Abstract
Objective
Maternal immunization with oxidized low-density-lipoprotein prior to pregnancy prevents pathogenic in utero programming by gestational hypercholesterolemia, but it is unknown whether gestational hypercholesterolemia and maternal immunization affect similar pathways.
Study Design
A lipidomic approach was used for unbiased plasma eicosanoid profiling in adult offspring of immunized and non-immunized normo- or hypercholesterolemic rabbit mothers.
Results
Gestational hypercholesterolemia was associated with increased levels of some eicosanoids formed by the cyclooxygenase and 12-lipoxygenase pathways only (including TXB2, PGF2α, PGE2, and PGD2). Immunization of hypercholesterolemic or normocholesterolemic mothers reduced 9 of 14 eicosanoids of the cyclooxygenase pathway, 21 of 23 eicosanoids of the 5- and 12-lipoxygenase pathways (e.g. 5-HETE, HXB3, 12-HETE), 8 of 19 eicosanoids of the cytochrome P-450 pathway and all metabolites of the nonenzymatic pathway.
Conclusion
Maternal immunization not only counteracts in utero programming by gestational hypercholesterolemia but reduces a broad range of eicosanoid modulators of immunity and inflammation in offspring.
Keywords: Cardiovascular disease, developmental programming, eicosanoids, immunization, inflammation, rabbits
INTRODUCTION
It is now well established that in utero conditions influence the susceptibility to cardiovascular disease later in life 1;2, but the mechanisms involved are largely unknown. Maternal hypercholesterolemia, even if limited to pregnancy, is associated with enhanced formation of fatty streaks in arteries of premature human fetuses and increased atherogenesis in normocholesterolemic children 3–5. Diet-induced gestational hypercholesterolemia causes similar atherogenic programming in genetically more homogeneous animal models. Increased atherogenesis in offspring of humans, rabbits and mice differs between arterial sites, and may be either spontaneous or require the presence of additional postnatal risk factors to manifest itself 6–9. In models resistant to atherosclerosis, e.g. rats, maternal high-fat, high-cholesterol diets program reduced vascular reactivity and increased insulin resistance in offspring 10–12.
Oxidative stress plays an important role in atherogenic in utero programming. Maternal hypercholesterolemia is associated with increased lipid peroxidation, formation of reactive oxygen species and inflammation, and affects oxidative stress in both the placenta and fetus 13. Conversely, cholesterol-lowering or antioxidant treatment during pregnancy reduce atherogenic programming, even though antioxidants do not affect maternal cholesterol levels 6;7;14. Protection against oxidative stress may also contribute to the marked reduction of atherosclerosis observed in offspring of rabbits and mice whose mothers were immunized with oxidized LDL (OxLDL) prior to pregnancy 15. Such immunization gives rise to high-titered antibodies to oxidation-specific epitopes, which form immune complexes with LDL particles carrying such epitopes on their apolipoproteins or oxidized phospholipids, and thus eliminate them from the circulation 16.
Although maternal-fetal cholesterol transport mechanisms have been elucidated 17;18, almost nothing is known about the fetal cells that are programmed by hypercholesterolemia or other gestational dysmetabolic conditions, nor about the mechanisms by which developmental programming actually modulates offspring diseases. Proposed mechanisms include epigenetic programming 19;20, altered mRNA expression or activity of antioxidant enzymes 8, altered cell differentiation and proliferation induced by the in utero environment alone or in conjunction with inherited genetic traits,21;22 altered mitochondrial function 12, growth restrictions due to protein restrictions 23, and in utero immune programming 15. Indeed, maternal OxLDL-immunization prior to pregnancy not only reduces atherogenesis in offspring, but also programs B-cell dependent immune responses in offspring 15. These include increased splenic presence of specific B cell populations, increased formation of oxidation-specific IgM antibodies and IgM-LDL complexes, and increased specific IgG and IgM responses in naïve offspring subjected to OxLDL challenge. Although increased titers of such antibodies may have contributed, the substantial reduction of atherosclerosis in offspring of immunized mothers 15 indicates the involvement of other antiatherogenic mechanisms, e.g. attenuation of inflammation. Identifying the mechanisms through which gestational hypercholesterolemia and maternal immunization may affect postnatal disease is rendered difficult by the fact that neither the nature of the in utero programming nor the cells involved are known. Systemic factors programmed in utero, such as those modulating immunity and inflammation, are of particular interest, because they may influence not just atherogenesis but also as insulin resistance and diabetes.
To determine whether in utero programming affects systemic levels of bioactive lipids in adult offspring, to identify candidate compounds for future investigations of causality, and to establish whether programming by gestational hypercholesterolemia and maternal immunization affects similar cells and pathways, we analyzed plasma levels of eicosanoids in adult rabbit offspring, using a lipidomic approach.
