Abstract
PURPOSE
Multimodal CT with CT angiography (CTA) and CT perfusion (CTP) are increasingly used in stroke triage. Our aim was to identify parameters most predictive of hemorrhagic transformation (HT), especially symptomatic intracerebral hemorrhage (SICH).
METHODS
This retrospective study included patients evaluated by baseline multimodal CT ≤ 9hours from ictus with acute nonlacunar middle cerebral artery (MCA) territory infarction. Two readers independently evaluated CTP maps for ischemic severity and CTA source images (CTA-SI) for infarct extent (as measured by ASPECTS). Presence of proximal occlusion (ICA or M1) and degree of collateralization (collateral score) were also assessed on CTA. HT was defined as SICH if associated with deterioration ≥ 4-points on NIHSS. Multivariate logistic regression analysis identified independent predictors of SICH. ROC curves selected optimal thresholds.
RESULTS
Of 84 patients reviewed, HT occurred in 22 (26.2%) and SICH in 8 (9.5%). Univariate predictors for SICH were proximal occlusion (OR = 8.65, P = .049), collateral score (OR = .34, P = .017), ASPECTS (OR = .46, P = .001), and CBV (OR = .001, P = .005). Multivariate analysis revealed ASPECTS as the only independent predictor with optimal threshold ≤ 5 and sensitivity and specificity of 75.0% and 85.5%, respectively.
CONCLUSION
For acute MCA infarcts ≤ 9 hours, the strongest predictor of SICH on multimodal CT was ASPECTS on CTA-SI.
Keywords: Alberta Stroke Program Early CT Score, CT perfusion, CT angiography, hemorrhagic transformation, acute stroke
Introduction
There remains an urgency to identify the baseline pretreatment clinical or radiological variables that are most predictive of hemorrhagic transformation (HT) of acute ischemic stroke. When symptomatic, HT further increases the risk of disability and death beyond that of the base infarct.1 A comprehensive multimodal CT protocol consisting of CT angiography (CTA) and CT perfusion (CTP) in addition to the standard noncontrast head CT (NCCT) is a promising imaging tool set for triage.2-4 Our aim was to determine the imaging parameter(s) from multimodal CT that are most predictive of HT and in particular, symptomatic intracerebral hemorrhage (SICH).
Materials and Methods
Patients
This was an IRB-approved retrospective study of patients evaluated by a multimodal CT stroke protocol at time of emergency department triage. Informed consent was waived for collection and analysis of preexisting data. Patients were included in this study if (1) age was ≥18 years; (2) time from symptom ictus to CT imaging was ≤ 9 hours; (3) the baseline NCCT showed no evidence for hemorrhage; and (4) middle cerebral artery (MCA) territory ischemia was identified on baseline CTP. Subjects were excluded if (1) there were severe motion artifacts or poor contrast injection on the CTA or CTP; (2) the venous time attenuation curve was truncated before returning to baseline on CTP; and (3) there were no follow-up imaging of the brain to evaluate for HT.
Scanning Protocols
All CT examinations were performed on 16-slice multidetector scanners (Lightspeed, GE Healthcare, Milwaukee, WI, USA). Imaging parameters for the comprehensive CT stroke protocol were as follows: NCCT–axial scanning mode of the head, 120 kVp, 285 mAs, 5-mm slice thickness at 5-mm interval; CTP–cine scanning mode centered at the basal ganglia, 80 kVp, 200 mAs, 4 × 5 mm slices centered over the mid-basal ganglia, 50 mL of nonionic iodinated contrast injected at a rate of 5 mL/second with an initial 5 second delay for 60-second cine duration; CTA–helical scanning mode of the head and neck (from aortic arch to vertex), 120 kVp, 200 mAs, 1.25-mm slice thickness at 1.25-mm interval, 90mL of nonionic iodinated contrast injected at a rate of 4 mL/second with bolus tracking.
For patients who received follow-up MRI of the brain, which were performed on 1.5 Tesla scanners (HDx, GE Healthcare), a gradient-recalled echo (GRE) sequence was performed to evaluate for HT with TR of 700 msec, TE of 15 msec, 256 × 192 matrix, flip angle of 20°, 5-mm slice thickness and 0-mm slice interval.
