Abstract
AIM
Paediatric drug prescriptions are known for their high percentages of off-label and unlicensed use. In paediatric oncology data available are scarce. The aim of this paper is an analysis of the licensing and labelling status of all prescribed medication over a 2 week period in a Dutch paediatric oncology centre.
METHODS
An analysis of the delivery of medication by the hospital pharmacy to patients admitted to the paediatric oncology centre was carried out.
RESULTS
In total 268 precriptions were filed for 39 patients. In 87% of children unlicensed medication was used. Fifty-nine per cent of the children received at least two unlicensed drugs. In total 72% of the drugs were used licensed and on-label was found in 57% of the prescriptions. There was a trend that in younger children percentages were lower. International and local guidelines necessitated in many cases unlicensed use, e.g. intrathecal prednisolone, low dose medication such as heparin, ethanol and vancomycin for locking intravenous devices and higher intravenous vancomycin dosages. There were no major differences with respect to type of malignancy.
CONCLUSION
Our figures are substantially higher than the figures reported from adult oncology. Comparison with other paediatric reports are cumbersome, due to different percentages of diseases in the reports and other rules to dispense medication in the outpatient setting. Our data are in line with reports mentioning the higher percentages of unlicensed and off-label use. Our data further underpin the need for more research on suitable formulations, dosages, safety and efficacy in these children.
Keywords: children, drug licensing, drug off-label use, oncology, pharmacotherapy
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
High percentages of off-label and unlicensed drug use are common in paediatrics.
Data in paediatric oncology patients hardly exist.
Extemporaneously prepared formulations are common.
WHAT THIS STUDY ADDS
Off-label and unlicensed use is substantially higher for cytostatic drugs in paediatric as compared with adult oncology.
Comparison with other paediatric reports on drug use cannot be made due to different percentages of diseases in the reports and other rules to dispense medication in the out-patient setting.
There is an urgent need for suitable formulations, licensing of dosages and provision of data on safety and efficacy in children with malignancies.
Introduction
Drugs are authorized on the basis of quality, safety and efficacy. Until recently the pharmaceutical industry was free to choose for which indication and for which age ranges marketing authorization was requested. As a result paediatric licensing and paediatric labelling is often lacking. Many drugs are used in children for indications without marketing authorization (unlicenced) or administrated not according to the approved formulation and/or dosing (off-label use).
Unlicensed and off-label use are widespread, and are mentioned in general acknowledged and accessible databases such as national formularies [1]. In a recent Dutch study only 55% of general physicians and 40% of paediatricians indicated that they were aware of the labelling and licensing status of medication (http://www.rivm.nl/bibliotheek/rapporten/370050001.html).
Unlicensed and off-label drugs have not been assessed with respect to safety and efficacy, as is done in a licensing process. Extrapolation from adults is often practised without consideration of changes in body composition, and ontogeny of metabolizing capability and excretion. Not only the drug itself may be a source of adverse effects, but also the other components of the formulation may introduce complications. From the past serious consequences are well known. Grey baby syndrome due to chloramphenicol and phocomelia due to thalidomide are even known to the general public. In primary care, figures differ from country to country. In the Netherlands up to 16% were unlicensed and 22% were used off-label [2]. In France 33% of unlabelled or unlicensed use is reported [3]. For neonatology and intensive care rates are even higher; i.e. two thirds of prescriptions were either off-label or unlicensed involving 90% of children [4]. For paediatric oncology data are scarce, but a UK report mentions 45% of unlicensed or off-label use [5]. This not only means that there is no public risk/benefit analysis, but even PK data of (cytostatic) drugs administered to children are very limited or confined to a few studies for a specific age group. In some instances only data based on case reports linked to adverse effects are available. Examples are reports on the excessive neurotoxicity of vincristine, resulting in hypotonia, feeding difficulties and paralysis of respiratory muscles [6–8]. Unexpected side effects during chemotherapeutic treatment of Wilms' tumours have resulted in the recommendation to decrease the vincristine dosages to 50% [9]. As a result in many protocols and some textbooks the rationale for dose recommendations is less clear and sources often are not indicated [10]. In most protocols dose reductions are proposed either given as a percentage according to age or calculated based on body weight instead of the body surface area. Since liver volume is correlated with body surface area and not weight dosing according to body surface area would be more relevant for drugs with hepatic clearance only. Also the impact of ontogeny on the metabolic capacity is completely neglected this way [11]. Even in a specific protocol for infants with acute lymphoblastic leukaemia (ALL) substantial dose reductions are mentioned irrespective of the drug involved [12, 13]. The pharmacokinetic relevance of this is doubtful [14, 15]. In this manuscript we report on the licensing and labelling status of prescriptions used in a paediatric oncology ward.