Eicosanoids are formed from polyunsaturated fatty acids by enzymatic or non-enzymatic oxygenation 24;25. Strictly defined, eicosanoids are derived from twenty-carbon fatty acids, but the term is now more broadly used to include related metabolites from virtually all long-chained polyunsaturated fatty acids. Eicosanoids are produced by most cells, typically in response to stimulation, and regulate a wide range of physiological processes through complex interrelated signaling networks 26. Three major enzymatic pathways lead to eicosanoid production, i.e., the cyclooxygenase (COX), lipoxygenase (LOX), and epoxygenase or cytochrome P450 (CYP) pathways. Arachidonic acid (AA) is a major precursor of eicosanoids, and the three pathways are therefore collectively known as the arachidonate cascade. In addition, autoxidation of polyunsaturated fatty acids through non-enzymatic pathways also generates bioactive lipids commonly used as biomarkers of oxidative stress 27. Prostanoids, including prostaglandins (PGs) and thromboxanes (TXs), are derived from the COX pathway (the target of non-steroidal anti-inflammatory drugs) 28;29. Leukotrienes, lipoxins and hepoxilins are produced by individual or sequential actions of the various lipoxygenases, and hydroxylated and epoxygenated metabolites of AA are formed by the enzymes of the CYP pathway 30.
We previously developed a liquid chromatography/mass spectrometry (LC/MS) method for the quantitative analysis of the entire spectrum of eicosanoids. This method was now used to assess the in utero programming effects of gestational hypercholesterolemia and maternal immunization on these modulators of immunity and inflammation, vasoconstriction and platelet aggregation. Plasma samples were from a previous large experiment, which had established that maternal hypercholesterolemia enhances and maternal immunization reduces atherosclerosis in rabbits 15.
METHODS
Ethics statement
All animal work was carried out under a protocol conforming with all applicable guidelines and approved by the Institutional Animal Care and Use Committee of UCSD (Protocol #S00041).
Experimental design
Plasma eicosanoids were determined in 4 representative subgroups of NZW rabbits (n=8–10 each, both males and females) from a large study on developmental immune programming 15. Groups differed only with regard to maternal treatment: 1. offspring of non-immunized normocholesterolemic mothers (NC); 2. offspring of non-immunized mothers with diet-induced hypercholesterolemia before and during gestation (HC); 3. offspring of OxLDL-immunized normocholesterolemic mothers (immNC); and 4. offspring of OxLDL-immunized hypercholesterolemic mothers (immHC).
Detailed descriptions of diets, antigen preparation and immunizations, as well as the assays used to assess the immunological effects of immunization in mothers and offspring and to measure aortic atherosclerosis in offspring are provided in reference 15. In brief, mothers fed regular chow were immunized with an equal mixture of homologous malondialdehyde-modified LDL and copper-oxidized LDL containing a broad spectrum of oxidation-specific epitopes, including oxidized phospholipids 31;32. The primary immunization consisted of s.c. injections of 200 μg OxLDL per kg body weight in complete Freund’s adjuvant (FA) and was followed by 2–3 biweekly i.m. boosts with the same amount of antigen in incomplete FA. Once a marked increase in antibody titer had been confirmed after the last boost, mothers of the HC groups were switched to a diet containing 9% fat, 17% protein, 57% carbohydrate, and 16% fibers (Harlan Teklad diet 7009 supplemented with 6.5% corn oil). Initially, 0.15% cholesterol was added to the diet, dissolved in ether. Total plasma cholesterol (TC) was determined after two weeks and the cholesterol concentration added to the diet was individually adjusted, if needed to maintain the animal in the target TC range (300–400 mg/dl). Females were then mated and TC during pregnancy was determined after 2.5 weeks. After delivery, all mothers were switched to the same atherogenic diet later fed to offspring (Harlan Teklad Rabbit Diet #7009 supplemented with 1.5% corn oil) containing 4% total fat, 17% protein, 57% carbohydrate, and 16% fibers).
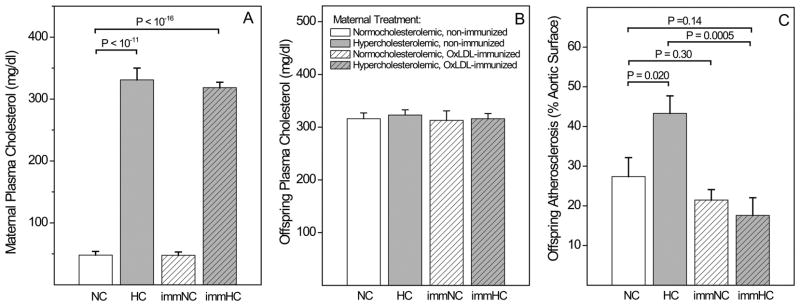
Offspring of all maternal groups were weaned at 4 weeks and then fed the above diet (with individually adjusted cholesterol, if needed to maintain a target TC of 350 mg/dl) for 5 months. Plasma samples used in the present study were obtained under a rigorous operating procedure. Blood was smoothly drawn into EDTA-containing sterile syringes from a rabbit ear vein, centrifuged for 10 minutes at 4° C, aliquoted, frozen and stored at −80°C until use. Atherosclerosis in the entire aorta was determined at age 6 months 15. Offspring groups contained roughly equal numbers of males and females, which were analyzed together, because previous studies did not indicate gender differences in atherosclerosis in rabbits. The maternal and offspring cholesterol and atherosclerosis data of the subgroups used for the present study are shown in Fig. 1.
Fig. 1. Characterization of the study population: Effect of maternal immunization and gestational cholesterol level on offspring atherosclerosis.