Image Processing
The CTP source images were retrieved from our institutions' picture archiving and communications system and loaded onto an offline workstation (Advantage Windows 4.0, GE Healthcare) equipped with deconvolution-based postprocessing software (CT Perfusion 3.0, GE Healthcare). Parametric color maps of cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) in 512 × 512 matrix were constructed following previously published guidelines,5 with arterial input and venous output functions obtained from the anterior cerebral artery and superior sagittal sinus, respectively. CTA source images (CTA-SI) were also retrieved and loaded onto the same offline workstation where they were reconstructed to thick (20 mm) axial and coronal maximum intensity projections (MIPs).
Data Acquisition
Baseline clinical data (see Table 1) including the National Institutes of Health Stroke Scale (NIHSS) score were retrieved from the admission record of each patient. Information was also gathered regarding time of stroke ictus, time of multimodal CT, and any treatment with tissue plasminogen activator (tPA) through either intravenous (IV) or intraarterial (IA) routes. All measurements and scoring of baseline CT variables were performed while blinded to clinical and follow-up data.
Table 1.
Univariate Logistic Regression Analysis for Hemorrhagic Transformation (HT)
| Variable | No HT (n = 62) | HT (n = 22) | OR | P |
|---|---|---|---|---|
| Female, no. (%) | 35 (56.5) | 9 (40.9) | .534 | .213 |
| Age (years), median (IQR) | 73 (65-82) | 64 (54-80) | .979 | .199 |
| Systolic BP (mm Hg), mean (SD) | 144 (28.1) | 147 (27.4) | 1.004 | .682 |
| Glucose (mmol/L), mean (SD) | 6.9 (2.1) | 8.4 (3.5) | 1.011 | .035 |
| Platelets (×103/μL), mean (SD) | 259 (100) | 244 (77.6) | .998 | .508 |
| Diabetes mellitus, no. (%) | 11 (17.7) | 4 (18.2) | 1.030 | .963 |
| Hypertension, no. (%) | 37 (59.7) | 10 (45.5) | .563 | .251 |
| Hyperlipidemia, no. (%) | 25 (40.3) | 6 (27.3) | .555 | .279 |
| Atrial fibrillation, no. (%) | 23 (37.1) | 9 (40.9) | 1.174 | .752 |
| Smoker, no. (%) | 16 (25.8) | 5 (22.7) | .846 | .775 |
| NIHSS score, median (IQR) | 12 (8-15) | 15.5 (11-19) | 1.149 | .006 |
| Proximal occlusion, no. (%) | 25 (40.3) | 16 (72.7) | 3.947 | .012 |
| Collateral score, median (IQR) | 2 (2-3) | 2 (1-3) | .440 | .008 |
| ASPECTS, median (IQR) | 7 (6-8) | 6 (5-7) | .731 | .021 |
| CBF (mL/100g/min), mean (SD) | 4.7 (4.2) | 2.1 (1.6) | .675 | .009 |
| CBV (mL/100g), mean (SD)* | .8 (.4) | .3 (.2) | .003 | <.001 |
| Time to CT (hours), mean (SD) | 3.8 (2.0) | 4.0 (1.8) | 1.026 | .824 |
| IV or IA tPA, no. (%) | 31 (50.0) | 8 (36.4) | .571 | .273 |
Significant at < .05 on multivariate analysis, ASPECTS = Alberta Stroke Program Early CT Score; BP = blood pressure; CBF = minimal cerebral blood flow; CBV = minimal cerebral blood volume; IA = intraarterial; IQR = interquartile range; IV tPA = intravenous tissue plasminogen activator; NIHSS = National Institutes of Health stroke scale; OR = odds-ratio; SD = standard deviation.