Methods
In the Netherlands all patients suffering from a malignancy are treated in one of the eight paediatric oncology departments. The paediatric oncology ward at the Emma Children Hospital AMC is one of the largest centres. In order to collect data on administered drugs to oncology patients, the prescription ordering system was analyzed for all medication orders given during the first 2 weeks of April 2008. Patient characteristics, disease, age, brand name and posology of all drugs were collected. Medical and nursing staff were not allowed to administer any medication not ordered via this system. The collected data were screened for licensing status and labelling according to national authorization (http://www.cbg-meb.nl) and European authorization (http://www.ema.europa.eu). Medication was categorized as unlicensed when the drug was contraindicated for use in children, drugs formulations were home or hospital pharmacy prepared (extemporaneous) and there was a lack of posology guidelines for children in the summary of product characteristics (SmPC). Off-label used drugs were drugs in which a discrepancy of the prescription was deviant in respect to authorized age (or weight), daily dosage and frequency, dosage form, route of administration, indication or contraindication against use in a particular patient.
All patients admitted to the paediatric oncology ward of the Emma Children Hospital AMC in the first 2 weeks of April 2008 were eligible. This ward is the only location in the Academic Medical Centre where cytostatic drugs are administered both for hospitalized as well as for children treated at the day-care centre. Medication used in the home setting was not addressed since these drugs, including cytostatics, are deliverd by the local home town pharmacies.
For statistical analysis of numerical differences the chi-square test was used.
Results
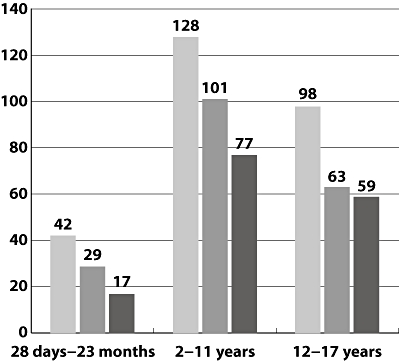
In total 268 drug prescriptions were filed for 39 patients. The number of prescriptions ranged from 1 to 15. Ages ranged from 0.25–17 years and the median age was 6 years. Distribution (according ICH E11 [16] categories) were 4, 23 and 11 patients in the age ranges 28 days–23 months, 2–12 years and 12–17 years, respectively. Distribution of patients was as follows: four brain tumour, one Ewing tumour, four germ cell tumour, two histiocytosis, one Hodgkin's disease, 11 leukaemia, one neuroblastoma, four non-Hodgkin lymphoma, two osteosarcoma, six nephroblastoma and three soft tissue tumour patients.
Per patient
In 34 children (87%) unlicenced medication was used at least once. In the remaining five patients only a limited number of drugs was prescribed (range 1 to 6, median 2 prescriptions), which was substantially lower than in the indicated 34 children (P = 0.015). Twenty-three (59%) of the children received at least two or more unlicensed medications. The number of prescriptions of the various medications used is depicted in Table 1. In this table the absolute numbers of medication for which license was given and the number of administrations given according to the SmPC are given in the last two columns.
Table 1.