(A) Maternal total plasma cholesterol levels. (B) Time-averaged cholesterol level of offspring during the 5 months of dietary intervention. (C) Atherosclerosis in the entire aorta in 6 months old offspring, measured as percentage of Sudan-positive surface area. Data represent the subgroup of those animals from reference 15 in which eicosanoids were profiled.
Eicosanoid extraction
All solvents were of chromatography purity. Eicosanoids used for primary standards in standard curves as well as their deuterated analogues were from Cayman Chemicals and Biomol. The potential formation of eicosanoids during sample collection and processing and the effect of freezing/thawing were tested with a mix of COX and LOX inhibitors. For this purpose, blood was collected from several animals and drawn directly into a mixture consisting of EDTA, the COX inhibitor ketorolac (10 μM final) and the non-specific LOX inhibitor NDGA (10 μM final). In parallel, blood was collected into EDTA without these inhibitors, and both plasma preparations were subjected to three cycles of freezing and thawing. Eicosanoid levels were then examined as outlined below. No significant differences in eicosanoid levels were detected between plasma prepared with or without inhibitors. To test the effect of long-term storage on eicosanoid profiles, identical plasma samples stored at −80°C were analyzed six months apart. We did not detect any significant changes in eicosanoid levels attributable to storage 33. None of the samples showed evidence of hemolysis, which may lead to platelet activation and increase COX eicosanoids. For extraction, plasma was thawed on ice and 0.9 ml were centrifuged at 6000 x g for 1 min to remove any cellular constituents. To the cleared plasma, 100 μl of a cocktail of internal standards consisting of 26 deuterated eicosanoids was added, each at 10 ng dissolved in ethanol. Samples were then purified by solid phase extraction on Strata-X 33μ polymeric reversed phase columns (Phenomenex). Columns were activated with methanol, washed with water and the samples were applied. After washes with 10% methanol, samples were eluted with 1 ml of 100% methanol. The eluant was dried under vacuum, redissolved in 100 μl of Buffer A consisting of water:acetonitril:formic acid = 63:37:0.02 (v/v/v) and immediately used for LC/MS analysis.
Reverse-phase liquid chromatography (LC) and mass spectrometry (MS)
Eicosanoids were separated by reverse-phase LC on a Synergy C18 column (2.1×250 mm, Phenomenex) at a flow rate of 300 μl/min at 50 °C 34. The column was equilibrated in Buffer A and 40 μl of sample was injected via a Pal auto-sampler (Leap Technologies) set to maintain samples at 4°C to minimize degradation of eicosanoids during queuing for analysis. Samples were eluted with a step gradient to 100% Buffer B (acetonitril:isopropanol=50:50 (v/v) over a period of 20 min. The eluted eicosanoids were analyzed using an on-line tandem quadruple mass spectrometer (ABI 4000 Q-Trap, Applied Biosystems) operated in negative ion mode via multiple reaction monitoring (MRM) with the electrospray voltage set at −4.5 kV, and the turbo ion spray source at 525°C. Collisional activation of eicosanoid precursor ions used nitrogen as a collision gas. Eicosanoids were measured using precursor/product (MRM) pairs, and the MS analysis was divided into periods consisting of 90 sec each to maximize the duty cycle. The declustering potential and collision energy for each eicosanoid was optimized for maximal signal strength. Eicosanoids were identified by matching their MRM signal and LC retention time with those of pure standards. A complete list of standards, instrument settings and MRM pairs can be found on www.lipidmaps.org under “resources”.
Quantitation of eicosanoids
Eicosanoids were quantitated by the stable isotope dilution method 35. Identical amounts of deuterated internal standards were added to each sample and to all the primary standards used to generate standard curves. The use of internal standards neutralizes differences in extraction efficiencies and mass spectrometry ion suppression. To calculate the amount of eicosanoids in a sample, ratios of peak areas between endogenous eicosanoids and matching deuterated internal eicosanoids were calculated. Ratios were converted to absolute amounts by linear regression analysis of standard curves generated under identical conditions. 140 primary standards and 26 deuterated internal standards were applied to generate standard curves for quantitation. Extraction controls were performed to offset small amounts of unlabeled eicosanoid contaminations in the deuterated internal standards. Final values were normalized to plasma volume and expressed as pmol/ml of plasma. Currently, we can quantitate over 140 eicosanoids at fmol levels.
Statistics
Data were analyzed with SSPS version 16.0. Results are expressed as the mean±SEM. Normal-distributed data were compared by unpaired T-test. P values < 0.05 were considered significant.
RESULTS
Characterization of the study population
Data on maternal and offspring cholesterol and atherosclerosis in the subgroups of the present study (Fig. 1A–C) were in line with those published for the larger cohort 15. In particular, maternal TC was similar in the non-immunized and immunized normocholesterolemic groups (47.7± 6.1 and 47.8± 5.3 mg/dl) and hypercholesterolemic groups (318.4±8.7 and 330.6±19.4 mg/dl respectively), but significantly higher in the latter. The time-averaged TC levels of all four offspring groups were very similar, thus ruling out differences in cholesterol metabolism as an explanation for differences in eicosanoid levels. Aortic atherosclerosis reflected a marked atherogenic effect of gestational hypercholesterolemia, in agreement with preceding animal and human studies 3–5;7;9;10;14;15;36 which was prevented by maternal immunization prior to pregnancy 3–5;7;9;10;14;15;36. Note that the protective effect of maternal immunization on atherosclerosis was significant in offspring of hypercholesterolemic mothers (17.6±4.4. % in the immHC vs. 43.3±4.4 % in the HC group, P=0.0005), but not in the NC group (21.5±2.5 % in the immNC vs. 27.4±4.8 % in the NC group, P=0.30).