For CTA, three variables were reviewed: (1) the presence or absence of proximal arterial occlusion (in the distal internal carotid artery and/or M1 segment of the MCA); (2) the Alberta Stroke Program Early CT Score (ASPECTS)6; and (3) the collateral score.4 Proximal arterial occlusion was determined on CTA-SI and 3-dimensional volume renderings. ASPECTS was obtained on CTA-SI using the conventional 10-point topographical scale in which 1-point was subtracted from a base score of 10 for every region that showed decreased enhancement compared to the contralateral homologous region.7,8 In short, the 10 stations of the ASPECTS template are: “C”–caudate head; “L”–lentiform nucleus; “IC”–internal capsule, “I”–insula; “M1”–anteroinferior frontal lobe; “M2”–anterosuperior temporal lobe; “M3”–posterior temporal lobe; “M4”–anterosuperior frontal lobe; “M5”–posterior frontal lobe; and “M6”–parietal lobe. The CTA collateral score was graded upon reviewing the source images and the thick MIPS using a 4-point scale from 0 to 3 as previously described.4 In short, “0”–absent collateral supply; “1”–collateral supply filling >0 but ≤ 50%; “2”–collateral supply filling >50 but <100%; and “3”–collateral supply filling 100% of the occluded MCA territory compared to the contralateral side. Presence of arterial occlusion, ASPECTS, and collateral scores were determined independently by two neuroradiologists familiar with these scoring systems. Any difference in the independent scoring was resolved by consensus review.
For CTP, the depth of ischemia was determined by region of interest (ROI) analysis of the CBF and CBV maps. A qualitative assessment was first performed on the available slice locations of the most severe perfusion deficit and then quantitatively evaluated by the manual placement of four 15-mm diameter circular ROIs. The minimal CBF and minimal CBV values from these ROIs were recorded for each patient and then averaged between the two readers. Taking the extreme perfusion values from multiple ROI samplings was previously shown to be a reliable method of obtaining the perfusion characteristics of a lesion.9 A 15-mm diameter circular ROI was chosen because: (1) 15-mm represents the conventional lower bound threshold for the diameter of a nonlacunar infarct; (2) taking the perfusion value from an ROI smaller than 15-mm may be heavily affected by random pixel noise; and (3) taking the mean perfusion value from a free-hand ROI of the entire region of hypoperfusion may “average out” that of a subregion of severe ischemia, which may represent the nidus of the hemorrhagic risk.
Follow-up clinical and imaging data consisting of the dates, times, and results of all imaging exams and any worsening neurological deficit during the hospital course were tabulated. Follow-up NCCT and GRE were examined for the presence or absence of HT. If present, HT was categorized according to the second European Cooperative Acute Stroke Study10 criteria as hemorrhagic infarction (HI) or parenchymal hematoma (PH). In particular, PH (but not HI) was defined as HT with space-occupying effect. SICH was identified as PH with worsening of neurological deficit ≥ 4 points on the NIHSS that was temporally related to its imaging appearance.
Statistical Analysis
All statistical operations were performed using MedCalc for Windows, version 10.4 (MedCalc Software, Mariakerke, Belgium). The study cohort was dichotomized into two groups first based on HT and then based on SICH as the outcome variable. For each outcome, a univariate logistic regression analysis was performed for all clinical and CT variables. Those that were significant at the P -value < .05 level were then entered into a multivariate logistic regression model constructed using the backward elimination method. Variables with P -value < .05 in the multivariate analyses were considered independent predictors. Odds-ratios (OR) with 95% confidence intervals (in brackets) were calculated. Receiver-operating characteristics (ROC) curves were implemented to identify the optimal accuracy thresholds and associated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Results
Study Population, Treatment, and Recanalization
Eighty-four patients were included. The mean time from symptom onset to CT was 3.9 ± 2.0 hours (range .5 hours-8.9 hours) and the median NIHSS score was 12 (range 2-25). Of the 84 patients, 61 (72.6%) had an arterial occlusion on baseline CTA and of these, 41 (67.2%) were proximal (supraclinoid or terminal ICA and/or M1 segment). Thirty-nine (46.4%) patients received tPA (38 by IV,8 by both IV+IA, and 1 by IA alone). Fourteen (16.7%) received IA thrombectomy. Overall, 44 (52.4%) patients received thrombolytic or mechanical therapy. Of the 61 patients with arterial occlusion on baseline CTA, 24 (39.3%) demonstrated posttreatment recanalization on digital subtraction angiography or on follow-up MRA or CTA. All patients had one or more follow-up imaging exams.
