Abstract
We recently demonstrated that sub-chronic low-dose kanamycin (KM, 300 mg/kg sc, 2x/day, 10 days) dramatically reduces permanent noise-induced hearing loss (NIHL) and hair cell loss in 1 month old CBA/J mice (Fernandez et al., 2010, J. Assoc. Res. Otolaryngol. 11, 235–244). Protection by KM remained for at least 48 hours after the last dose, and appeared to involve a cumulative effect of multiple doses as part of a preconditioning process. The first month of life lies within the early ‘sensitive period’ for both cochlear noise and ototoxic injury in mice, and CBA/J mice appear exquisitely vulnerable to noise during this period (Ohlemiller et al., 2011, Hearing Res. 272, 13–20). From our initial data, we could not rule out 1) that less rigorous treatment protocols than the intensive one we applied may be equally—or more—protective; 2) that protection by KM is tightly linked to processes unique to the sensitive period for noise or ototoxins; or 3) that protection by KM is exclusive to CBA/J mice. The present experiments address these questions by varying the number and timing of fixed doses (300 mg/kg sc) of KM, as well as the age at treatment in CBA/J mice. We also tested for protection in young C57BL/6J (B6) mice. We find that nearly complete protection against at least 2 hours of intense (110 dB SPL) broadband noise can be observed in CBA/J mice at least for ages up to 1 year. Reducing dosing frequency to as little as once every other day (a four-fold decrease in dosing frequency) appeared as protective as twice per day. However, reducing the number of doses to just 1 or 2, followed by noise 24 or 48 hrs later greatly reduced protection. Notably, hearing thresholds and hair cells in young B6 mice appeared completely unprotected by the same regimen that dramatically protects CBA/J mice. We conclude that protective effects of KM against NIHL in CBA/J mice can be engaged by a wide range of dosing regimens, and are not exclusive to the sensitive period for noise or ototoxins. While we cannot presently judge the generality of protection across genetic backgrounds, it appears not to be universal, since B6 showed no benefit. Classical genetic approaches based on CBA/J × B6 crosses may reveal loci critical to protective cascades engaged by kanamycin and perhaps other preconditioners. Their human analogs may partly determine who is at elevated risk of acquired hearing loss.
Keywords: Noise-induced permanent threshold shifts, hair cells, development, susceptibility, genetics, CBA/J, C57BL/6J, aminoglycosides, ototoxicity
INTRODUCTION
Young humans and animals appear particularly vulnerable to both noise and ototoxins (Saunders and Chen, 1982; Li, 1992a; Pujol, 1992; Henley and Rybak, 1995). Scattered evidence, moreover, supports the expectation that sub-injurious noise and sub-clinical ototoxic exposure will interact synergistically to produce hearing loss in young humans and animals (Bernard, 1981; Henley and Rybak, 1995; Li and Steyger, 2009). However, in a recent attempt to document this type of synergy in young CBA/J mice (Fernandez et al., 2010), we unexpectedly observed dramatic protection against permanent noise-induced hearing loss (NIHL) by sub-chronic application of low-dose kanamycin (KM, 300 mg/kg sc, 2x/day, postnatal day 20–30). The protection remained undiminished up to 48 hours after the final dose, yet no protection was provided by a single dose of KM. Thus we suggested that KM engages ‘preconditioning’ protective processes not unlike those engaged in the cochlea by heat shock (Yoshida et al., 1999), hypoxia (Gagnon et al., 2007), moderate sound (Yoshida and Liberman, 2000; Niu and Canlon, 2002), and restraint (Wang and Liberman, 2002), as well as those demonstrable in the brain and eye by local ischemia and other triggers (Dirnagl et al., 2003; Eisen et al., 2004; Ran et al., 2005; Gidday, 2006). As with some forms of preconditioning, protection by KM may build with repeated application over time, then dissipate slowly.
The finding of protection by an ototoxin early in life potentially holds implications for the nature of noise/ototoxin interactions in humans, both early and later in life. Moreover, the dramatic protection we found may mean that KM initiates robust protective cascades for which pharmacologic mimics may be sought. The broad relevance of KM preconditioning depends on several factors not examined in our original study. Among these, we used an intensive treatment protocol that may itself have caused some injury, and we did not determine whether less intensive treatments could retain efficacy. Second, we only tested mice during the early ‘sensitive period’ for NIHL and ototoxicity (Henry, 1982b; Chen and Saunders, 1983; Ohlemiller et al., 2000; Wu et al., 2001; Kujawa and Liberman, 2009), and could not rule out that the protection was somehow limited to this period. Third, a host of mouse strain differences have been described with regard to the effects of noise, ototoxins, and aging (Willott, 1991; Erway and Willott, 1996; Zheng et al., 1999; Wu et al., 2001; Ohlemiller, 2006, , 2009), as well as ‘protectability’ by preconditioning (Gagnon et al., 2007). We therefore could not assume that our findings would generalize to all—or even most—inbred lines. In the present study we address these issues in turn, by testing effects of less rigorous treatment paradigms, and by determining the extent of protection in older CBA/J mice, as well as young C57BL/6J (B6) mice. We show that KM protection against NIHL in CBA/J is highly permissive with regard to dosing regimen and age of treatment, but does not work equally well in CBA/J and B6 mice. B6 mice may carry alleles that either promote net injury by KM or impair the engagement of protective cascades.
METHODS
Animals
Our study encompassed 67 CBA/J that received KM or saline under various 10–11 day dosing regimens (Table I) with noise exposure on postnatal day (PND) 29 or 30. Also included were 13 CBA/J mice aged 2 mos at the start of a 10 day treatment, and 8 CBA/J mice aged 1 yr at start of a 10 day treatment. Archival data from 14 additional untreated 1 month old and four 1 year old CBA/J mice were used to evaluate the normalcy of some ABR and hair cell data sets (Figs. 4,5). A total 21 C57BL/6J (B6) mice also underwent 11 day KM or saline injection, then received noise at PND 30. All groups were randomly mixed by gender. Sample sizes by experiment and group are given in each graph. Inbred mice were purchased from The Jackson Laboratory (JAX) or bred from these. B6 mice were used to test the generality of protection against NIHL by kanamycin. Although neither B6 nor any other single strain can conclusively resolve this issue, B6 mice were chosen based on the wealth of noise injury data available on these (Henry, 1982a; Li, 1992b; Erway and Willott, 1996; Davis et al., 2001; Ohlemiller and Gagnon, 2007), and on the non-overlapping origins of B6 and CBA/J from their inception as inbred lines (Fox et al., 1997). In addition, extant data suggest that these two strains show different capacities for protection by some preconditioning paradigms (Gagnon et al., 2007).
Table I.