Number of administered, licensed and on-label medications
| Medication group | Medication | Number of prescriptions | Licensed use | On-label use |
|---|---|---|---|---|
| Oncolytic | ||||
| Amsacrine | 1 | 1 | 1 | |
| Asparaginase | 2 | 2 | 2 | |
| Bleomycin | 1 | 1 | 0 | |
| Cytarabin | 10 | 10 | 6 | |
| Carboplatin | 3 | 0 | 0 | |
| Chloormethine | 1 | 0 | 0 | |
| Cyclofosfamide | 2 | 2 | 1 | |
| Cladribin | 1 | 0 | 0 | |
| Dacarbazine | 1 | 1 | 1 | |
| Dactinomycin | 1 | 1 | 1 | |
| Doxorubicine | 6 | 6 | 4 | |
| Etoposide | 8 | 1 | 1 | |
| Gemcitabine | 1 | 0 | 0 | |
| Ifosfamide | 2 | 0 | 0 | |
| Irinotecan | 1 | 0 | 0 | |
| Mercaptopurine | 1 | 1 | 1 | |
| Methotrexate | 10 | 10 | 10 | |
| Oxaliplatin | 1 | 0 | 0 | |
| Topotecan | 1 | 0 | 0 | |
| Vinorelbine | 3 | 0 | 0 | |
| Vinblastin | 2 | 1 | 1 | |
| Vincristine | 12 | 12 | 12 | |
| Biologicals | ||||
| Rituximab | 1 | 0 | 0 | |
| Corticosteroids | ||||
| Solumedrol | 1 | 1 | 1 | |
| Prednisolone | 10 | 2 | 1 | |
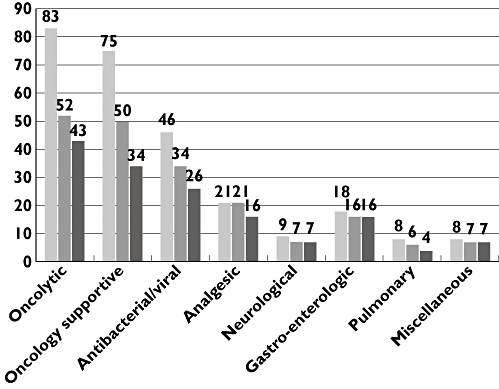
| Subtotal | 83 | 52 | 43 | |
| Oncology supportive care medication | ||||
| Allopurinol | 1 | 1 | 1 | |
| Enoxaparine | 1 | 0 | 0 | |
| Dexamethasone | 12 | 12 | 8 | |
| Domperidone | 6 | 6 | 4 | |
| Folinic acid | 3 | 3 | 2 | |
| Heparin | 24 | 0 | 0 | |
| Hypromellose | 4 | 4 | 4 | |
| Urometoxan | 2 | 2 | 2 | |
| Filgrastrim | 2 | 2 | 2 | |
| Polyvidon | 1 | 1 | 1 | |
| Ondansetron | 19 | 19 | 10 | |
| Subtotal | 75 | 50 | 34 | |
| Antibacterial and antiviral medication | ||||
| Aciclovir | 1 | 1 | 1 | |
| Amoxicillin | 2 | 2 | 2 | |
| Amoxicillin + clavulanic acid | 2 | 2 | 1 | |
| Cotrimoxazole | 11 | 11 | 10 | |
| Feniticilline | 1 | 1 | 0 | |
| Ciprofloxacin | 2 | 2 | 2 | |
| Colistin | 7 | 0 | 0 | |
| Flucloxacillin | 1 | 1 | 0 | |
| Gentamycin | 3 | 3 | 2 | |
| Itrakonazole | 1 | 0 | 0 | |
| Miconazole | 1 | 1 | 1 | |
| Nystatin | 4 | 4 | 4 | |
| Penicillin | 2 | 2 | 2 | |
| Trisporal | 3 | 0 | 0 | |
| Vancomycin | 3 | 3 | 0 | |
| Famciclovir | 1 | 0 | 0 | |
| Chickenpox vaccine | 1 | 1 | 1 | |
| Subtotal | 46 | 34 | 26 | |
| Analgesic | ||||
| Acetaminophen (paracetamol) | 17 | 17 | 14 | |
| Tramadol | 4 | 4 | 2 | |
| Subtotal | 21 | 21 | 16 | |
| Neurological | ||||
| Carbamazepine | 1 | 1 | 1 | |
| Chloral hydrate | 1 | 0 | 0 | |
| Depakine | 2 | 2 | 2 | |
| Diazepam | 3 | 3 | 3 | |
| Midazolam | 1 | 0 | 0 | |
| Gabapentin | 1 | 1 | 1 | |
| Subtotal | 9 | 7 | 7 | |
| Gastro-enterologic | ||||
| Colex clysma | 1 | 1 | 1 | |
| Forlax | 9 | 9 | 9 | |
| Loperamide | 1 | 1 | 1 | |
| Lactulose | 1 | 0 | 0 | |
| Omeprazole | 1 | 1 | 1 | |
| Sodium laurylsulfoacetate + sodiumcitrate + sorbitol | 3 | 3 | 3 | |
| Esomeprazole | 2 | 1 | 1 | |
| Subtotal | 18 | 16 | 16 | |
| Pulmonary | ||||
| Ipratropium | 2 | 2 | 2 | |
| Montelukast | 1 | 1 | 0 | |
| Salbutamol | 2 | 1 | 1 | |
| Xylomethazolin | 3 | 2 | 1 | |
| Subtotal | 8 | 6 | 4 | |
| Miscellaneous | ||||
| Clemastine | 1 | 1 | 1 | |
| Epinephrine | 1 | 1 | 1 | |
| Atropine | 1 | 1 | 1 | |
| Hydrocortisone | 2 | 1 | 1 | |
| Ethinylestradiol/levonorgestrel | 2 | 2 | 2 | |
| Desmopressin | 1 | 1 | 1 | |
| Subtotal | 8 | 7 | 7 | |
Medication in italics was used in at least 25% either unlicensed or off-label.
