 1.1 to 11.5 years following enrollment in the WHI, with interquartile range 4.0 to 7.0 years.
Women were grouped according to weight gain (≥ 5% gain), weight loss (≥ 5% loss), or remaining stable prior to WHISCA enrollment and their subsequent mean levels of cognitive function (standardized scores) were estimated with full covariate-adjustment (Table 3). Women with prior weight loss had mean (standard error) standardized global cognitive function scores of -0.04 (0.02), which were slightly lower than for women who had been weight stable or who had gained weight: 0.02 (0.01) and 0.03 (0.02), respectively, with p=0.04 for differences among groups. Differences among weight change groups were statistically significant (p≤0.05) for verbal knowledge, verbal fluency, and fine motor speed. Table 3 also included results from pairwise comparisons of mean cognitive function scores among weight change groups. When differences existed, these tended to be between the weight loss and weight stable groups, although for fine motor speed, the differences between the weight loss and weight gain groups also reached statistical significance. For no measure were differences between the weight stable and weight gain groups statistically significant.
Table 3.
Relationships between percent change in weight and standardized measures of average test-specific cognitive function by weight loss (≥5% loss), weight gain (≥5% gain), or stable weight.
| Mean (SE) | p-value1 | Significant Pairwise Differences2 | |
|---|---|---|---|
| 3MS | |||
| Weight loss | -0.02 (0.02) | ||
| Stable weight | 0.03 (0.02) | 0.09 | |
| Weight gain | 0.04 (0.03) | ||
| Verbal Knowledge | |||
| Weight loss | 0.09 (0.02) | ||
| Stable weight | 0.14 (0.02) | 0.002 | WL vs. SW |
| Weight gain | 0.14 (0.02) | ||
| Verbal Fluency | |||
| Weight loss | 0.05 (0.02) | ||
| Stable weight | 0.09 (0.02) | 0.02 | |
| Weight gain | 0.06 (0.03) | ||
| Figural Memory | |||
| Weight loss | 0.07 (0.02) | ||
| Stable weight | 0.11 (0.02) | 0.13 | |
| Weight gain | 0.13 (0.03) | ||
| Verbal Memory3 | |||
| Weight loss | -0.15 (0.02) | ||
| Stable weight | -0.12 (0.02) | 0.31 | |
| Weight gain | -0.14 (0.03) | ||
| Attention and Working Memory | |||
| Weight loss | 0.03 (0.02) | ||
| Stable weight | 0.04 (0.02) | 0.61 | |
| Weight gain | 0.02 (0.03) | ||
| Spatial Ability | |||
| Weight loss | 0.15 (0.02) | ||
| Stable weight | 018 (0.02) | 0.36 | |
| Weight gain | 0.16 (0.03) | ||
| Fine Motor Speed | |||
| Weight loss | 0.05 (0.02) | WL vs. SW | |
| Stable weight | 0.10 (0.02) | 0.01 | WL vs. WG |
| Weight gain | 0.13 (0.03) | ||
| Global Cognitive Function | |||
| Weight loss | 0.06 (0.02) | ||
| Stable weight | 0.10 (0.02) | 0.004 | WL vs. SW |
| Weight gain | 0.11 (0.02) | ||
WL = weight loss; WG = weight gain; SW = stable weight.
Mean standardized score over follow-up in standard deviation units. Adjustments were made for treatment assignment, baseline 3MS, a quadratic term for on-WHISCA time to control for a learning effect and all variables listed in Table 1.