Treatment schedule for young CBA/J and C57BL/6J
| Postnatal Day (doses/day) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Strain | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | ABR Day | Protection |
| Fernandez et al. (2010) | CBA/J | *2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 1x+Noise | 40 | Full | ||
| Fernandez et al. (2010) | CBA/J | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | Noise | 41 | Full | ||
| Fernandez et al. (2010) | CBA/J | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | Noise | 42 | Full | ||
| Fernandez et al. (2010) | CBA/J | 1x+Noise | 40 | None | ||||||||||||
| A (Present study) | CBA/J | 1x | 1x | 1x | 1x | 1x | 1x | 1x | 1x | 1x | 1x+Noise | 40 | Full | |||
| B (Present study) | CBA/J | 1x | 1x | 1x | 1x | 1x | Noise | 40 | Full | |||||||
| C (Present study) | CBA/J | 1x | 1x | 1x | 1x | Noise | 40 | Partial | ||||||||
| D (Present study) | CBA/J | 1x | Noise | 40 | Partial | |||||||||||
| E (Present study) | CBA/J | 1x | Noise | 40 | None | |||||||||||
| F (Present study) | CBA/J | 1x | 1x | Noise | 40 | Partial | ||||||||||
| G (Present study) | C57BL/6J | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 1x+Noise | 44 | None | ||
All doses 300 mg/kg, sc
Administered 12 hrs apart
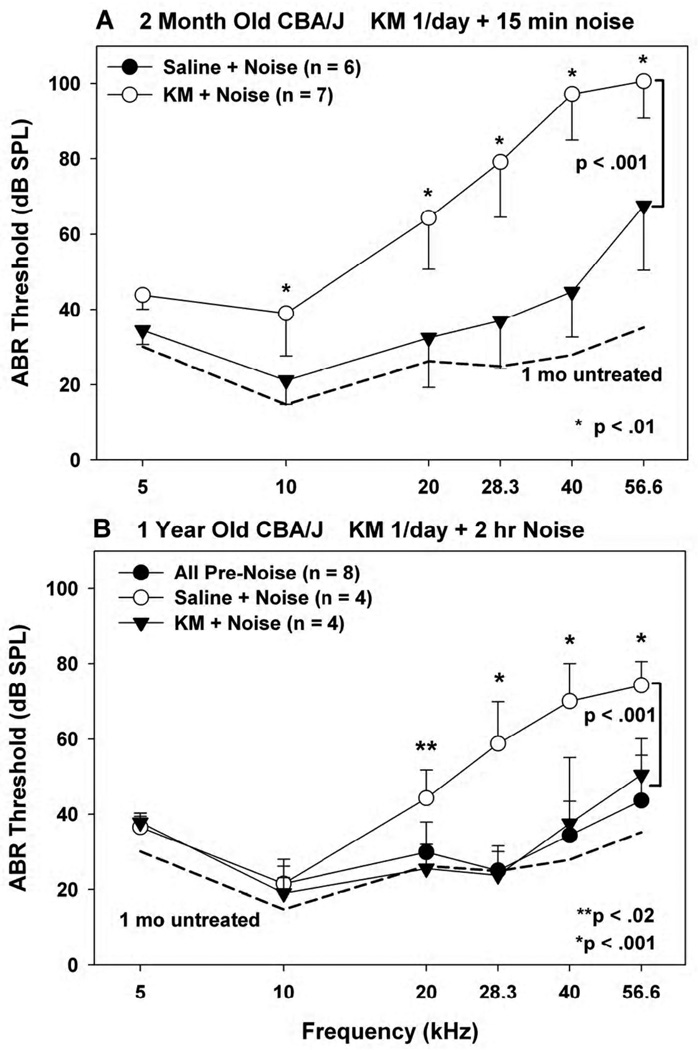
Figure 4.
(A) Mean(−SD) ABR thresholds in 2 month old noise-exposed CBA/J mice receiving KM or saline once/day for 10 days prior to noise. (B) Mean(+SD) ABR thresholds in 1 year old noise-exposed CBA/J mice receiving KM or saline once/day for 10 days prior to noise. In B, thresholds were obtained at the end of the dosing regimen, but prior to noise. These were similar in KM- and saline-treated mice, and have been combined. In all cases KM dosing was held constant at 300 mg/kg. Typical ABR results for young CBA/J are included for comparison in both graphs (dotted lines).
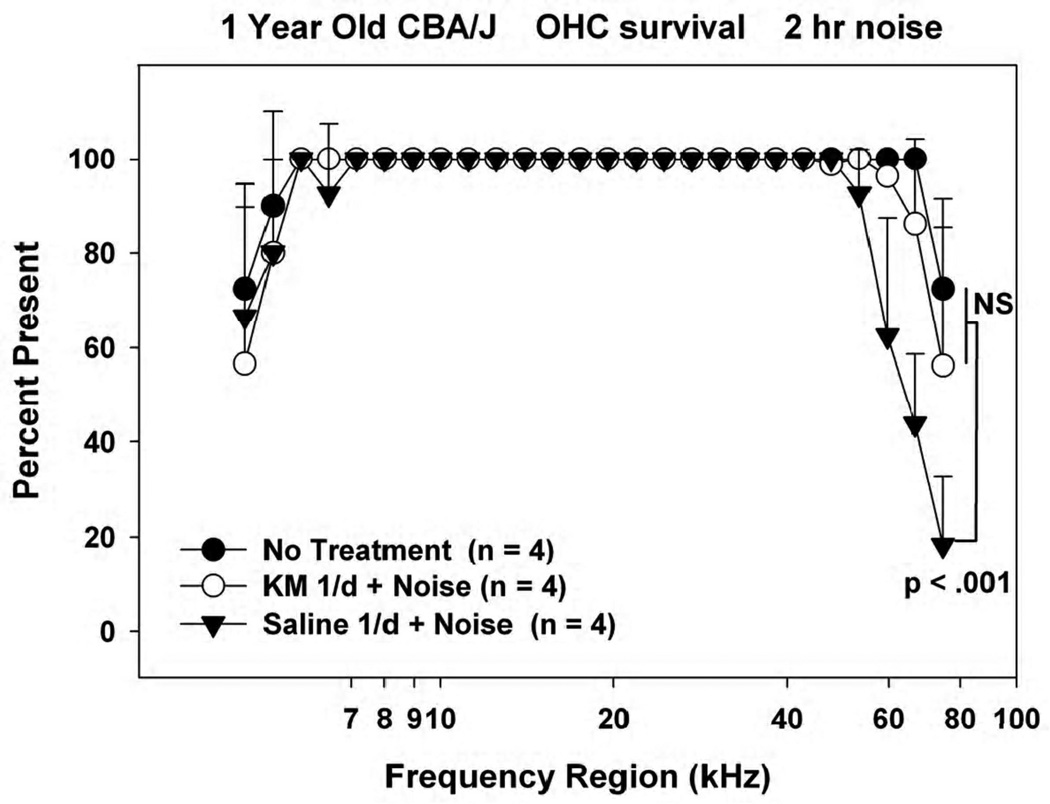
Figure 5.
Mean(+SD) outer hair cell counts in 1 year old noise-exposed and control CBA/J mice receiving KM or saline for 10 days prior to noise. Accompanying ABR data are shown in Figure 4.
Drug treatments
In all cases each dose of KM was delivered subcutaneously at 300 mg/kg, and only the timing of dosing varied. The choice of 300 mg/kg as a subclinical dose was based on a comparison of relative kanamycin ototoxicity in young CBA/J, B6, and BALB/c mice (Wu et al., 2001).