Hemorrhagic Transformation
Twenty-two (26.2%) patients exhibited some HT on follow-up NCCT or MRI, with 12 HI and 10 PH. Table 1 outlines the baseline clinical and CT variables as well as tPA treatment for the subgroups with and without HT. Univariate logistic regression revealed significant associations between HT and serum glucose (OR = 1.011 [1.001-1.022], P = .035), NIHSS score (OR = 1.149 [1.040-1.269], P = .006), proximal occlusion (OR = 3.947 [1.358-11.468], P = .012), collateral score (OR = .440 [.241-.804], P .008), ASPECTS (OR = .731 [.561-.953], P = .021), CBF (OR = .675 [.503-.905], P = .009), and CBV (OR = .003 [.0002 -.046], P < .0001). Multivariate analysis identified CBV as the only independent predictor of any HT. The area under the ROC curve was .866 [.774-.931], with an optimal CBV threshold ≤ .5 mL/100 g. Using this threshold, HT was predicted with sensitivity of 86.4% (65.1-97.1), specificity of 77.4% (65.0-87.1), PPV of 57.6% (39.2-74.5), and NPV of 94.1% (83.8-98.8). See Figure 1 for one of the 22 patients with HT.
Fig 1.
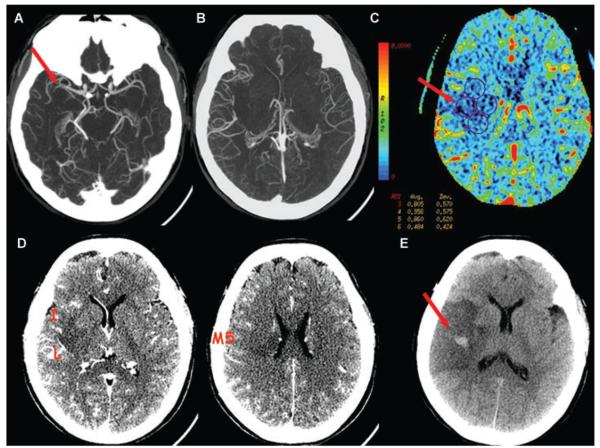
A 49-year-old female presented at 6 hours 25 minutes from onset of left hemiparesis. NIHSS score was 24. (A) CTA thin MIP shows clot at the distal M1 segment of the right MCA. (B) CTA thick MIP shows collateral supply that fills >50% but < 100% of the vessels compared to the contralateral left MCA branches, for a collateral score of 2. (C) CBV map shows hypoperfusion to the proximal right MCA territory with a minimal CBV sampling of .48 mL/100 g (ROI no.6). (D) CTA-SI shows hypoattenuation in the lentiform nucleus (L), insula (I), and M5, for ASPECTS of 7. No treatment was attempted. (E) Follow-up NCCT at 21 hours shows type 2 hemorrhagic infarction, that was asymptomatic.
Symptomatic Intracerebral Hemorrhage
Eight (9.5%) patients developed SICH, all of which were PH. Table 2 outlines the baseline clinical and CT variables as well as tPA treatment for the subgroups with and without SICH. Univariate logistic regression revealed significant associations between SICH and proximal occlusion (OR = 8.647 [1.014-73.757], P = .049), collateral score (OR = .340 [.140-.824], P = .017), ASPECTS (OR = .462 [.292-.730], P = .001), and CBV (OR = .001 [< .0001-.065], P = .005). Multivariate analysis identified ASPECTS as the only independent predictor of SICH. The area under the ROC curve was .890 [.803- .948], with an optimal ASPECTS threshold at ≤ 5. Using this threshold, SICH was predicted with sensitivity of 75.0% (34.9-96.8), specificity of 85.5% (75.6-92.5), PPV of 35.3% (14.2-61.7), and NPV of 97.0% (95% CI: 89.6-99.6). Treatment with tPA was entered into the model as a separate variable but no significant association with SICH was found (P .346). However, the interaction term of tPA × ASPECTS ≤ 5 was significant (P = .008). See Figure 2 for one of the 8 patients with SICH.