Differences among weight change groups
P<0.05 based on Bonferroni-adjustment for three pairwise comparisons
Additional adjustment for CVR
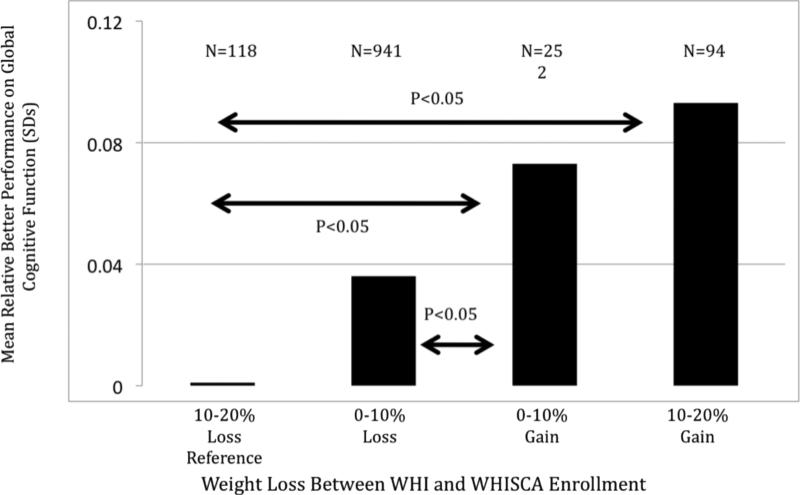
Figure 1 portrays results from analyses for different groupings of weight change. Here women are grouped according to the degree of any weight loss (<10% or ≥10%) or weight gain (<10% or ≥10%) and mean global cognitive function scores are computed with full covariate adjustment. As indicated on the figure, women with ≥10% weight loss performed significantly worse than either weight gain group, and those with 10% weight loss performed significantly worse than women gaining <10%. There was not a significant difference between the two weight gain groups.
Figure 1. Association between weight changes and global cognitive function.
Presented are fitted mean standardized global cognitive function scores from the full covariate model for women grouped according to prior changes in weight (p=0.002, overall). The pairwise differences between weight change groups that reach statistical significance (Bonferroni-adjusted p<0.05) are between 10-20% weight loss and each other weight change group and between 0-10% weight loss and 0-10% weight gain.
At WHI enrollment, women had a mean waist circumference of 89.2 cm with interquartile range 79.0 to 98.0 cm, with average percent change 0.0% with interquartile range - 3.6% to 3.4% prior to WHISCA. Changes in weight and waist circumference were modestly correlated r=0.35. Among the factors listed in Table 1, only two were significantly associated with changes in waist circumference. Women with waist hip ratios <0.80 averaged 1.37 cm increases while others averaged 0.71 cm decreases (p<0.0001). Mean waist circumference increased among women on placebo (0.47 cm for the CEE-Alone trial and 0.41cm for the CEE+MPA trial) and decreased for women on active therapy (-0.51 cm for CEE-Alone and -0.34 for CEE+MPA) (p = 0.008). Changes in waist circumference were not related to any of the tests of cognitive function, with or without full covariate adjustment (data not shown). The associations between weight changes and cognition were not materially altered by including waist circumference changes as an additional covariate and no interactions between weight and waist circumference changes in joint models of associations with cognition were significant.
Percent weight changes were inversely associated with baseline BMI: mean (SE) changes were 0.11 (0.84) for BMI < 20 kg/m2(N=53), 1.10 (0.25) for BMI 20-24 kg/m2 (N=621), 0.05 (0.21) for BMI 25-29 kg/m2 (N=814), -0.30 (0.27) for BMI 30-34 kg/m2 (N=491), and -1.32 (0.36) for BMI ≥35 kg/m2 (N=295). At WHI enrollment, 10.6% of women reported that they were currently following a low calorie diet. Reporting a low calorie diet was associated with lower cognitive test scores for attention and working memory (p < 0.001) and higher scores on fine motor speed (p = 0.03). When included in the regression models underlying Table 3, low calorie diet had no influence on estimates or inference tests and did not have significant interactions with associations involving weight changes.
The reported (FFQ) mean caloric intake of women at WHI enrollment was 1599 kilocalories (interquartile range 1146 to 1929 kilocalories). When quartile of intake (coded 1-4) was included as a covariate in regression models, it was significantly associated with global cognitive function (p=0.002), figural memory (p=0.002), verbal memory (p<0.001), and fine motor speed (p=0.04). In each case, the direction of association was positive: higher reported caloric intake was associated with better cognitive performance. Including this covariate did not materially influence the estimated regression coefficients relating changes in weight to mean cognitive performance. No interactions between weight changes and kilocalorie intake reached statistical significance.
DISCUSSION
In this cohort of generally healthy, community-dwelling, post-menopausal women, we find no differences in cognitive performance between women who gained weight or whose weight remained stable over an average of 3.5 years (range 1- 5.6 years). Worse cognitive performance, however, was associated with weight loss, potentially signaling increased risk for cognitive impairment, as weight loss is common in pre-dementia stages. The relationships were not modified by initial BMI, self-reported caloric intake or dieting.