1 Month Old CBA/J
Variations from our original twice per day dosing regimen included progressively reducing the frequency of KM/saline dosing to 1/day, every other day, and every 3rd day (Table I.), always beginning dosing on PND 20. To prevent a prolonged gap after the final dose, the last dose for the every-3rd-day paradigm was given on PND 30 rather than PND 29. Varying dose interval over the same total duration also meant that each group received a different number of doses as follows: once/day (10 doses), every other day (5 doses), every 3rd day (4 doses). Additional experiments in CBA/J tested the efficacy of a single dose applied either on PND 28 or 29, followed by noise on PND 30. The final experiment in 1 month old CBA/Js probed further the minimal effective protocol, applying KM on both PND 27 and 29, followed by noise on PND 30.
Older CBA/J
The sensitivity period to aminoglycosides in mice ends around 1 month of age (Henry et al., 1981; Wu et al., 2001), while that for noise extends to at least 4 months (Henry, 1982b; Kujawa and Liberman, 2009). To test the significance of these windows for preconditioning processes, and the effect of age in general, additional experiments were conducted in mice that were either 2 months or 1 year old at the time of noise exposure. Noting, as we will show, that a single application of KM per day is highly effective in young CBA/J, and not wishing to stress the 1 year old CBA/J mice any more than necessary, these received KM or saline once/day for 10 days, followed by noise on day 11.
1 Month Old B6
Having no information at the outset what might constitute the optimal treatment protocol for B6, we determined to recapitulate the original intensive approach taken in CBA/J. Thus these mice received KM twice/day from PND 20–30, with noise exposure on PND 30. The logic of applying the most intensive protocol was to maximize the odds of either clear protection or overt harm by KM.
Noise exposure
All exposures utilized broadband noise (4–45 kHz, 110 dB SPL), produced and filtered with General Radio 1310 generators and Krohn-Hite 3550 filters, respectively. Pairs of mice from different treatment groups were placed in a wire cage suspended among four Motorola KSN1020A piezo ceramic speakers placed at 0, 90, 180, and 270 degrees azimuth in a foam-lined single-walled sound-proof booth (Industrial Acoustics, Bronx, NY). The cage was rotated at 0.013 Hz during the exposure to achieve a homogeneous sound field. The duration of exposure varied by strain and age, in accordance with previous work indicating marked differences in noise vulnerability between young (6 wk old) CBA/J and B6, and between young and older CBA/J (6 wks vs. 6 mos) (Ohlemiller et al., 2011). In each case the goal was to inflict a permanent moderate (20–50 dB) hearing loss at most ABR test frequencies. At PND 30–31 CBA/J mice are near peak vulnerability, requiring as little as 30 s of noise to manifest substantial NIHL. Therefore, 30 s exposures were applied to these mice, while B6 mice of the same age received 30 min of noise. By 2 months, CBA/Js can withstand longer exposures, so that these received 15 min of noise. Finally, because the effects of a moderate broadband 2 hr exposure in older (8–10 mos) CBA/J have been characterized (Ohlemiller and Gagnon, 2007), this exposure was selected for 1 year old CBA/J.
ABR recording
ABR testing was performed 10–14 days after noise exposure, depending on the experiment, by an operator blinded to condition. Because of the risk of losing fragile weanling mice in multiple tests, ABR tests in CBA/J and B6 mice noise exposed at 1 month were performed only after noise, so that the extent of NIHL was judged by comparison of treatment groups. Because of the limited number of 1 year old CBA/J mice available, these were tested both before treatment and after noise. Testing was performed using Tucker-Davis Technologies (TDT) System II hardware and software. Animals were anesthetized with a solution of ketamine and xylazine (80/15 mg/kg, i.p.) and positioned dorsally in a custom headholder. Subdermal platinum needle electrodes (Grass) were placed in the mid-back (ground), behind the right pinna (active), and at the vertex (reference). Body temperature was monitored throughout testing using a rectal probe, and maintained at 37.5 ± 1.0°C using a DC current-based isothermal pad (FHC). The right ear of each mouse was stimulated in freefield using a TDT ES-1 speaker placed at 7 cm distance along the interaural axis. Stimuli were 5 ms tonebursts (1000 repetitions, 20/s, 1.0 ms rise/fall time) at frequencies of 5, 10, 20, 28.3, 40, and for later experiments, 56.6 kHz. To eliminate contributions to the ABR by the unstimulated ear, the left external meatus was compressed using a spring-loaded clip. Responses were amplified ×100,000 and filtered at 100–10,000 Hz. Thresholds were taken to be the lowest sound level for which Wave I could be identified, using a 5 dB minimum step size. Data were analyzed by 2 way ANOVA (threshold by group, frequency), followed by Bonferroni multiple comparisons tests.
Sample preparation
One cochlea from each of 3–9 mice/group was prepared for hair cell counts after recording (See related figures for sample sizes.). For sacrifice, animals were overdosed using Pentobarbital and perfused transcardially with cold 2.0% paraformaldehyde/2.0% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). Each cochlea was rapidly isolated, immersed in the same fixative, and the stapes was removed. Complete infiltration of the cochlea by fixative was facilitated by drilling a small hole at the apex of the cochlear capsule, and gently circulating the fixative over the cochlea using a transfer pipet. After decalcification in sodium EDTA for 72 hours, cochleas were post-fixed in buffered 1% osmium tetroxide, dehydrated in an ascending acetone series, and embedded in Epon. Cochleae were dissected using fine blades into half-turn segments, immersed in mineral oil in a depression slide, and examined as surface preparations by Nomarski optics using a 20× oil objective and a calibrated grid ocular. The percent outer hair cells (OHCs) and inner hair cells (IHCs) missing (as judged by the absence of nuclei) was estimated in contiguous 200 µm segments, and data were recorded separately by cell type as a function of distance from the basal tip. For each hair cell type, distance versus percent present was plotted as a function of frequency based on Muller et al. (Muller et al., 2005). Hair cell survival differences by group were analyzed by 2 way ANOVA (cell loss by group, cochlear place), followed by Bonferroni multiple comparisons tests. Because only IHC loss was minimal, and varied little by group, only OHC results are presented.