Table 2.
Univariate Logistic Regression Analysis for Symptomatic Intracerebral Hemorrhage (SICH)
| Variable | No SICH (n = 76) | SICH (n = 8) | OR | P |
|---|---|---|---|---|
| Female, no. (%) | 41 (54.0) | 3 (37.5) | .512 | .382 |
| Age (years), median (IQR) | 71.5 (62-82) | 68.5 (60-75) | .980 | .381 |
| Systolic BP (mm Hg), mean (SD) | 145 (28.5) | 144 (22.0) | .999 | .939 |
| Glucose (mmol/L), mean (SD) | 7.4 (2.7) | 6.8 (1.1) | .994 | .542 |
| Platelets (×103/μL), mean (SD) | 255 (95.0) | 253 (96.3) | 1.000 | .946 |
| Diabetes mellitus, no. (%) | 14 (18.4) | 1 (12.5) | .633 | .680 |
| Hypertension, no. (%) | 42 (55.3) | 5 (62.5) | 1.349 | .696 |
| Hyperlipidemia, no. (%) | 30 (39.5) | 1 (12.5) | .219 | .165 |
| Atrial fibrillation, no. (%) | 29 (38.2) | 3 (37.5) | .972 | .971 |
| Smoker, no. (%) | 17 (22.4) | 4 (50.0) | 3.471 | .101 |
| NIHSS score, median (IQR) | 12 (9-16) | 14 (12-17) | 1.087 | .221 |
| Proximal occlusion, no. (%) | 34 (44.7) | 7 (87.5) | 8.647 | .049 |
| Collateral score, median (IQR) | 2 (2-3) | 1 (1-2) | .340 | .017 |
| ASPECTS, median (IQR)* | 7 (6-8) | 5 (3-5) | .462 | .001 |
| CBF (mL/100g/min), mean (SD) | 4.3 (4.0) | 1.6 (.5) | .508 | .070 |
| CBV (mL/100g), mean (SD) | .7 (.4) | .2 (.1) | .0001 | .005 |
| Time to CT (hours), mean (SD) | 3.9 (2.0) | 3.9 (1.2) | .989 | .951 |
| IV or IA tPA, no. (%) | 34 (44.2) | 5 (62.5) | 2.058 | .338 |
significant at < .05 on multivariate analysis, ASPECTS = Alberta Stroke Program Early CT Score; BP = blood pressure; CBF = minimal cerebral blood flow; CBV = minimal cerebral blood volume; IA = intraarterial; IQR = interquartile range; IV tPA = intravenous tissue plasminogen activator; NIHSS = National Institutes of Health stroke scale; OR = odds-ratio; SD = standard deviation.
Fig 2.
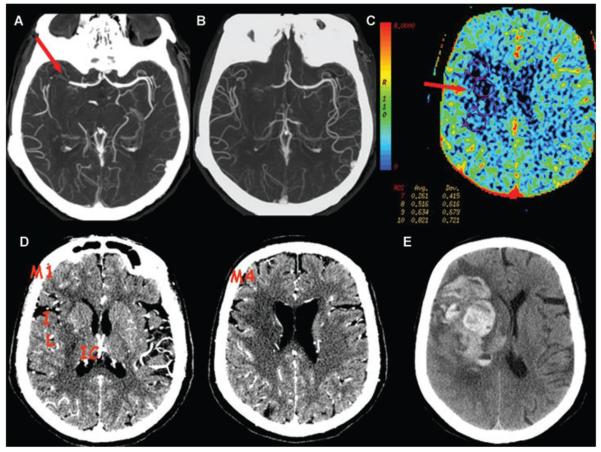
A 62-year-old female presented at 5 hours and 20 minutes from onset of left hemiparesis. NIHSS score was 16. (A) CTA thin MIP shows clot at the distal M1 segment extending into the M2 branches of the right MCA. (B) CTA thick MIP shows collateral supply that fills >50% but < 100% of the vessels compared to the contralateral left MCA branches, for a collateral score of 2. (C) CBV map shows hypoperfusion to the proximal right MCA territory with a minimal CBV sampling of .26 mL/100 g (ROI no.7). (D) CTA-SI shows hypoattenuation in the lentiform nucleus (L), internal capsule (IC), insula (I), M1, and M4, for ASPECTS of 5. No treatment was attempted. (E) Follow-up NCCT at 46 hours was obtained for acute mental status change and shows a type 2 parenchymal hematoma.