Per prescription
From the 268 prescriptions 72% were licensed. Off–label use was found in 43%. Of the licensed drugs 21% were administered off-label. The various drugs were grouped as oncologic (also including biologicals and corticosteroids used for haematologic malignancies), oncologic supportive (drugs used to prevent/combat side effects of cytostatics), anti-bacterial and anti-viral medication (including anti-fungal therapy), analgesic, neurological, gastro-enterological, pulmonary and miscellaneous medication. The distribution of prescriptions according to these subgroups is given in Figure 1. Statistical analysis for individual medication as well as in each subgroup revealed no statistical differences for each subgroup. Analysis with respect to age did not reveal statistical differences for percentage and absolute numbers among the various age groups (see Figure 2). Table 2 summarizes the medication used per disease category No statistical differences were noted between the various disease groups.
Figure 1.
Distribution of prescriptions per therapeutic area. Number of prescriptions ( ); licensed (
); licensed ( ); on-label (
); on-label ( )
)
Figure 2.
Distribution of prescriptions per age group. Number of prescriptions ( ); licensed (
); licensed ( ); on-label (
); on-label ( )
)
Table 2.
Use of medication per disease
| Medication group | Number of prescriptions | Licensed use | On-label use |
|---|---|---|---|
| Brain tumours | |||
| Oncologic | 5 | 2 | 2 |
| Oncology supportive | 7 | 6 | 4 |
| Antibacterial and antiviral | 6 | 5 | 3 |
| Analgesic | 2 | 2 | 2 |
| Neurological | 2 | 2 | 2 |
| Miscellaneous | 2 | 1 | 1 |
| Ewing family tumours | |||
| Oncologic | 1 | 0 | 0 |
| Antibacterial and antiviral | 2 | 2 | 0 |
| Analgesic | 3 | 3 | 3 |
| Neurological | 1 | 1 | 1 |
| Gastro-enterologic | 2 | 2 | 2 |
| Germ cell tumours | |||
| Oncologic | 6 | 1 | 1 |
| Oncology supportive | 8 | 5 | 4 |
| Antibacterial and antiviral | 3 | 3 | 3 |
| Analgesic | 2 | 2 | 1 |
| Neurological | 1 | 0 | 0 |
| Gastro-enterologic | 3 | 3 | 3 |
| Pulmonary | 1 | 1 | 0 |
| Miscellaneous | 1 | 1 | 1 |
| Histiocytosis | |||
| Oncologic | 7 | 5 | 5 |
| Oncology supportive | 4 | 4 | 3 |
| Antibacterial and antiviral | 7 | 6 | 3 |
| Analgesic | 1 | 1 | 0 |
| Miscellaneous | 1 | 1 | 1 |
| Hodgkin's disease | |||
| Oncologic | 2 | 1 | 1 |
| Oncology supportive | 1 | 0 | 0 |
| Leukaemia | |||
| Oncologic | 29 | 22 | 18 |
| Oncology supportive | 19 | 13 | 10 |
| Antibacterial and antiviral | 15 | 11 | 6 |
| Analgesic | 10 | 9 | 8 |
| Neurological | 4 | 3 | 3 |
| Gastro-enterologic | 6 | 5 | 5 |
| Pulmonary | 2 | 1 | 1 |
| Miscellaneous | 1 | 1 | 1 |
| Neuroblastoma | |||
| Oncologic | 4 | 3 | 3 |
| Oncology supportive | 4 | 4 | 3 |
| Antibacterial and antiviral | 3 | 0 | 0 |
| Gastro-enterologic | 1 | 1 | 1 |
| Non-Hodgkin's lymphoma | |||
| Oncologic | 11 | 5 | 5 |
| Oncology supportive | 10 | 3 | 3 |
| Antibacterial and antiviral | 6 | 6 | 4 |
| Analgesic | 2 | 2 | 2 |
| Neurological | 2 | 2 | 2 |
| Gastro-enterologic | 2 | 2 | 2 |
| Miscellaneous | 2 | 2 | 2 |
| Osteosarcoma | |||
| Oncologic | 2 | 1 | 1 |
| Oncology supportive | 10 | 10 | 6 |
| Neurological | 1 | 1 | 1 |
| Miscellaneous | 1 | 1 | 1 |
| Renal tumours | |||
| Oncologic | 9 | 4 | 1 |
| Oncology supportive | 10 | 6 | 4 |
| Antibacterial and antiviral | 3 | 2 | 2 |
| Analgesic | 1 | 1 | 0 |
| Gastro-enterologic | 3 | 3 | 3 |
| Pulmonary | 5 | 4 | 3 |
| Soft tissue tumours | |||
| Oncologic | 1 | 0 | 0 |
| Oncology supportive | 5 | 3 | 5 |
| Anti-bacterial – anti-viral | 2 | 1 | 1 |
| Neurological | 1 | 1 | 0 |
Discussion