A recent study by Kanaya and colleagues (11) reports no relationships between obesity and cognition in women, even after controlling for potential explanatory links, including metabolic risk factors, adipocytokines and sex hormone levels. Moreover, the Framingham study found little prospective evidence for both obesity and hypertension as risk factors for cognitive decline over a 4-6 year period in middle-aged and older women (37, 38), while the Health ABC study reported trends toward less cognitive change in relation to obesity (11). Our findings are largely in line with the above-mentioned studies – we report no negative relationships between weight gain and cognitive performance in our sample of relatively healthy older post-menopausal women.
Some have suggested that central obesity (waist circumference) is a better measure of obesity in older adults (39), given that BMI may not adequately represent fat accumulation due to concurrent decrease in muscle mass and bone density, and a proportionally higher increase in abdominal compared to peripheral fat with age. Changes in waist circumference were not related to global or domain-specific cognitive performance, although the results could potentially be affected by the loss in power given fewer available waist circumference data points.
Other potential limitations lie in the fact that our sample is not population-based, all are female and that we can not address possible effects in younger postmenopausal women. Also, we don't know whether the observed weight loss is a consequence of or predates age-related impairment or whether there was an increase in morbidity or mortality associated with weight loss that might signal changes due to some underlying pathology as opposed to the normal aging process. These limitations, however, should not undermine the unique aspects of our study, namely longitudinal assessments of a broad range of cognitive functions in a large number of high-functioning, community-dwelling older women who were assessed annually and extensively characterized through detailed prospective follow-up.
The literature suggests several possible mechanisms that may provide a clue to the causal pathways (12, 40). Adipose tissue, being the largest endocrine organ in the body and a hormonally active tissue, secretes a number of protein hormones and adipokines, all of which cross the blood-brain barrier, enter the central nervous system where there are appropriate receptors, and play a direct role in the development of insulin resistance and endothelial dysfunction, maintenance and regulation of body fat, and in learning and memory. Still, the link between obesity and body weight in general, and cognition is poorly understood. Future studies focusing on mechanisms linking body weight in isolation and obesity as a part of the metabolic syndrome (16) and cognition are imperative given that obesity is a modifiable potential risk factor.
In conclusion, the magnitudes of associations between weight change and cognitive performance in our cohort of older, post-menopausal women were small and of little clinical significance for an individual woman. Additional longitudinal studies are needed to confirm the associations, or the lack there of, between obesity and cognitive performance with age. Moreover, a larger number of participants or follow-up visits over longer intervals will be crucial in determining the sensitivity of different psychometric measures to weight change with greater confidence.
ACKNOWLEDGEMENTS
Lists of participating centers and investigator groups appear in articles cited. Short list of WHISCA investigators included the following: National Institute of Aging program office, Alan Zonderman, Susan M. Resnick; WHISCA central coordinating center, Sally Shumaker, principal investigator; Stephen Rapp, Mark Espeland, Laura Coker, Deborah Farmer, Anita Hege, Patricia Hogan, Darrin Harris, Cynthia McQuellon, Anne Safrit, Lee Ann Andrews, Candace Warren, Carolyn Bell, Linda Allred. WHISCA clinical sites included: WHI (Durham, NC), Carol Murphy; (Rush Presbyterian-St. Luke's Medical Center, Chicago, IL), Linda Powell; (Ohio State University Medical Center, Columbus, OH), Rebecca Jackson; (University of California at Davis, Sacramento, CA), John Robbins; (University of Iowa College of Medicine, Des Moines, IA), Robert Wallace; (University of Florida, Gainesville/Jacksonville, FL), Marian Limacher; (University of California at Los Angeles, Los Angeles, CA), Howard Judd; (Medical College of Wisconsin, Milwaukee, WI), Jane Kotchen; (The Berman Center for Outcomes and Clinical Research, Minneapolis, MN), Karen Margolis; (University of Nevada School of Medicine, Reno, NV), Robert Brunner; (Albert Einstein College of Medicine, Bronx, NY), Sylvia Smoller; (The Leland Stanford Junior University, San Jose, CA), Marcia Stefanick; (The State University of New York, Stony Brook, NY), Dorothy Lane; (University of Massachusetts/Fallon Clinic, Worcester, MA), Judith Ockene. The following investigators were the original investigators for these sites: Mary Haan, Davis, CA; Richard Grimm, Minneapolis, MN; Sandra Daugherty, deceased (Reno, NV). The WHI program office (National Heart, Lung, and Blood Institute, Bethesda, MD) included Barbara Alving, Jacques Rossouw, Linda Pottern. The WHI central coordinating center investigators (Fred Hutchinson Cancer Research Center, Seattle, WA) included Deborah Bowen, Gretchen VanLom, Carolyn Burns.