RESULTS
Reduced dosing frequency in young CBA/J
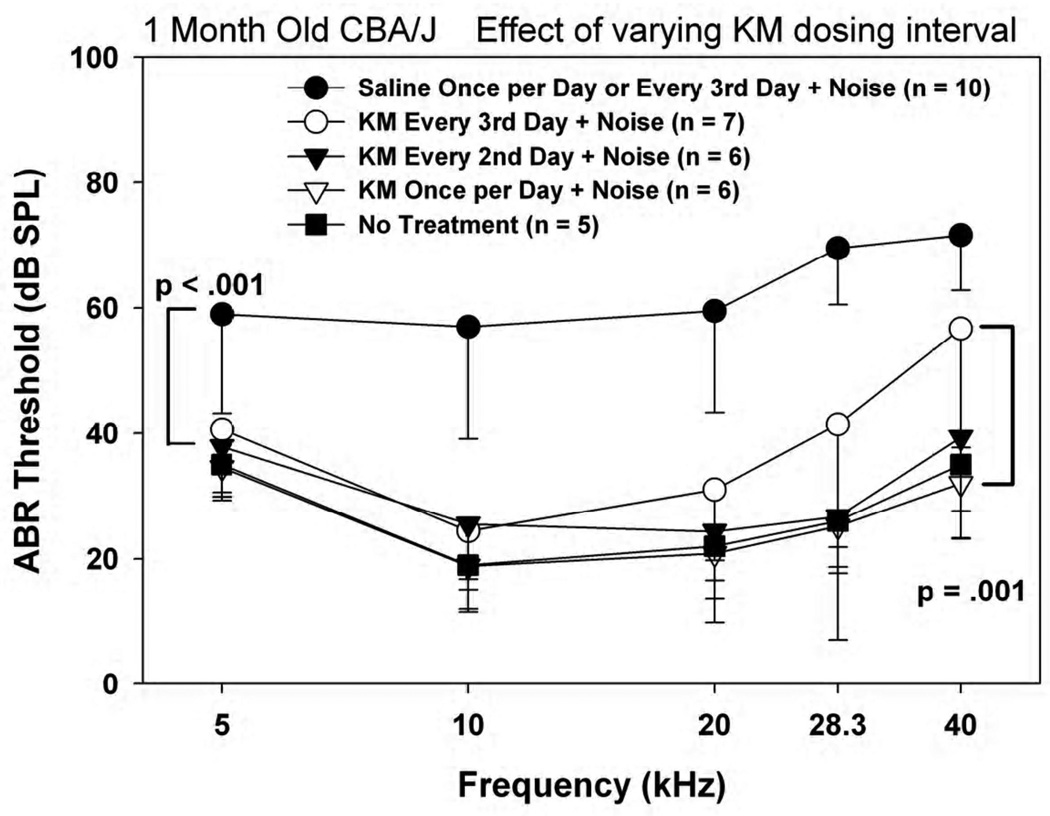
The previous data indicated that a twice/day KM injection regimen is nearly completely protective against otherwise substantial NIHL in 1 month old CBA/J (Fernandez et al., 2010). Figure 1 shows the result of progressively reducing dosing frequency to 1/day, every other day, then every third day. Saline control animals receiving injections 1/day or every 3rd day showed similar results, so that data from these have been collapsed. Only when KM was applied as infrequently as every third day was protection against 30 s of noise clearly less than complete for frequencies up to 40 kHz.
Figure 1.
Mean(−SD) ABR thresholds in 1 month old noise-exposed and control CBA/J mice as a function of KM dosing frequency, keeping each dose constant at 300 mg/kg.
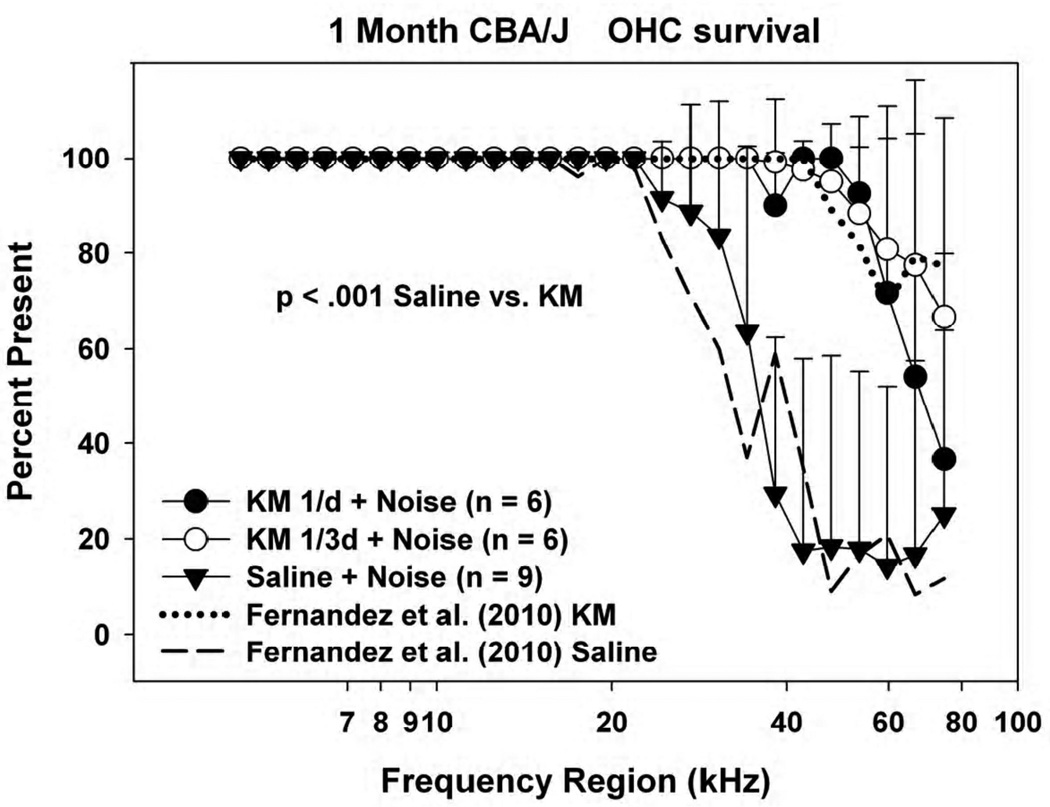
Corresponding outer hair cell counts are shown in Figure 2. Because CBA/J mice less than 8 months old show essentially no age-associated hair cell loss (Sha et al., 2008), any loss is assumed to indicate either noise injury or direct KM toxicity. Saline control and 2/day KM data from Fernandez et al. (2010) are also reproduced for comparison. OHC loss was limited in all cases to the lower cochlear base, yet was further restricted to the hook region in all KM-treated groups. Comparing the previous and present animals, we could discern no difference in OHC survival between mice receiving KM twice/day, once per day, or every 3rd day. There also appeared to be no systematic improvement in OHC survival in the hook region as the frequency of KM injections was decreased. From these data we conclude that the original twice per day KM regimen was at least four times more intensive than required for essentially complete protection of frequency regions below 40 kHz in the young CBA/J mice. The hook region (approximately the most basal 0.8 mm), however, appeared refractory to protection by KM at any dose. The relative constancy of OHC loss across KM dosing schedules suggests that the OHC loss in the hook region was un-remedied noise injury, and not injury by KM.
Figure 2.
Mean(+SD) outer hair cell counts in 1 month old noise-exposed and control CBA/J mice as a function of KM dosing frequency, with overlaid data from Fernandez et al. (2010). Accompanying ABR data are shown in Figure 1.