As mentioned in the introduction medication used in children is in many instances off-label or unlicensed [17, 18]. This is due to the free choice of pharmaceutical companies to apply for a specific indication. Formerly companies did not need to apply for potential indications in paediatrics. In respect to any new indications and age ranges they also have to make a cost-benefit calculation in order to decide if it was worthwhile to apply for an indication in often a very specialized area. A major point in such a calculation is the remaining period of marketing exclusivity and the projected increase in profits in selling the drug, in case a paediatric license is obtained. Major barriers for more licensing and adequate labelling of medication for children are, for example, small market size, fewer chronic illnesses, greater complexity of drug development [19]. This complexity is due to several factors, such as low incidence, ethical and legal restraints in children, patient accrual and heterogeneity of diseases.
For many of the products generics are available. Grants (EU and FDA) are available in order to promote studies on pharmacokinetic, pharmacodynamic, safety and efficacy data for these old drugs (http://cordis.europa.eu/fp7). Despite these efforts, this will not sort out all questions and in the future we will still have to write prescriptions for many drugs without data scrutinized by the registration authorities. The introduction of the recent US and EU regulation, requiring paediatric investigation plans for the requested indications might resolve in due time many of the mentioned points for newly introduced medications (http://www.ema.europa.eu). Regarding decisions to apply for new indications a positive factor is the extension of patent protection as well as market exclusivity. Regarding publication of data, the European Paediatric Regulation (regulation EC, nr 1901/2006, http://ec.europa.eu/health/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf) makes provision for publication of paediatric trials and results which will happen in the near future. A positive effect of the US measures has already been noted for older children. For children below the age of 6 years the effect has not been objectified [20]. In respect to the problem of the low incidence, which is especially prominent in paediatrics, the orphan drug legislation additionally provides marketing protection. It promotes development of specific drugs in very tiny niches.
In paediatric oncology extemporaneous medication is also a major source of off-label use. Methotrexate and 6-mercaptopurine are the most prominent examples. Important problems using extemporaneous formulations are the instability, the often short shelf-life and the unknown bioavailability. Non-optimal palatability might increase the non-compliance of these products, which might be an important factor in treatment failure [21]. In international trials, which are frequent in paediatric oncology, this non-compliance can interfere with the outcome of the studies [22]. It is peculiar, that treatment according to well defined and closely monitored protocols, also in paediatric oncology, can be an impediment for general access to pharmacologic data. Often, the gathered data will only appear in the scientific literature and will not be incorporated in the publically accessible SmPC. As such it is warranted to provide these data to the pharmaceutical industry to complete the SmPCs. In relation to this report on paediatric oncology patients the several types of malignancies and the low incidences will be a persisting obstacle for licensing. Diseases occurring in adults as well, such as acute leukaemia, Ewing sarcoma, osteosarcoma, etc., run probably less risk.