Effect of in utero conditions on plasma levels of arachidonic acid
The induction of phospholipase A2 and subsequent release of arachidonic acid from membrane phospholipids of leukocytes is a hallmark of inflammation 25;37. Most of the free arachidonic acid remains in the cell and constitutes the major precursor of eicosanoids, but some of it is secreted and may serve as an indicator of inflammatory responses 38. Based on the observation of increased plasma levels of several oxidized fatty acids, including 10-OH arachidonic acid, in offspring of hypercholesterolemic mothers 6;7, we expected gestational hypercholesterolemia to be associated with proinflammatory conditions and increased levels of free arachidonic acid. Gestational hypercholesterolemia tended to be associated with an increase in offspring plasma free arachidonic acid, whereas maternal immunization reduced it (Fig. 2). This was particularly pronounced in the immunized hypercholesterolemic group, whose levels were lower than those of both hypercholesterolemic and normocholesterolemic controls (P=0.002 and P=0.005, respectively).
Fig. 2.

Effect of maternal immunization and gestational hypercholesterolemia on plasma levels of arachidonic acid in offspring at age 6 months.
Eicosanomics analysis of the effect of in utero programming
Comprehensive profiling by LC/MS identified 64 eicosanoids at detectable levels, which were grouped into the four major pathways leading to their formation, i.e., COX, LOX, CYP, and non-enzymatic pathways. For initial analysis, the effect of gestational hypercholesterolemia was assessed as the relative change of each plasma eicosanoid in offspring of nonimmunized hypercholesterolemic mothers over that of normocholesterolemic mothers (HC/NC ratio) (Fig. 3). Similarly, the effect of prior maternal immunization on normocholesterolemic pregnancy was assessed as the immNC/NC ratio, and the effect of immunization on hypercholesterolemic pregnancy as the immHC/HC and immHC/NC ratios. Relative comparisons were made between the mean values of various groups. Complete plasma concentrations for all eicosanoids are listed in Table 1. (Note to the Copy Editor: Please place the table, which was submitted as a “supplement” after all figures, not here)
Fig. 3. Relative changes in plasma eicosanoids induced by maternal immunization and gestational hypercholesterolemia.
Heat map indicating relative changes in eicosanoids generated by the COX, LOX, CYP, and nonenzymatic pathways. The effect of maternal hypercholesterolemia (column left of divider) is indicated as the ratio of plasma levels in offspring of non-immunized hypercholesterolemic mothers over those of normocholesterolemic controls (HC/RC). The effects of immunization prior to pregnancy were assessed as immNC/NC, immHC/HC, and immHC/NC ratios (columns right of divider). Relative comparisons were made between the mean values of various groups.
Marked effects of in utero programming were apparent for a surprisingly high proportion of eicosanoids. Maternal hypercholesterolemia increased plasma levels of major COX-derived prostaglandins and some LOX metabolites. Within the COX pathway, the relative increase was most prominent for thromboxane B2 (TXB2). TXB2 is formed by non-enzymatic hydrolysis of the biologically active but short-lived TXA2, and is therefore used to estimate thromboxane secretion. Strong activation of the thromboxane arm of the COX pathway in offspring of hypercholesterolemic mothers was further confirmed by increased levels of 12-hydroxyheptatrienoic acid (12-HHT), one of the primary AA metabolites generated by thromboxane synthase in platelets, concurrently with TXA2 39. Other major prostaglandins and their catabolites were also significantly increased, including prostaglandin E2 (PGE2). Prostaglandins F2α (PGF2α) and D2 (PGD2) also tended to be increased. The effect of gestational hypercholesterolemia on the LOX pathway was limited to 12/15 LOX products, whereas 5 LOX metabolites were not increased. In contrast to gestational hypercholesterolemia, which only affected selected pathways, maternal immunization resulted in a striking reduction of the vast majority of eicosanoids generated by all four pathways (Fig. 3).
Comparative effects of in utero programming on specific eicosanoid pathways
For a direct quantitative comparison of the impact of maternal immunization and gestational hypercholesterolemia on eicosanoid plasma levels, important metabolites generated by each of the four pathways were then considered separately. Gestational hypercholesterolemia generally raised primary COX metabolites, TXB2, PGF2α, PGE2, and PGD2. In contrast, maternal immunization consistently reduced the levels of these pro-inflammatory mediators (Fig. 4A–D). As already noted for AA, this was particularly impressive in the immunized hypercholesterolemic group, whose levels were often lower not only than those of the nonimmunized hypercholesterolemic group, but also those of normocholesterolemic controls. The same was true for many downstream metabolites (Table 1). To facilitate interpretation of the results, we considered maternal immunization to have reduced a particular eicosanoid when either immHC, or immNC, or both, showed a statistically significant reduction, compared to non-immunized control groups. This was the case for 9 of 14 eicosanoids of the COX pathway. Of note, prostanoids undergo multiple steps of rapid catabolic conversions. Despite the resulting variability in some downstream metabolites, results indicate that maternal hypercholesterolemia increased and maternal immunization decreased primary eicosanoids of the COX pathway.