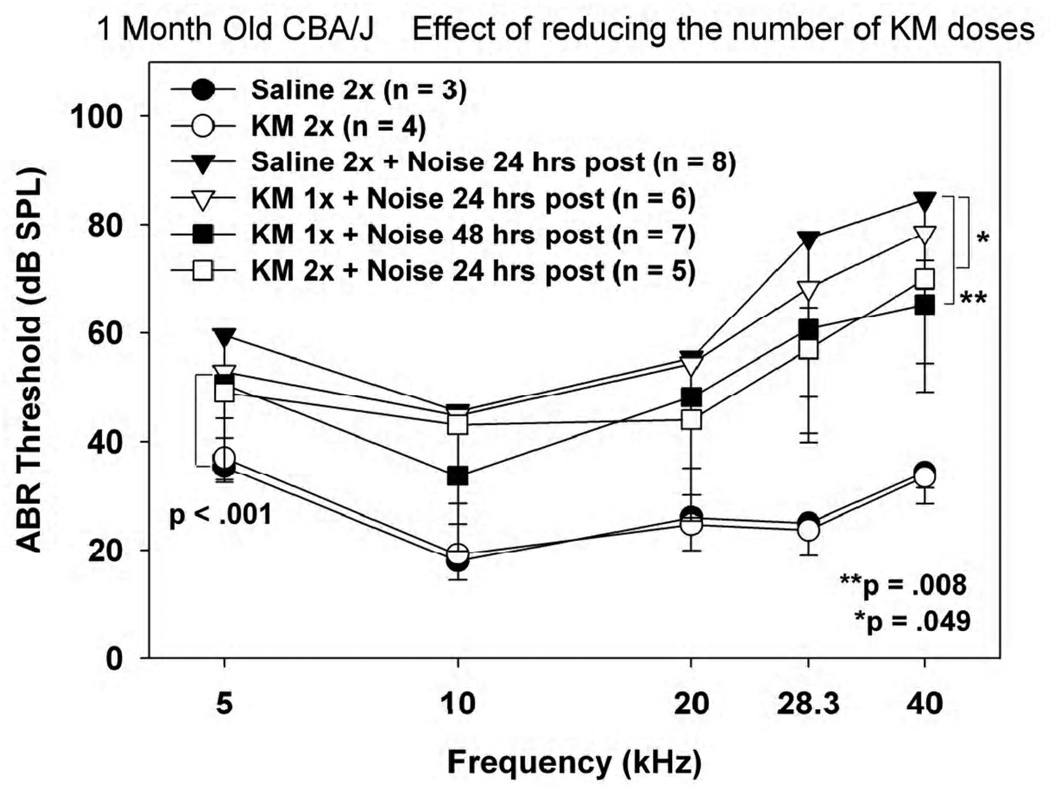
Minimal dosing requirements in young CBA/J
Neither the results of our previous paper (Fernandez et al., 2010) nor those presented in the previous section rule out substantial efficacy in 1 month old CBA/J mice of a single dose of KM followed by noise exposure 24 or 48 hours later. Figure 3 shows an explicit test of the protective power of a single KM application either 24 or 48 hrs prior to noise, or 2 doses applied 48 hours apart with noise following the next day. Saline controls covered only the 2 dose condition. Although there were minor—albeit statistically significant—differences among the KM groups, all differed substantially from thresholds in mice receiving KM or saline alone. Although these tests do not specifically reveal the minimal KM dosing regimen needed for optimal efficacy, we infer such a regimen to entail at least three 300 mg/kg injections, spaced no more than 48 hours apart. The minimal regimen is thus sub-chronic in nature, in keeping with a preconditioning mechanism that requires some time to engage.
Figure 3.
Mean(−SD) ABR thresholds in 1 month old noise-exposed and control CBA/J mice as a function of the number and timing of KM injections, keeping each dose constant at 300 mg/kg. Animals received 1 or 2 doses of KM or saline, either 24 or 48 hrs prior to noise.
Efficacy of KM in older CBA/J
The protective potential of KM against noise was further tested in CBA/J mice receiving noise at 2 mos or 1 yr of age (Fig. 4). In each case, either KM or saline was administered once per day. At 2 mos, KM treated mice sustained significantly less NIHL than saline controls (Fig. 4A). Although concurrent no-treatment controls were not run, typical age-matched archival data (dashed line) indicate that the protection was not complete. Because of the limited availability 1 year old mice, both KM- and saline-treated animals underwent initial ABR testing at the end of their injections. At this point the two groups completely overlapped, suggesting the KM alone was not toxic, and data from these have been combined (Fig. 4B). Juxtaposed with the young archival data, it is apparent that these animals all had near-normal hearing, as expected at this age in CBA/J (Ohlemiller et al., 2010), so that pre-existing hearing loss was not a factor in any subsequent NIHL. Two hours of noise imparted 20–40 dB threshold shifts to the saline-treated animals, consistent with previous data (Ohlemiller and Gagnon, 2007). Under these conditions, the KM-treated mice showed essentially complete threshold protection up to 56.6 kHz.
Figure 5 shows accompanying OHC survival data from the 1 year old animals. Owing to age, all mice evidenced OHC loss in the extreme base and apex (Sha et al., 2008; Ohlemiller et al., 2010). The untreated and KM-treated animals were similar in this respect, suggesting that KM treatment prevented all noise-related OHC loss in these mice. By contrast, basal OHC losses in the saline-treated mice, while still limited to the hook, extended significantly further into this region. From these tests we conclude that protection against noise by KM in CBA/J is limited neither to the sensitive period for ototoxic injury in mice, nor to any age up to one year.
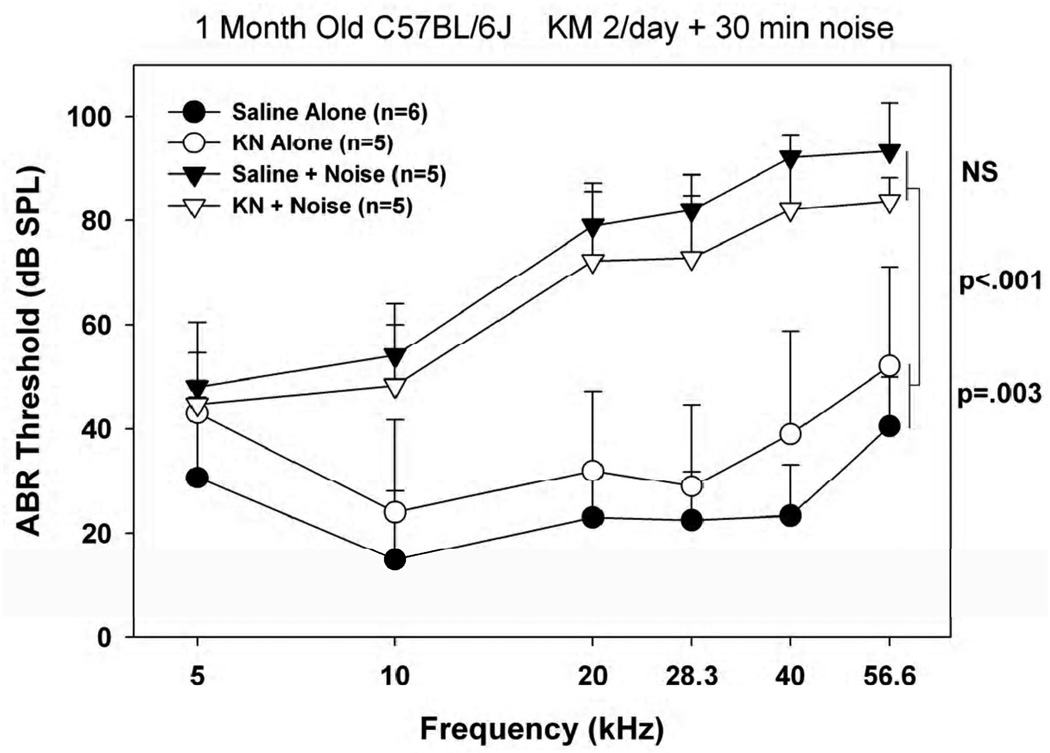
Efficacy of KM in young C57BL/6
Figure 6 shows results when our original KM treatment paradigm was applied to 1 month old B6 mice. Unlike CBA/J, mice receiving KM alone exhibited modest (~10 dB) but statistically significant hearing loss. Also unlike CBA/J, animals receiving KM prior to noise fared no better against noise than did saline controls. The small protective effect that might be hinted in these data may reflect protection afforded by the initial threshold shift.