Discussion
We aimed to determine the imaging parameters from multimodal CT that are most predictive of HT and SICH in acute MCA territory stroke. We hypothesized that the severity of ischemia would be important and multivariate logistic regression revealed that the development of any hemorrhage was independently predicted by CBV. An optimal threshold of ≤ .5 mL/100 g selected by ROC curve analysis yielded good sensitivity (86.4%) and specificity (77.4%) for HT. While the PPV was fair (57.6%), the NPV (94.1%) was high. Amongst the HTs, the majority (63.6%, 14 of 22) was asymptomatic and discovered on routine follow-up. It remains uncertain if there is utility in anticipating asymptomatic HT since some investigators have shown it to be deleterious toward functional recovery,11-13 while others believe it to solely be an epiphenomenon.14 It should be noted that predicting HT by measurements of low CBV was also previously reported using perfusion weighted MRI (PWI). Alsop et al15 found that extremely low CBV of <5% compared to the contralateral gray matter represented tissue at risk for postthrombolytic HT. This result was achieved in a limited series of 20 patients and showed that CBV outperformed apparent diffusion coefficient values in predicting HT but did not account for other clinical or imaging variables. Reduced CBV on CTP was also described as the only independent risk factor for HT after IA therapy and recanalization.16 Low CBV is the best indicator of tissue destined to infarct17-19 on CTP and serves as the CT correlate to the diffusion weighted imaging (DWI) lesion. However, a threshold at ≤.5 mL/100 g found in our study is much lower than that which has been previously attributed to irreversible ischemia18 (≤2.0 mL/100g) and represents virtually little to no contrast ever arriving to the ROI. Not only is the tissue not survivable with such limited perfusion, we speculate that the integrity of the blood-brain barrier may also be compromised, either functionally or structurally.
Univariate analysis showed HT was also associated with higher serum glucose, higher NIHSS score, proximal arterial occlusion, lower collateral score, lower ASPECTS, and lower CBF. Hyperglycemia and clinical stroke severity are well-known clinical predictors of HT20 and we were not surprised to find them in this study. Proximal arterial occlusion and suboptimal collateral supply are potential risk factors for HT most likely because they favor a larger infarct volume (and thus lower ASPECTS). Yet, by defining all HT as a single outcome, variables such as proximal occlusion, collateral score, and ASPECTS may not account for the cases in which the infarct is comparatively small but the local tissue is severely damaged. This may explain why a regional sampling of low perfusion, particularly low CBV, showed more significance for HT than the other variables.
For SICH, univariate logistic regression analysis revealed associations with proximal occlusion, lower collateral score, lower CBV, and lower ASPECTS with the latter as the only independent predictor. This result suggests that for development of a symptomatic hematoma, ischemic extent is more important than ischemic depth even though the two are often covariates.21 Pretreatment infarct volume as measured on DWI has already been shown in several studies21,22 including the Diffusion and Perfusion Imaging Evaluation For Understanding Stroke Evolution (DEFUSE)23 to independently predict SICH. ASPECTS is a semiquantitative gauge of ischemic extent with the advantage of functional weighting and it has been noted since its original conception on NCCT to be a predictor of SICH.6,24 We opted to grade ASPECTS on CTA-SI since previous studies have shown that CTA-SI is better at depicting early ischemic change than NCCT and therefore offer a more accurate score.7,8,25 However, some authors have suggested that CTA-SI acquired on a 64-slice or higher configuration scanner may be more “blood-flow” than “blood-volume” weighted, in which case the source images may delineate the extent of ischemia but not necessarily the infarct core.26 The data acquired for this study were performed on a 16-slice scanner, and thus we believe that ASPECTS was reflective of the core extent.27 But with increasingly larger scanners coming into the market, and consequently improved z-directional coverage for the dynamic CTP acquisition, we believe ASPECTS should be assessed on the CBV map rather than CTA-SI.28 While we are not aware of another study that explicitly shows ASPECTS on CTA-SI to be a predictor of SICH, a recent large multiinstitutional retrospective study has shown pretreatment ASPECTS on DWI to predict post-thrombolytic SICH.29 Unlike that study, which apriori used an ASPECTS cutpoint of ≤7, we found an optimal threshold at ≤5 using ROC analysis with good sensitivity (75.0%) and specificity (85.5%) for SICH. While the PPV was limited (35.3%), the NPV (97.0%) was notably high. In our cohort, treatment with tPA alone was not associated with an increased rate of SICH but the interaction of tPA and ASPECTS≤5 was significant, suggesting a potentiating effect between them. This is a novel result in the context of an imaging variable on multimodal CT, yet it is also fully compatible with the well-known risk of tPA-induced hemorrhage with infarcts larger than one-third of the MCA territory.