Fig. 4. In utero programming effects on offspring plasma levels of major eicosanoids generated by the cyclooxygenase pathway.
(A) Thromboxane B2 (TXB2). (B) Prostaglandin F2α (PGF2α). (C) Prostaglandin E2 (PGE2). (D) Prostaglandin D2 (PGD2). n=8–10 per group.
The effects of maternal hypercholesterolemia on products of the LOX pathway were more selective than those of the COX pathway. None of the 5-LOX-derived metabolites increased in offspring of hypercholesterolemic mothers. In fact, 5-HETE, was actually lower than in offspring of normocholesterolemic mothers (Fig. 5A). We also screened for all leukotrienes, including leukotriene B4 (LTB4), LTC4, LTD4 and LTE4, but did not detect any of these metabolites formed by enzymatic conversion of LTA4. However, we found low levels of non-enzymatic products of the short-lived precursor of all leukotrienes, LTA4, including trans leukotriene B4 (6t LTB4). Maternal hypercholesterolemia did not alter plasma levels of 6t LTB4 in offspring (Fig. 5B). Maternal immunization, however, significantly reduced the levels of both 5-HETE and 6t LTB4 (Figs. 5A,B). In contrast to 5-LOX, gestational hypercholesterolemia tended to activate 12-LOX activity, as shown for two representative metabolites, 12-HETE and hepoxilin B3 (HXB3) (Fig. 5C,D). Maternal immunization robustly suppressed these metabolites. Altogether, significant reductions by maternal immunization were seen for 20 of the 23 LOX eicosanoids (Table 1).
Fig. 5. In utero programming effects on offspring plasma levels of major eicosanoids generated by the lipoxygenase (LOX) pathway.
(A) 5-hydroxyeicosatetraenoic acid (5-HETE), the main metabolite of 5-LOX. (B) 6-trans leukotriene B4 (6t LTB4), a surrogate of LTA4, the precursor of all leukotrienes. (C) 12-hydroxyeicosatetraenoic acid (12-HETE) and (D) hepoxilin (HXB3), two major metabolites of the 12-LOX pathway.
The CYP pathway was least affected by maternal treatment. Although a majority of metabolites showed a trend towards an increase by gestational hypercholesterolemia, none reached statistical significance. In contrast, 6 of 18 metabolites showed a significant effect of immunization, including 5,6-EET, 8,9-EET, and 14,15 EET (Fig. 6A–C). All 7 metabolites generated by the nonenzymatic pathway (e.g., 5iso PGF2α VI, Fig. 7), were also consistently decreased in offspring of immunized mothers, suggesting that maternal immunization reduced offspring oxidative stress.
Fig. 6. In utero programming effects on offspring plasma levels of major eicosanoids generated by the cytochrome P-450 (CYP) pathway.
(A) 5,6-epoxyeicosatrienoic acid (5,6-EET). (B) 8,9-EET. (C) 14,15 EET.
Fig. 7.

In utero programming effects on offspring plasma levels of 5iso PGF2α VI, an eicosanoid generated by the nonenzymatic pathway.
Correlation between plasma eicosanoids and atherosclerosis
The source of plasma eicosanoids cannot be determined, as most cells are capable of generating them 24. As shown in Fig. 1C, maternal interventions resulted in marked differences of aortic atherosclerosis in offspring, which may be both a consequence of increased pro-atherogenic eicosanoids in the circulation and a contributor to their plasma levels (via secretion by endothelial cells or leakage from intimal leukocytes). When mean data for each group were used, the level of free arachidonic acid correlated with atherosclerosis (R2=0.96, P<0.02). Similarly, all major COX metabolites were correlated (R2=0.83 for TXB2, 0.98 for PGF2α, 0.99 for PGE2, 0.96 for PGD2, 0.88 for PGB2, and 0.78 for PGJ2;P<0.02 for all except TXB2). Some 12-LOX metabolites showed similar associations (R2=0.98 for 12-HETE, 0.93 for 12-HETE, and 0.91 for HXB3)with lesser R2 values for most others. 5-LO metabolites showed very poor correlations, and CYP metabolites showed no consistent association. Correlation coefficients were considerably lower when individual data were used.
COMMENT
Results established that in utero programming has a profound impact on the plasma levels of many eicosanoids affecting inflammation, immunity, vasoconstriction and coagulation. Although the biological half-life of many eicosanoid metabolites is short and the physiological effect differs even among metabolites of the same pathway, data clearly revealed two distinct patterns of effects: one for gestational hypercholesterolemia, an opposite and much broader one for maternal immunization prior to pregnancy. Both involved a surprisingly large number of metabolites generated by more than one pathway, and were broadly consistent with the proatherogenic effect of maternal hypercholesterolemia and the athero-protective effect of maternal immunization. One of the challenges of lipidomics is the integration of large amounts of data into pathophysiological disorders via causally linked pathways. The lipidomic approach has, however, identified a number of eicosanoids that are programmed in utero and that are candidates for further studies of causality, because the programmed changes were extensive and because their known biological effects (discussed below) were consistent with the overall detrimental effect of gestational hypercholesterolemia and the protective effect of immunization.