Figure 6.
Mean(+SD) ABR thresholds in 1 month old noise-exposed and control C57BL/6J mice receiving KM or saline twice/day prior to noise, keeping each dose constant at 300 mg/kg.
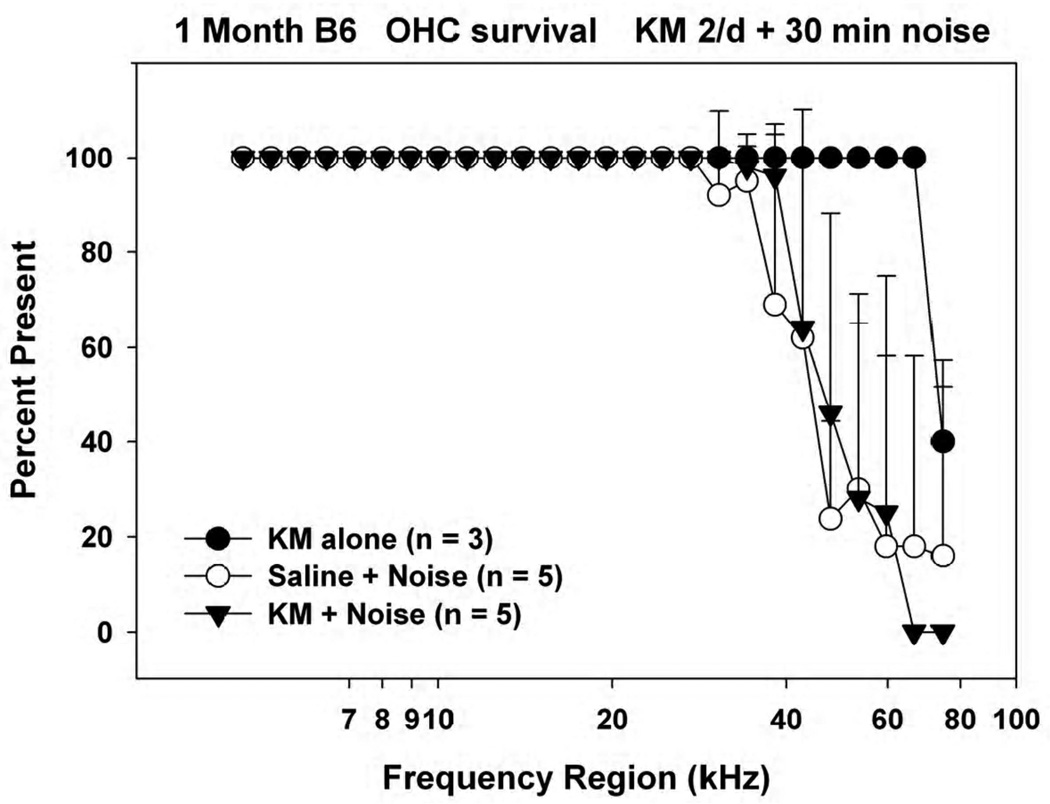
Figure 7 shows corresponding OHC counts. The B6 mice receiving KM alone showed cell loss in the extreme base that is probably associated with the earliest effects of the Cdh23Ahl allele (Johnson et al., 1997). By contrast, both saline- and KM-treated animals showed additional OHC loss that extended well into the lower cochlear basal turn. From these tests we conclude that the same KM injection regimen that shows dramatic protection in young CBA/J mice protects neither hearing thresholds nor outer hair cells in young B6 mice, and may in fact be harmful.
Figure 7.
Mean(+SD) outer hair cell counts in 1 month old noise-exposed and control C57BL/6J mice receiving KM or saline twice/day for 10 days prior to noise. Accompanying ABR data are shown in Figure 6.
DISCUSSION
Our data confirm the findings of Fernandez et al. (2010), and further indicate that the protective effects of sub-chronic KM against NIHL extend down to dosing levels that cause neither threshold elevation nor hair cell loss in CBA/J mice. The present data support nearly complete protection against noise exposures ranging up to 2 hrs, exposures that otherwise inflict moderate NIHL and hair cell loss. In fact, the degree of protection we observed over a range of conditions exceeds that reported for some pharmacotherapies (Ohlemiller, 2008) so that the cellular and molecular mechanisms are well worth exploring. The apparent requirement for multiple KM applications over days to obtain maximal protection supports the contention that KM initiates a preconditioned protective state that probably shares properties with other preconditioning paradigms in the cochlea, as well as in brain, retina, and other tissues. While the seeming lack of protection in B6 mice suggests that KM protection may not work on all genetic backgrounds, we do not yet know which case is more typical. Moreover, classical genetic approaches brought to bear on this glaring strain difference may ultimately reveal genes that are critical to innate protective processes and which may predispose some individuals to acquired hearing loss.
Permissive character of KM protection in CBA/J mice
Table I shows that minor variations in the application of KM do not impair its protective effects in CBA/J mice, apparently as long as more than 2 doses are applied no more than 48 hrs apart. The extent of protection was affected little by the duration of the noise exposure, or by the age at which the experiments were conducted. These findings were not necessarily anticipated. During the first months of life CBA/J mice are reportedly more vulnerable to both noise and ototoxins than are B6 mice (Wu et al., 2001; Ohlemiller et al., 2011). Processes unique to CBA/J that are active only during this period could therefore have limited KM’s protective effects to the sensitive period for noise, ototoxins, or both.
Lack of protection young C57BL/6 mice
We could not demonstrate protection by KM in 1 month old B6 mice. Our experiments do not prove that KM cannot elicit protection in B6, since there may be suitable conditions we did not test. Nevertheless, contrasted with the permissive nature of protection in CBA/J, the B6 data suggest that there are fundamental, genetically related differences in the internal response to low-dose KM among inbred strains.
Among the molecular mechanisms implicated in various forms of preconditioning (see below), some will overlap. Thus it is worth considering whether there exists an established pattern of unresponsiveness to preconditioning in B6. The literature on preconditioning against NIHL includes few inbred mouse strain comparisons, yet among these are two comparisons of B6 versus CBA/J or CBA/CaJ. Both B6 and CBA/CaJ gain protection from restraint stress (Peppi et al., 2011); however, only B6 showed no benefit from hypoxia (Gagnon et al., 2007). The basis of preconditioning is an up-regulation of innate protective cascades by a noxious, but not permanently harmful, stressor. There may be a precarious balance between the amount of harm inflicted by the preconditioning stimulus and the strength of the internal response. The response to low-dose KM presumably involves some level of injury to hair cells and other cells that engages protective and repair machinery. Our ABR data suggest that B6 mice are actually harmed, rather than helped, by kanamycin. It may be the relative strength of these two processes—injury versus the engagement of protections—that distinguishes CBA/J from B6 mice. The suggestion in our data that systemic KM at 300 mg/kg may be more ototoxic to young B6 mice than to young CBA/Js seems to diverge from an earlier report showing the opposite (Wu et al., 2001). The variance in results may reflect a difference in the age at which KM treatment began (3 wks vs. 5 wks).