The biggest limitations of this study are its retrospective design and limited sample size. The cohort is also heterogeneous with presentation times that ranged from 30 minutes to just under 9 hours. This time window was selected for potential candidates of therapy and thus relevant to the goal of identifying pretreatment risk factors for HT and SICH. The CTP maps in this study were constructed using a deconvolution algorithm based on standard singular value decomposition (SVD), and thus tracer delay effects may have occurred.30,31 However, one of our main results was the emergence of the CBV parameter as being significant for evaluating HT risks and CBV is generally unaffected by tracer delay.30 Future studies should nevertheless utilize software algorithms that are either delay-insensitive or delay-corrected.
References
- 1.Hacke W, Donnan G, Fieschi C, et al. ATLANTIS Trials Investigators. ECASS Trials Investigators. NINDS rt-PA Study Group Investigators Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 2.Schramm P, Schellinger PD, Klotz E, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke. 2004;35:1652–1658. doi: 10.1161/01.STR.0000131271.54098.22. [DOI] [PubMed] [Google Scholar]
- 3.Kloska SP, Nabavi DG, Gaus C, et al. Acute stroke assessment with CT: do we need multimodal evaluation? Radiology. 2004;233:79–86. doi: 10.1148/radiol.2331030028. [DOI] [PubMed] [Google Scholar]
- 4.Tan JC, Dillon WP, Liu S, et al. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007;61:533–543. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- 5.Sanelli PC, Lev MH, Eastwood JD, et al. The effect of varying user-selected input parameters on quantitative values in CT perfusion maps. Acad Radiol. 2004;11:1085–1092. doi: 10.1016/j.acra.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 7.Coutts SB, Lev MH, Eliasziw M, et al. ASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcome. Stroke. 2004;35:2472–2476. doi: 10.1161/01.STR.0000145330.14928.2a. [DOI] [PubMed] [Google Scholar]
- 8.Camargo EC, Furie KL, Singhal AB, et al. Acute brain infarct: detection and delineation with CT angiographic source images versus nonenhanced CT scans. Radiology. 2007;244:541–548. doi: 10.1148/radiol.2442061028. [DOI] [PubMed] [Google Scholar]
- 9.Wetzel SG, Cha S, Johnson G, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology. 2002;224:797–803. doi: 10.1148/radiol.2243011014. [DOI] [PubMed] [Google Scholar]
- 10.Larrue V, von Kummer RR, Muller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 11.Kent DM, Hinchey J, Price LL, et al. In acute ischemic stroke, are asymptomatic intracranial hemorrhages clinically innocuous? Stroke. 2004;35:1141–1146. doi: 10.1161/01.STR.0000125712.02090.6e. [DOI] [PubMed] [Google Scholar]
- 12.Dzialowski I, Pexman JH, Barber PA, et al. CASES Investigators Asymptomatic hemorrhage after thrombolysis may not be benign: prognosis by hemorrhage type in the Canadian Alteplase for Stroke Effectiveness Study registry. Stroke. 2007;38:75–79. doi: 10.1161/01.STR.0000251644.76546.62. [DOI] [PubMed] [Google Scholar]
- 13.Kimura K, Iguchi Y, Shibazaki K, et al. Hemorrhagic transformation of ischemic brain tissue after t-PA thrombolysis as detected by MRI may be asymptomatic, but impair neurological recovery. J Neurol Sci. 2008;272:136–142. doi: 10.1016/j.jns.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Thomalla G, Sobesky J, Köhrmann M, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke. 2007;38:313–318. doi: 10.1161/01.STR.0000254565.51807.22. [DOI] [PubMed] [Google Scholar]