Gestational hypercholesterolemia was associated with increased plasma levels of free arachidonic acid and many eicosanoids formed by the COX and 12-LOX pathways. Release of arachidonic acid from cell membranes of platelets and endothelial cells occurs in response to inflammatory signals 40;41 and may therefore be indicative of proinflammatory conditions 42. It may also reflect increased intracellular levels of arachidonic acid. This assumption is supported by the increase of COX metabolites, in particular TXA2 (measured by its more stable metabolite, TXB2), 12-HHT, and PGE2. TXA2 formed in platelets and endothelial cells by cyclooxygenase 1 promotes platelet adhesion and aggregation 43;44.
Gestational hypercholesterolemia did not increase leukotrienes or other metabolites formed by 5-LOX. Leukotrienes are best known for their role in asthma and bronchitis but are involved in many other inflammatory conditions. None of the leukotrienes were increased to detectable levels by gestational hypercholesterolemia, and 5-HETE, the precursor of the bioactive 5-oxo eicosatetraenoic acid (5-oxo ETE), was actually decreased. In contrast, gestational hypercholesterolemia increased major metabolites of 12-LOX, such as 12-HETE. Manipulations of 12/15-LOX activity in animal models have indicated an important role of this pathway in the progression of atherosclerosis 45. Increased levels of 12/15-LOX eicosanoids may also influence diabetes and hypertension. Mice lacking 12/15-LOX activity are resistant to streptozotocin-induced diabetes 46, whereas increased production of 12/15-LOX metabolites was observed in ob/ob diabetic mice 47. The role of this class of lipid mediators in the regulation of blood pressure is also well established. Prostacyclin (PGI2), an important endothelium-derived vasorelaxant factor, was not detected and its non-enzymatic hydrolysis product 6-keto PGF1α was not significantly affected.
In contrast to the selective increases of COX and 12-LOX derived eicosanoids associated with gestational hypercholesterolemia, maternal OxLDL-immunization prior to pregnancy resulted in a marked reduction of arachidonic acid and eicosanoids formed by all four major pathways. Prior immunization of hypercholesterolemic or normocholesterolemic mothers significantly reduced offspring levels of 9 of 14 eicosanoids of the COX pathway, 20 of 23 eicosanoids formed by either the 5-LOX or 12-LOX pathways, 8 of 19 CYP eicosanoids, and all 7 metabolites of the nonenzymatic pathway. Thus, maternal immunization not only prevented the increase of eicosanoids induced by maternal hypercholesterolemia, but also reduced the baseline level of many eicosanoids in offspring of normocholesterolemic mothers. Together with the reduction of metabolites generated by pathways not activated by gestational hypercholesterolemia (5-LOX, CYP and nonenzymatic pathways), this suggests that beneficial immunization-induced in utero programming involves a greater range of cells or genes than the atherogenic programming by gestational hypercholesterolemia. Most cells and tissues are capable of generating eicosanoids. Therefore, we do not know whether, and to what extent, the plasma levels reflect in utero programming of circulating immune cells, platelets, vascular cells, or cells of other tissues. We also do not know whether the effects are due to programming of specific cells, or whether they are secondary to a global effect of developmental programming on inflammation in adult offspring.
In addition to the biological effects already discussed, eicosanoids regulate important aspects of immunity, including cell differentiation, cell migration, cytokine production, and antigen presentation, and have been implicated in both acute inflammatory responses and chronic inflammatory disorders. We have previously shown that maternal immunization programs specific humoral immune responses in offspring, not only in the rabbits of the present experimental cohort, but also in mice 15. Similar immune responses were also seen in naïve offspring (i.e. offspring neither boosted with the maternal immunogen nor fed an atherogenic diet that enhances the spontaneous formation of OxLDL). Programming was independent of transplacental passage of maternal IgG antibodies to OxLDL, but was dependent on B-lymphocytes and led to increased production of oxidation-specific IgM antibodies later in life 15. The present data suggest that changes in immune responses stemming from maternal immunization are not limited to B-cells, but that eicosanoids may also play a role in upregulating protective immune responses and/or decreasing proinflammatory ones. In addition, a recent report implicated COX metabolites in germinal center formation and antibody class switch, linking innate immune cell function and humoral immune responses 48. Finally, antibody-mediated reductions of the ligands of scavenger receptors and receptors for advanced glycation endproducts (RAGE) may directly influence inflammatory conditions in utero 49.
Eicosanoids are considered local hormones that trigger specific effects on nearby target cells, mainly by binding to specific G protein-coupled receptors 50. In addition to extracellular signaling, eicosanoids can bind to and activate nuclear receptors 51. Their short half-live and susceptibility to further enzymatic or non-enzymatic modifications (usually accompanied by changes in pharmacological potency) complicate the assessment of the effects of individual eicosanoids on immunity and inflammation. Plasma eicosanoid levels are generally low in healthy individuals, whereas local or systemic inflammation is accompanied by a significant increase. Nevertheless, not all eicosanoids that increase during inflammatory pathology are proinflammatory. Whereas some, such as PGD2, have been associated with acute inflammation, others have pro- as well as anti-inflammatory properties. For example, a study in humans showed an association between type 2 diabetes mellitus and a single- nucleotide polymorphism of PGE synthase 2, indicating that moderately elevated plasma levels of PGE2 may be protective 52. Other eicosanoids are more closely associated with the resolution phase, and are therefore termed resolvins 53. Furthermore, in addition to changes in the level of a given eicosanoid, the global eicosanoid expression pattern may change depending on the type of insult or inflammation. For all of the above reasons, a complete plasma profile of eicosanoids and their breakdown products should provide a more accurate metabolic snapshot than measurements of selected proinflammatory metabolites, and present a better tool for the assessment of inflammatory responses in health and disease.