Genes and mechanisms
Demonstrated forms of cochlear preconditioning against NIHL include pre-treatment by heat (Yoshida et al., 1999), hypoxia (Gagnon et al., 2007), moderate sound (Yoshida and Liberman, 2000; Niu and Canlon, 2002), and restraint (Wang and Liberman, 2002). Glucocorticoid pathways have been implicated in the case of sound and restraint (Wang and Liberman, 2002; Canlon et al., 2007), and a key transcription factor (PLZF) has been recently identified (Peppi et al., 2011). Heatshock protein HSP70 largely mediates protection by elevation of body temperature (Yoshida et al., 1999), while our own work using hypoxia (Gagnon et al., 2007) suggested a role for HIF-1α. The preconditioning literature across systems and tissues further implicates antioxidants, TNFα, NFκB, HO-1, and Nrf2 (Dirnagl et al., 2003; Ran et al., 2005; Gidday, 2006; Lehotsky et al., 2009),. All the above factors are known to participate in cochlear responses to stress (Rybak, 2007; So et al., 2008; Matsunobu et al., 2009; Yamamoto et al., 2009; Chance et al., 2010), some to aminoglycosides specifically (Jiang et al., 2005; Chen et al., 2008; Taleb et al., 2009). The friend-or-foe nature of some factors, in particular TNFα and NFκB (Shi and Nuttall, 2007; Yamamoto et al., 2009), may depend on when and where they are expressed (Ohlemiller, 2008). The critical genetic differences that distinguish CBA/J and B6 mice with regard to KM ‘protectability’ may include genes that encode one or more of the factors just listed, or a downstream target. There is probable commonality among genes that mediate preconditioning, genes that regulate innate cellular protective and repair processes, and ‘susceptibility’ genes that render some individuals more likely to exhibit acquired hearing loss. It may be possible to use mouse strain differences like those reported here as the basis for mapping studies to uncover genes and mechanisms common to all three categories. Genes thus revealed in mice may play the same role in humans.
Critical site of protection
Depending on exposure parameters, permanent cochlear noise injury in CBA/CaJ and CBA/J mice may encompass the organ of Corti, spiral ganglion, and lateral wall (Wang et al., 2002; Hirose and Liberman, 2003; Ohlemiller and Gagnon, 2007; Kujawa and Liberman, 2009). At least in these strains, the latter targets do not appear to influence threshold recovery, although for any given region the health of the spiral ligament may influence the survival of adjacent hair cells (Ohlemiller, 2008). Nevertheless, in kanamycin preconditioning as well as other methods, the key effects are probably improved hair cell function and survival. Significant preservation of OHCs was found in all KM-protected groups, but notably, not in B6. Typical of mice (Ou et al., 2000; Wang et al., 2002; Fernandez et al., 2010), the range of frequencies exhibiting noise-induced ABR threshold shifts exceeded those corresponding to cochlear locations with hair cell loss. Most likely, KM promoted repair and retention of function in hair cells not killed by noise. In CBA/J and B6 we would therefore predict that the critical mediators of KM preconditioning are expressed—or not—in the organ of Corti or adjacent spiral ligament.
Research Highlights.
CBA/J mice can be nearly completely protected from noise-induced hearing loss by sub-chronic application of low-dose kanamycin (300 mg/kg, sc).
Maximum protection can be obtained from a few doses spaced at 48 hr intervals, and is not dependent on mouse age.
Protection by kanamycin in CBA/J is associated with significant preservation of outer hair cells.
Dosing paradigms that work well in CBA/J mice do not protect hearing thresholds or outer hair cells in C57BL/6J mice.
Acknowledgements
Supported by P30 DC004665 (R. Chole), P30 NS057105 (D. Holtzman), R01 DC03454 (KKO), R01 DC08321 (KKO), TL1 RR024995, T35 DC008765, WUSM Department of Otolaryngology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernard PA. Freedom from ototoxicity in aminoglycoside treated neonates: A mistaken notion. Laryngoscope. 1981;91:1985–1994. doi: 10.1288/00005537-198112000-00001. [DOI] [PubMed] [Google Scholar]
- Canlon B, Meltser I, Johansson PYT. Glucocorticoid receptors modulate auditory sensitivity to acoustic trauma. Hearing Res. 2007;226:61–69. doi: 10.1016/j.heares.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Chance MR, Chang J, Liu S, Gokulrangan G, Chen DH-C, Lindsay A, Geng R, Zheng QYKA. Proteomics, bioinfomatics and targeted gene expression analysis reveals up-regulation of cochlin and identifies other potential biomarkers in the mouse model for deafness in usher syndrome type 1F. Hum. Mol. Genet. 2010;19:1515–1527. doi: 10.1093/hmg/ddq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-S, Saunders JC. The sensitive period for ototoxicity of kanamycin in mice: Morphological evidence. Arch. Otorhinolaryngol. 1983;238:217–223. doi: 10.1007/BF00453932. [DOI] [PubMed] [Google Scholar]
- Chen F, Sha S-H, Schacht J. Stress-induced changes in mitochondrial peroxiredoxin in mouse cochlear hair cells. Abstr., Assn. Res. Otolaryngol. 2008;31:50. [Google Scholar]
- Davis RR, Newlander JK, Ling X-B, Cortopassi GA, Kreig EF, Erway LC. Genetic basis for susceptibility to noise-induced hearing loss in mice. Hearing Res. 2001;155:82–90. doi: 10.1016/s0378-5955(01)00250-7. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. TINS. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Eisen A, Fisman EZ, Rubenfire M, Freimark D, McKechnie R, Tenenbaum A, Motro M, Adler Y. Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis. 2004;172:201–210. doi: 10.1016/S0021-9150(03)00238-7. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF. Genetic Susceptibility to Noise-Induced Hearing Loss. In: Axelsson A, editor. Scientific Basis of Noise-Induced Hearing Loss. New York: Thieme; 1996. pp. 56–64. [Google Scholar]
- Fernandez EA, Ohlemiller KK, Gagnon PM, Clark WW. Protection against noise-induced hearing loss in young CBA/J mice by low-dose kanamycin. J. Assoc. Res. Otolaryngol. 2010;11:235–244. doi: 10.1007/s10162-009-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RR, Witham BA, Neleski LA, editors. Handbook of genetically standardized JAX mice. Bar Harbor ME: The Jackson Laboratory; 1997. [Google Scholar]
- Gagnon PM, Simmons DD, Bao J, Lei D, Ortmann AJ, Ohlemiller KK. Temporal and genetic influences on protection against noise-induced hearing loss by hypoxic preconditioning in mice. Hearing Res. 2007;226:79–91. doi: 10.1016/j.heares.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nature Rev. Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Henley CM, Rybak LP. Ototoxicity in developing animals. Brain Research Reviews. 1995;20:68–90. doi: 10.1016/0165-0173(94)00006-b. [DOI] [PubMed] [Google Scholar]