- 15.Alsop DC, Makovetskaya E, Kumar S, et al. Markedly reduced apparent blood volume on bolus contrast magnetic resonance imaging as a predictor of hemorrhage after thrombolytic therapy. Stroke. 2005;36:746–750. doi: 10.1161/01.STR.0000158913.91058.93. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt A, Vora NA, Thomas AJ, et al. Lower pretreatment cerebral blood volume affects hemorrhage risks after intra-arterial revascularization in acute stroke. Neurosurgery. 2008;63:874–879. doi: 10.1227/01.NEU.0000333259.11739.AD. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer PW, Roccatagliata L, Ledezma C, et al. First-pass quantitative CT perfusion identifies thresholds for salvageable penumbra in acute stroke patients treated with intra-arterial therapy. AJNR Am J Neuroradiol. 2006;27:20–25. [PMC free article] [PubMed] [Google Scholar]
- 18.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 19.Murphy BD, Fox AJ, Lee DH, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–1777. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 20.Lansberg MG, Albers GW, Wijman CA. Symtpomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stoke: a review of the risk factors. Cerebrovasc Dis. 2007;24:1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- 21.Selim M, Fink JN, Kumar S, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002;33:2047–2052. doi: 10.1161/01.str.0000023577.65990.4e. [DOI] [PubMed] [Google Scholar]
- 22.Singer OC, Humpich MC, Fiehler J, et al. MR Stroke Study Group Investigators Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008;63:52–60. doi: 10.1002/ana.21222. [DOI] [PubMed] [Google Scholar]
- 23.Lansberg MG, Thijs VN, Bammer R, et al. DEFUSE Investigators Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007;38:2275–2278. doi: 10.1161/STROKEAHA.106.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzialowski I, Hill MD, Coutts SB, et al. Extent of early ischemic changes on computed tomography (CT) before thrombolysis: prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke. 2006;37:973–978. doi: 10.1161/01.STR.0000206215.62441.56. [DOI] [PubMed] [Google Scholar]
- 25.Parsons MW, Pepper EM, Chan V, et al. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol. 2005;58:672–679. doi: 10.1002/ana.20638. [DOI] [PubMed] [Google Scholar]
- 26.Konstas AA, Goldmakher GV, Lee TY, et al. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: theoretical basis. AJNR Am J Neuroradiol. 2009;30:662–668. doi: 10.3174/ajnr.A1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke. 2001;32:2021–2028. doi: 10.1161/hs0901.095680. [DOI] [PubMed] [Google Scholar]
- 28.Lin K, Rapalino O, Law M, et al. Accuracy of the Alberta Stroke Program Early CT Score during the first 3 h of middle cerebral artery stroke: comparison of noncontrast CT, CT angiography source images, and CT perfusion. AJNR Am J Neuroradiol. 2008;29:931–936. doi: 10.3174/ajnr.A0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer OC, Kurre W, Humpich MC, et al. MR Stroke Study Group Investigators Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke. 2009;40:2743–2748. doi: 10.1161/STROKEAHA.109.550111. [DOI] [PubMed] [Google Scholar]
- 30.Kudo K, Sasaki M, Ogasawara K, et al. Difference in tracer delay-induced effect among deconvolution algorithms in CT perfusion analysis: quantitative evaluation with digital phantoms. Radiology. 2009;251:241–249. doi: 10.1148/radiol.2511080983. [DOI] [PubMed] [Google Scholar]
- 31.Konstas AA, Goldmakher GV, Lee TY, et al. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: technical implementations. AJNR Am J Neuroradiol. 2009;30:885–892. doi: 10.3174/ajnr.A1492. [DOI] [PMC free article] [PubMed] [Google Scholar]