In summary, the present findings indicate that in utero programming, in particular immune programming, exerts a profound effect on the formation of eicosanoids that may influence offspring diseases through modulation of inflammation and immunity, vasoconstriction or aggregation. Maternal immunization prior to pregnancy affects a broader panel of bioactive lipids and may protect offspring against immunomodulated conditions not only by counterbalancing pathogenic in utero programing by gestational hypercholesterolemia, but also by affecting a greater number of genes.
Supplementary Material
Acknowledgments
This study was supported by NIH grants HL-067792 (W.P.), HL-089559 (W.P.), Ellison Medical Foundation Senior Scholar Award AG-SS 1851-07 (W.P.), and the NIH LIPID MAPS Large Scale Collaborative Grant GM069338 (E.D., O.Q.).
Footnotes
No reprints will be available.
Disclosures: None of the authors declares a conflict of interest.
Reference List
- 1.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–17. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 2.Palinski W, Nicolaides E, Liguori A, Napoli C. Influence of Maternal Dysmetabolic Conditions During Pregnancy on Cardiovascular Disease. J Cardiovasc Transl Res. 2009;2:277–85. doi: 10.1007/s12265-009-9108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napoli C, D’Armiento FP, Mancini FP, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–90. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354:1234–41. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- 5.Napoli C, Witztum JL, de Nigris F, Palumbo G, D’Armiento FP, Palinski W. Intracranial arteries of human fetuses are more resistant to hypercholesterolemia-induced fatty streak formation than extracranial arteries. Circulation. 1999;99:2003–10. doi: 10.1161/01.cir.99.15.2003. [DOI] [PubMed] [Google Scholar]
- 6.Napoli C, Witztum JL, Calara F, de Nigris F, Palinski W. Maternal hypercholesterolemia enhances atherogenesis in normocholesterolemic rabbits, which is inhibited by antioxidant or lipid-lowering intervention during pregnancy: an experimental model of atherogenic mechanisms in human fetuses. Circ Res. 2000;87:946–52. doi: 10.1161/01.res.87.10.946. [DOI] [PubMed] [Google Scholar]
- 7.Palinski W, D’Armiento FP, Witztum JL, de Nigris F, Casanada F, Condorelli M, et al. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Circ Res. 2001;89:991–96. doi: 10.1161/hh2301.099646. [DOI] [PubMed] [Google Scholar]
- 8.Napoli C, de Nigris F, Welch JS, Calara FB, Stuart RO, Glass CK, et al. Maternal hypercholesterolemia during pregnancy promotes early atherogenesis in LDL receptor-deficient mice and alters aortic gene expression determined by microarray. Circulation. 2002;105:1360–67. doi: 10.1161/hc1102.106792. [DOI] [PubMed] [Google Scholar]
- 9.Alkemade FE, Gittenberger-de Groot AC, Schiel AE, VanMunsteren JC, Hogers B, van Vliet LS, et al. Intrauterine exposure to maternal atherosclerotic risk factors increases the susceptibility to atherosclerosis in adult life. Arterioscler Thromb Vasc Biol. 2007;27:2228–35. doi: 10.1161/01.ATV.0000282193.31936.fd. [DOI] [PubMed] [Google Scholar]
- 10.Koukkou E, Ghosh P, Lowy C, Poston L. Offspring of normal and diabetic rats fed saturated fat in pregnancy demonstrate vascular dysfunction. Circulation. 1998;98:2899–904. doi: 10.1161/01.cir.98.25.2899. [DOI] [PubMed] [Google Scholar]
- 11.Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation. 2004;110:1097–102. doi: 10.1161/01.CIR.0000139843.05436.A0. [DOI] [PubMed] [Google Scholar]
- 12.Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Sare-Anane H, et al. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R134–R139. doi: 10.1152/ajpregu.00355.2004. [DOI] [PubMed] [Google Scholar]
- 13.Liguori A, D’Armiento FP, Palagiano A, Balestrieri ML, Williams-Ignarro S, de Nigris F, et al. Effect of gestational hypercholesterolaemia on omental vasoreactivity, placental enzyme activity and transplacental passage of normal and oxidised fatty acids. BJOG. 2007;114:1547–56. doi: 10.1111/j.1471-0528.2007.01510.x. [DOI] [PubMed] [Google Scholar]
- 14.Elahi MM, Cagampang FR, Anthony FW, Curzen N, Ohri SK, Hanson MA. Statin treatment in hypercholesterolemic pregnant mice reduces cardiovascular risk factors in their offspring. Hypertension. 2008;51:939–44. doi: 10.1161/HYPERTENSIONAHA.107.100982. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita T, Freigang S, Eberle C, Pattison J, Gupta S, Napoli C, Palinski W. Maternal immunization programs postnatal immune responses and reduces atherosclerosis in offspring. Circ Res. 2006;99:E51–E64. doi: 10.1161/01.RES.0000244003.08127.cc. [DOI] [PubMed] [Google Scholar]
- 16.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci US A. 1995;92:821–25. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woollett LA. Where does fetal and embryonic cholesterol originate and what does it do? Annu Rev Nutr. 2008;28:97–114. doi: 10.1146/annurev.nutr.26.061505.111311. [DOI] [PubMed] [Google Scholar]
- 18.Palinski W. Maternal-fetal cholesterol transport in the placenta: good, bad, and target for modulation. Circ Res. 2009;104:569–71. doi: 10.1161/CIRCRESAHA.109.194191. [DOI] [PubMed] [Google Scholar]