Considering the data on the various drugs in our report some drugs were unexpectedly high frequently used in an unlicensed manner. For instance prednisolone was used as intrathecal medication. Although the preparation used was allowed for local parenteral use, such as intra-articular infiltration, there was no license for intrathecal use. The low percentage for heparin is almost exclusively due to prophylactic administration in permanent intra-vascular devices to prevent occlusion when these devices are not in use. Surprisingly, the percentages of off-label use of licensed oncologic and oncology supportive medication were in line with other medications. In respect to this the relatively free phrasing of posology in SmPCs of cytostatic drugs has to be mentioned. In this category of drugs dosages are often left open at the discretion of the treating physician, using phrases such as: ‘prescriptions should be done by experienced physicians only’, ‘dosages can be modified in conjunction with other cytostatic drugs’ and ‘dosing depends on the tolerability in relation to bone marrow toxicity’. In the cytostatic supportive group the high unlicensed use for ondansetron is caused by the prolonged use of this drug in many patients. The high percentage of off-label use in the anti-microbial category is not unexpected since vancomycin is one of the most frequently used drugs. At initiation of therapy it is standardly overdosed to prevent initial low drug concentrations. This local practice is based on previous experience with low blood concentration determiniations in this patient group. These aberant blood concentrations on standard posology in paediatric oncology patients are in line with reports in the literature [23]. Other presciptions were to a large extent based on data available in the national Dutch formulary (http://www.kinderformularium.nl). Part of the data in this formulary were based on our inhospital guidelines for drug administration. There was no statistically significant difference between the age groups, although the percentages of licensed and on-label used drugs was lower at a younger age. An explanation for the lack of significance is probably the still rather limited number of prescriptions.
In the literature both for children in general practice as well as in intensive and medium care settings several reports on labelling and license status of drugs are available. For general practice, percentages of off-label use are mostly in the 20 to 30% range [2, 24]. Figures as high as 60% for off- label/unlicensed use are, however, mentioned [2–4, 25–28]. Extraction of paediatric oncological data in pharmacology/pharmacotherapy from these mentioned reports is hampered due to several problems. The use of medication by children suffering from malignancies constitutes only a small percentage of total medication prescribed for the total population [29]. An additional peculiarity of paediatric oncology is that many cytotoxic drugs are used with very limited data in respect to pharmacokinetics [30]. It is also sometimes even doubtful whether there is activity in combination chemotherapy for an individual drug [31]. Furthermore one should consider that children with malignancies represent a frail population treated with potentially very toxic medication and are, due to polypharmacy, at risk for many side effects. For paediatric oncology only limited data on licensing and approved use are reported in literature. In a report by Conroy et al. [5] 19% were unlicensed and 26% of licensed drugs were used in an off-label manner. Unlicensed preparations were noted in 40% of prescriptions for cytotoxic agents, due to a lack of commercially available formulations suitable for the paediatric patient. A French report mentions 75% on-label; with lower rates for cytostatic drugs in the younger age range [32]. Conroy et al.'s figures and the findings in our cohort are substantially higher as compared with adult oncology [18]. A point of difference considering the report of Conroy et al. is the different distribution of diseases. Their population consisted of 79% leukaemia cases whereas in our cohort only 28% of patients suffered from leukaemia. This will be related to the referral patterns of the various institutions. The leukaemia percentage of Conroy et al. does not reflect the normal distribution of malignancies in children. Also in our institution we are biased due to referral patterns by non-paediatric specialists. Since our hospital is one of the four bone tumour centres designated by the Ministry of Health, we have relatively high numbers of osteosarcomas and Ewing tumours. The MIBG-treatment facility, the presence of an important neurosurgery unit, and facilities to perform brachytherapy in children attract many neuroblastoma, brain tumour and soft tissue tumour patients. The absence of an ophthalmologist experienced in retinoblastoma results in a low percentage of this condition in our cohort. In contrast to Conroy et al.'s report, but similar to other hospitals our data do not reflect the use of all medication used by paediatric oncology patients. For insurance reasons our hospital pharmacy was not involved in the delivery of drugs in the home setting. Drugs such as mercaptopurine and methotrexate are very frequently administered in the home setting. In the hospital mercaptopurine and oral methotrexate are seldom used. For both drugs no adequate formulation is marketed for young children. As a result the high number of extemporaneous administration (mostly liquids) in the outpatient setting is known to the oncologists, but could not be quantified in our study. As a result the use of unlicensed prescriptions will be substantially higher for the patient group as a whole. This might explain the higher percentage of licensed use as compared with the data of Conroy et al. as they had a predominance of leukaemia patients and indicated that oral cytostatics were provided by their hospital pharmacy [5].