- Henry KR. Influence of genotype and age on noise-induced auditory losses. Behav. Genet. 1982a;12:563–573. doi: 10.1007/BF01070410. [DOI] [PubMed] [Google Scholar]
- Henry KR. Age-related changes in sensitivity of the postpubertal ear to acoustic trauma. Hearing Res. 1982b;8:285–294. doi: 10.1016/0378-5955(82)90020-x. [DOI] [PubMed] [Google Scholar]
- Henry KR, Chole RA, McGinn MD, Frush DP. Increased Ototoxicity in Both Young and Old Mice. Arch.Otolaryngol. 1981;107:92–95. doi: 10.1001/archotol.1981.00790380022006. [DOI] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J. Assoc. Res. Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Sha S, Schacht J. NF-kB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. J. Neurosci. Res. 2005;79:644–651. doi: 10.1002/jnr.20392. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hearing Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after 'temporary' noise-induced hearing loss. J. Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehotsky J, Burda J, Danielisova V, Gottlieb M, Kaplan P, Saniova B. Ischemic tolerance: The mechanisms of neuroprotective strategy. Anatom. Rec. 2009;292:2002–2012. doi: 10.1002/ar.20970. [DOI] [PubMed] [Google Scholar]
- Li H-S. Genetic influences on susceptibility of the auditory system to aging and environmental factors. Scand. Audiol. (Suppl.) 1992a;36:1–39. [PubMed] [Google Scholar]
- Li H, Steyger PS. Synergistic ototoxicity due to noise exposure and aminoglycoside antibiotics. Noise & Health. 2009;11:26–32. doi: 10.4103/1463-1741.45310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS. Influence of genotype and age on acute acoustic trauma and recovery in CBA/Ca and C57BL/6J mice. Acta Oto-Laryngol. 1992b;112:956–967. doi: 10.3109/00016489209137496. [DOI] [PubMed] [Google Scholar]
- Matsunobu T, Satoh Y, Ogawa K, Shiotani A. Heme oxygenase-1 expression in the guinea pig cochlea induced by intense noise stimulation Acta. Otolaryngol. 2009;129:18–23. doi: 10.1080/00016480902933056. [DOI] [PubMed] [Google Scholar]
- Muller M, von Hunerbein K, Hoidis S, Smolders JWT. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hearing Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Niu X, Canlon B. Protective mechanisms of sound conditioning. Adv. Otorhinolaryngol. 2002;59:96–105. doi: 10.1159/000059246. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Recent findings and emerging questions in cochlear noise injury. Hearing Res. 2008;245:5–17. doi: 10.1016/j.heares.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Genetic dependence of cochlear cells and structures injured by noise. Hearing Res. 2007;224:34–50. doi: 10.1016/j.heares.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Heidbreder AF. Vulnerability to noise-induced hearing loss in 'middle-aged' and young adult mice: A dose-response approach in CBA, C57BL, and BALB inbred strains. Hearing Res. 2000;149:239–247. doi: 10.1016/s0378-5955(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Dahl AR, Gagnon PM. Divergent aging characteristics in CBA/J and CBA/CaJ mouse cochleae. J. Assoc. Res. Otolaryngol. 2010;11:605–623. doi: 10.1007/s10162-010-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rybak Rice ME, Rellinger EA, Ortmann AJ. Divergence of noise vulnerability in cochleae of young CBA/J and CBA/CaJ mice. Hearing Res. 2011;272:13–20. doi: 10.1016/j.heares.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Bohne BA, Harding GW. Noise damage in the C57BL/CBA mouse cochlea. Hearing Res. 2000;145:111–122. doi: 10.1016/s0378-5955(00)00081-2. [DOI] [PubMed] [Google Scholar]
- Peppi M, Kujawa SG, Sewell W. A corticosteroid-responsive transcription factor, promeolytic leukemia zinc finger protein, mediates protection of the cochlea from acoustic trauma. J. Neurosci. 2011;31:735–741. doi: 10.1523/JNEUROSCI.3955-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R. Sensitive Developmental Period and Acoustic Trauma: Facts and Hypotheses. In: Dancer AL, editor. Noise-Induced Hearing Loss. St. Louis: Mosby; 1992. pp. 196–203. [Google Scholar]
- Ran R, Xu H, Lu A, Bernaudin M, Sharp FR. Hypoxic preconditioning in the brain. Dev. Neurosci. 2005;27:87–92. doi: 10.1159/000085979. [DOI] [PubMed] [Google Scholar]
- Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr. Opin. Otolaryngol. Head Neck Surg. 2007;15:364–369. doi: 10.1097/MOO.0b013e3282eee452. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Chen C-S. Sensitive periods of susceptibility to auditory trauma in mammals. Environ. Health Persp. 1982;44:63–66. doi: 10.1289/ehp.824463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha S-H, Kanicki A, Dootz GA, Talaska AE, Halsey K, Dolan DF, Altschuler RA. Age-related auditory pathology in the CBA/J mouse. Hearing Res. 2008;243:87–94. doi: 10.1016/j.heares.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Nuttall AL. Expression of adhesion molecular proteins in the cochlear lateral wall of normal and PARP-1 mutant mice. Hearing Res. 2007;224:1–14. doi: 10.1016/j.heares.2006.10.011. [DOI] [PubMed] [Google Scholar]
- So H-S, Kim HJK, Kim E, Pae H-O, Chung H-T, Kim H-J, Kwon K-B, Lee K-M, Lee H-Y, Moon S-K, Park R. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J. Assoc. Res. Otolaryngol. 2008;9:290–306. doi: 10.1007/s10162-008-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life span. J. Acoust. Soc. Am. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Taleb M, Brandon CS, Lee F-S, Harris KC, Dillmann WH, Cunningham LL. Hsp70 inhibits aminoglycoside-induced hearing loss and cochlear hair cell death. Cell Stress Chaperones. 2009;14:427–437. doi: 10.1007/s12192-008-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liberman MC. Restraint stress and protection from acoustic injury in mice. Hearing Res. 2002;165:96–102. doi: 10.1016/s0378-5955(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF. Aging and the Auditory System: Anatomy, Physiology, and Psychophysics Singular Publishing Group. San Diego: 1991. [Google Scholar]
- Wu W-J, Sha S, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL, and BALB mice and the Sprague-Dawley rat. Hearing Res. 2001;158:165–178. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Omelchenko I, Shi S, Nuttall AL. The influence of NfκB signal-transduction pathways on the murine inner ear by acoustic overstimulation. J. Neurosci. Res. 2009;87:1832–1840. doi: 10.1002/jnr.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Liberman MC. Sound conditioning reduces noise-induced permanent threshold shift in mice. Hearing Res. 2000;148:213–219. doi: 10.1016/s0378-5955(00)00161-1. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Kristiansen A, Liberman MC. Heat stress and protection from permanent acoustic injury in mice. J. Neurosci. 1999;19:10116–10124. doi: 10.1523/JNEUROSCI.19-22-10116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KH, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analysis. Hearing Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]