- 19.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci US A. 2008;105:17046–49. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palinski W, Napoli C. The fetal origins of atherosclerosis: maternal hypercholesterolemia, and cholesterol-lowering or antioxidant treatment during pregnancy influence in utero programming and postnatal susceptibility to atherogenesis. FASEB J. 2002;16:1348–60. doi: 10.1096/fj.02-0226rev. [DOI] [PubMed] [Google Scholar]
- 22.Palinski W. Sodium exposure induces stroke in a genetically susceptible model: new insights into early-life factors modulating adult disease. Circulation. 2009;119:1459–62. doi: 10.1161/CIRCULATIONAHA.109.849554. [DOI] [PubMed] [Google Scholar]
- 23.Bhasin KK, van NA, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes. 2009;58:559–66. doi: 10.2337/db07-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–75. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 25.Buczynski MW, Dumlao DS, Dennis EA. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–38. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–69. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–86. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 28.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Pratico D, Dogne JM. Vascular biology of eicosanoids and atherogenesis. Expert Rev Cardiovasc Ther. 2009;7:1079–89. doi: 10.1586/erc.09.91. [DOI] [PubMed] [Google Scholar]
- 30.Smith WL, Murphy RC. Cyclooxygenase, lipoxygenase, and epoxygenase pathways. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam: Elsevier; 2008. pp. 331–62. [Google Scholar]
- 31.Palinski W, Ylä-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, et al. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–35. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 32.Palinski W, Hörkkö S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–14. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deems R, Buczynski MW, Bowers-Gentry R, Harkewicz R, Dennis EA. Detection and quantitation of eicosanoids via high performance liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzymol. 2007;432:59–82. doi: 10.1016/S0076-6879(07)32003-X. [DOI] [PubMed] [Google Scholar]
- 35.Hall LM, Murphy RC. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J Am Soc Mass Spectrom. 1998;9:527–32. doi: 10.1016/S1044-0305(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 36.Goharkhay N, Sbrana E, Gamble PK, Tamayo EH, Betancourt A, Villarreal K, et al. Characterization of a murine model of fetal programming of atherosclerosis. Am J Obstet Gynecol. 2007;197:416.e1–416.e5. doi: 10.1016/j.ajog.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–59. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Buczynski MW, Stephens DL, Bowers-Gentry RC, Grkovich A, Deems RA, Dennis EA. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem. 2007;282:22834–47. doi: 10.1074/jbc.M701831200. [DOI] [PubMed] [Google Scholar]
- 39.Diczfalusy U, Falardeau P, Hammarstrom S. Conversion of prostaglandin endoperoxides to C17-hydroxy acids catalyzed by human platelet thromboxane synthase. FEBS Lett. 1977;84:271–74. doi: 10.1016/0014-5793(77)80704-7. [DOI] [PubMed] [Google Scholar]
- 40.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–71. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 41.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–87. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 42.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nature Reviews Immunology. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 43.Nasjletti A, Arthur C. Corcoran Memorial Lecture. The role of eicosanoids in angiotensin-dependent hypertension. Hypertension. 1998;31:194–200. doi: 10.1161/01.hyp.31.1.194. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, Lucitt MB, Stubbe J, Cheng Y, Friis UG, Hansen PB, et al. Prostaglandin F2alpha elevates blood pressure and promotes atherosclerosis. Proc Natl Acad Sci USA. 2009;106:7985–90. doi: 10.1073/pnas.0811834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, et al. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–31. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- 46.Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest. 1999;103:1431–36. doi: 10.1172/JCI5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatley ME, Srinivasan S, Reilly KB, Bolick DT, Hedrick CC. Increased production of 12/15 lipoxygenase eicosanoids accelerates monocyte/endothelial interactions in diabetic db/db mice. J Biol Chem. 2003;278:25369–75. doi: 10.1074/jbc.M301175200. [DOI] [PubMed] [Google Scholar]
- 48.Blaho VA, Buczynski MW, Dennis EA, Brown CR. Cyclooxygenase-1 orchestrates germinal center formation and antibody class-switch via regulation of IL-17. J Immunol. 2009;183:5644–53. doi: 10.4049/jimmunol.0901499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36:388–98. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–17. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 51.Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15:924–31. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boomgaarden I, Bosy-Westphal A, Muller MJ, Doring F. Influence of a type 2 diabetes associated prostaglandin E synthase 2 polymorphism on blood prostaglandin E2 levels. Prostaglandins Leukot Essent Fatty Acids. 2009;80:185–88. doi: 10.1016/j.plefa.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.