Despite insufficient data on safety and efficacy the use of off-label drugs and unlicensed use should not be condemned per se. They offer in many cases the best available treatment for a specific child, but our data underpin once more the necessity to have more research on suitable formulations, dosages, safety and efficacy in childhood pharmacotherapy.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Bua J, L'Erario I, Barbi E, Marchetti F. When off-label is a good practice: the example of paracetamol and salbutamol. Arch Dis Child. 2008;93:546–7. doi: 10.1136/adc.2008.139097. [DOI] [PubMed] [Google Scholar]
- 2.Schirm E, Tobi H, de Jong-van den Berg LT. Unlicensed and off label drug use by children in the community: cross sectional study. BMJ. 2002;324:1312–3. doi: 10.1136/bmj.324.7349.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalumeau M, Treluyer JM, Salanave B, et al. Off label and unlicensed drug use among French office based paediatricians. Arch Dis Child. 2000;83:502–5. doi: 10.1136/adc.83.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.'t Jong GW, Vulto AG, de HM, Schimmel KJ, Tibboel D, van den Anker JN. A survey of the use of off-label and unlicensed drugs in a Dutch children's hospital. Pediatrics. 2001;108:1089–93. doi: 10.1542/peds.108.5.1089. [DOI] [PubMed] [Google Scholar]
- 5.Conroy S, Newman C, Gudka S. Unlicensed and off label drug use in acute lymphoblastic leukaemia and other malignancies in children. Ann Oncol. 2003;14:42–7. doi: 10.1093/annonc/mdg031. [DOI] [PubMed] [Google Scholar]
- 6.Woods WG, O'Leary M, Nesbit ME. Life-threatening neuropathy and hepatotoxicity in infants during induction therapy for acute lymphoblastic leukemia. J Pediatr. 1981;98:642–5. doi: 10.1016/s0022-3476(81)80785-8. [DOI] [PubMed] [Google Scholar]
- 7.Allen JC. The effects of cancer therapy on the nervous system. J Pediatr. 1978;93:903–9. doi: 10.1016/s0022-3476(78)81209-8. [DOI] [PubMed] [Google Scholar]
- 8.Reaman G, Zeltzer P, Bleyer WA, De Lorenzo P, Hann I, De Rossi G, Felice M, Hovi L, LeBlanc T, Szczepanski T, Ferster A, Janka G, Rubnitz J, Silverman L, Stary, Campbell M, Li CK, Mann G, Suppiah R, Biondi A, Vora A, Valsecchi MG. Acute lymphoblastic leukemia in infants less than one year of age: a cumulative experience of the Children's Cancer Study Group. J Clin Oncol. 1985;3:1513–21. doi: 10.1200/JCO.1985.3.11.1513. [DOI] [PubMed] [Google Scholar]
- 9.Jones B, Breslow NE, Takashima J. Toxic deaths in the Second National Wilms' Tumor Study. J Clin Oncol. 1984;2:1028–33. doi: 10.1200/JCO.1984.2.9.1028. [DOI] [PubMed] [Google Scholar]
- 10.Reaman GH. Special considerations for the infant with cancer. In: Pizzo PA, Poplack DG, editors. Pediatric Oncology. Philadelphia, PA: J.B.Lippincott; 1989. pp. 263–74. [Google Scholar]
- 11.Murry DJ, Crom WR, Reddick WE, Bhargava R, Evans WE. Liver volume as a determinant of drug clearance in children and adolescents. Drug Metab Dispos. 1995;23:1110–6. [PubMed] [Google Scholar]
- 12.Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, Hovi L, LeBlanc T, Szczepanski T, Ferster A, Janka G, Rubnitz J, Silverman L, Stary, Campbell M, Li CK, Mann G, Suppiah R, Biondi A, Vora A, Valsecchi MG. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370:240–50. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 13.van der Linden MH, Valsecchi MG, De Lorenzo P, Moricke A, Janka G, LeBlanc TM, Felice M, Biondi A, Campbell M, Hann I, Rubnitz JE, Stary J, Szczepanski T, Vora A, Ferster A, Hovi L, Silverman LB, Pieters R. Outcome of congenital acute lymphoblastic leukemia treated on the Interfant-99 protocol. Blood. 2009;114:3764–8. doi: 10.1182/blood-2009-02-204214. [DOI] [PubMed] [Google Scholar]
- 14.Hempel G, Relling MV, de Rossi G, Stary J, De Lorenzo P, Valsecchi MG, Barisone E, Boos J, Pieters R. Pharmacokinetics of daunorubicin and daunorubicinol in infants with leukemia treated in the Interfant 99 protocol. Pediatr Blood Cancer. 2010;54:355–60. doi: 10.1002/pbc.22266. [DOI] [PubMed] [Google Scholar]