Funding/Support: WHISCA is funded by the National Institute on Aging Contract N01-AG-9-2115 and is supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services, through Contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. Wyeth Pharmaceuticals, Inc., St. Davids, Pennsylvania, provided the active and placebo hormone therapy formulations. Drs. Driscoll and Resnick are supported by the Intramural Research Program, National Institute on Aging, National Institutes of Health.
REFERENCES
- 1.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity. 2007 Jan;15(1):216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 3.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of body mass index and risk for Alzheimer's disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 4.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 5.Luchsinger JA, Gustafson DR. Adiposity and Alzheimer's disease. Curr Opin Clin Nutrit Metabolic Care. 2009;12:15–21. doi: 10.1097/MCO.0b013e32831c8c71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: a 27 years longitudinal population based study. BMJ. 2005;1330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cournot M, Marquié JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 9.Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass indices over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutrit. 2009;89:601–607. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 11.Kanaya AM, Lindquist K, Harris TB, et al. Health ABC Study. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch Neurol. 2009;66:329–335. doi: 10.1001/archneurol.2008.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life cognitive impairment: a population-based study. Neurology. 2001;56:1683–9. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 13.Launer LJ, Masaski K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 14.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia Aging Study. Neurobiol Aging. 2000;21:51–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 15.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 16.Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Dis Assoc Disord. 2007;21:167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- 17.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Kreuger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 18.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. The implications of body fat mass and fat distribution for cognitive function in the elderly. Obesity Res. 2004;12:1519–1526. doi: 10.1038/oby.2004.189. [DOI] [PubMed] [Google Scholar]
- 19.Kerwin D, Zhang Y, Kotchen JM, et al. The cross-sectional relationship between body mass index and cognitive function in postmenopausal women enrolled in the Women's Health Initiative (WHI). J Am Geriatr Soc. 2010;28:1427–1432. doi: 10.1111/j.1532-5415.2010.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late- life body mass index and dementia: The Kame Project. Neurology. 2009;72(20):1741–1746. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo HK, Jones RN, Milberg WP, et al. Cognitive function in normal-weight, overweight, and obese older adults: An analysis of the Advanced Cognitive Training for Independent and Vital Elderly Cohort. J Am Geriatr Soc. 2006;54:97–103. doi: 10.1111/j.1532-5415.2005.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nourhashemi F, Andrieu S, Gillette-Guyonmet S, et al. Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. J Am Geriatr Soc. 2002;50:1796–1801. doi: 10.1046/j.1532-5415.2002.50507.x. [DOI] [PubMed] [Google Scholar]
- 23.Nourashemi F, Deschmaps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–9. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 24.Brownbill RA, Ilich JZ. Cognitive function in relation with bone mass and nutrition: cross-sectional association in postmenopausal women. BMC Women's Health. 2004;4:1–8. doi: 10.1186/1472-6874-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deschamps V, Astier X, Ferry M, Rainfray M, Emeriau JP, Barberger-Gateau P. Nutritional status of health elderly persons living in Dordogne, France, and relation with mortality and cognitive or functional decline. Eur J Clin Nutr. 2002;56:305–312. doi: 10.1038/sj.ejcn.1601311. [DOI] [PubMed] [Google Scholar]
- 26.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 28.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 29.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 30.Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 31.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clinical Trials. 2004;1:440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 32.Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 33.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91(5):1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 34.Resnick SM, Espeland MA, An Y, et al. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab. 2009;94:4152–61. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 36.Burnam M, Wells K, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obesity. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 38.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino BD. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. NeurobiolAging. 2005;26S:S11–S16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Whitmer RA. The epidemiology of adiposity and dementia. Curr Alz Res. 2007;4(2):117–122. doi: 10.2174/156720507780362065. Review. [DOI] [PubMed] [Google Scholar]
- 40.Horber FF, Gruber B, Thomi F, Jensen EX, Jaeger P. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition. 1997;13(6):524–534. doi: 10.1016/s0899-9007(97)00031-2. [DOI] [PubMed] [Google Scholar]