- 15.Adamson PC. It's not easy being small. Pediatr Blood Cancer. 2010;54:341–3. doi: 10.1002/pbc.22343. [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration HHS. International Conference on Harmonisation; guidance on E11 clinical investigation of medicinal products in the pediatric population; availability. Notice. Federal Register. 2000:78493–4. [PubMed] [Google Scholar]
- 17.Poole SG, Dooley MJ. Off-label prescribing in oncology. Support Care Cancer. 2004;12:302–5. doi: 10.1007/s00520-004-0593-6. [DOI] [PubMed] [Google Scholar]
- 18.Leveque D, Michallat AC, Schaller C, Ranc M. [Off label drug use in adult patients treated by anticancer chemotherapy] Bull Cancer. 2005;92:498–500. [PubMed] [Google Scholar]
- 19.Milne CP, Bruss JB. The economics of pediatric formulation development for off-patent drugs. Clin Ther. 2008;30:2133–45. doi: 10.1016/j.clinthera.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Grieve J, Tordoff J, Reith D, Norris P. Effect of the paediatric exclusivity provision on children's access to medicines. Br J Clin Pharmacol. 2005;59:730–5. doi: 10.1111/j.1365-2125.2005.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lilleyman JS, Lennard L. Non-compliance with oral chemotherapy in childhood leukaemia. BMJ. 1996;313:1219–20. doi: 10.1136/bmj.313.7067.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brion F, Nunn AJ, Rieutord A. Extemporaneous (magistral) preparation of oral medicines for children in European hospitals. Acta Paediatr. 2003;92:486–90. doi: 10.1111/j.1651-2227.2003.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 23.Piro CC, Crossno CL, Collier A, Ho R, Koyama T, Frangoul H. Initial vancomycin dosing in pediatric oncology and stem cell transplant patients. J Pediatr Hematol Oncol. 2009;31:3–7. doi: 10.1097/MPH.0b013e31818b3520. [DOI] [PubMed] [Google Scholar]
- 24.Muhlbauer B, Janhsen K, Schoettler P. Off-label use of prescription drugs in childhood and adolescents. Dtsch Arztebl. 2009;106:25–31. doi: 10.3238/arztebl.2009.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar P, Walker JK, Hurt KM, Bennett KM, Grosshans N, Fotis MA. Medication use in the neonatal intensive care unit: current patterns and off-label use of parenteral medications. J Pediatr. 2008;152:412–5. doi: 10.1016/j.jpeds.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 26.Schirm E, Tobi H, de Jong-van den Berg LT. Risk factors for unlicensed and off-label drug use in children outside the hospital. Pediatrics. 2003;111:291–5. doi: 10.1542/peds.111.2.291. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell CP, Stone RJ, Morley CJ. Unlicensed and off-label drug use in an Australian neonatal intensive care unit. Pediatrics. 2002;110:e52. doi: 10.1542/peds.110.5.e52. [DOI] [PubMed] [Google Scholar]
- 28.Bavdekar SB, Gogtay NJ. Unlicensed and off-label drug use in children. J Postgrad Med. 2005;51:249–52. [PubMed] [Google Scholar]
- 29.Sturkenboom MC, Verhamme KM, Nicolosi A, Murray ML, Neubert A, Caudri D, Picelli G, Sen EF, Giaquinto C, Cantarutti L, Balardi P, Felisi MG, Ceci A, IC Wong, Teddy European Network of Excellence Drug use in children: cohort study in three European countries. BMJ. 2008;337:a2245. doi: 10.1136/bmj.a2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groninger E, Proost JH, de Graaf SS. Pharmacokinetic studies in children with cancer. Crit Rev Oncol Hematol. 2004;52:173–97. doi: 10.1016/j.critrevonc.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Bramwell VH, Burgers M, Sneath R, Souhami R, Van Oosterom AT, Voute PA, Rouesse J, Spooner D, Craft AW, Somers R. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: the first study of the European Osteosarcoma Intergroup. J Clin Oncol. 1992;10:1579–91. doi: 10.1200/JCO.1992.10.10.1579. [DOI] [PubMed] [Google Scholar]
- 32.LeGuyader N, Pouliquen A-L, Benoit G, Leverger G. Medicaments hors autorisation de mise sur le marche en hematologie-oncologie pediatrique. Arch Pediatr. 2006;13:1267, 1268. doi: 10.1016/j.arcped.2006.05.011. [DOI] [PubMed] [Google Scholar]


