Abstract
AIM
Our aim was to compare the practicability of six different potentially inappropriate medication (PIM) criteria in geriatric outpatients with polypharmacy.
METHODS
We analysed baseline data from the Medication Safety Review Clinic in Taiwanese Elders (MSRC-Taiwan) study. The prevalence and correlates of PIMs were determined on the basis of criteria developed in the USA, Canada, France, Norway, Ireland and Thailand. The percentage of PIMs considered as drug-related problems and the problem-solving rate are reported.
RESULTS
In the 193 participants, the prevalence of PIM varied from 24 to 73%. Application of the criteria revealed that a high number of chronic medications was a common risk factor for having at least one PIM. Of the 1713 medications reviewed, 5.6–14.8% were considered PIMs. Only 30–40% of the identified PIMs were reported as drug-related problems by the MSRC team experts. Criteria with a higher number of statements and a higher percentage of local market/institution drug availability tended to detect more PIMs.
CONCLUSIONS
The prevalence of PIM varied significantly when different criteria were applied. Caution should be exercised in applying PIM criteria developed in other regions when medication availability in the local market is limited.
Keywords: elderly patient, explicit criteria, polypharmacy, potentially inappropriate medication
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Many different criteria have been developed to detect potentially inappropriate medications (PIMs), with wide variations in prevalence estimates and inconsistent associations with health outcomes.
Without head-to-head comparisons, it is difficult to know whether some PIM criteria systematically detect more or fewer PIMs than others in the same cohorts.
WHAT THIS STUDY ADDS
Six sets of PIM criteria were applied to a single cohort, with PIM prevalence ranging from 24 to 73%.
Criteria with a higher number of statements and a higher percentage of local market/institution drug availability tended to detect more PIMs.
Caution should be exercised in applying PIM criteria developed in other regions when medication availability in the local market is limited.
Introduction
Drug-related problem (DRP) is a general term to describe an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes [1]. Drug-related problems are highly prevalent among elderly adults and may contribute to excessive morbidity and mortality, as well as increased health resource utilization [2].
Potentially inappropriate medications (PIMs), often defined as drugs with ineffectiveness or high risk–benefit ratio [3], are an important aspect of preventable DRPs. The Beers criteria developed in the USA, the latest version of which was published in 2003 [4] are widely applied in clinical studies in many countries as a geriatric healthcare quality indicator [5, 6]. However, the prevalence of PIMs detected according to the Beers criteria varied from 18 to 42% among studies performed in different countries [7–11]. It is difficult to determine whether the differences were due to the availability of the Beers drugs in local markets, the familiarity of the Beers criteria to prescribing physicians, or other study design issues. Many international researchers have cited difficulty in applying the Beers criteria in their own countries [12]. Also, several experts did not agree with some statements in the Beers lists [13]. Therefore, several country-specific PIM criteria have been developed to improve the prescription quality for older adults in different regions [4, 13–17].
Compared with the Beers criteria, the newly developed ‘Screening Tool of Older Person's Prescriptions’ (STOPP) in Ireland could help detect more PIMs in a sample of 1329 community-dwelling elderly adults [10]. Likewise, the PIM criteria established for France identified more PIMs than the Beers criteria in 30 683 non-institutionalized volunteers in France [18]. So far, published studies have only presented comparisons of one set of criteria against the Beers criteria. There was a lack of data comparing the performance of multiple (three or more) sets of criteria in one cohort. Such comparisons are important to determine whether some PIM criteria systematically detect more or fewer PIMs than others. Theoretically, criteria with fewer statements might detect fewer PIMs than those using criteria with more statements. However, using criteria with more statements might be more time consuming. Empirical data are necessary for enhancing the comparability of different PIM criteria on PIM prevalence and their associations with health outcomes. In our pervious review of seven sets of published explicit criteria for PIM, we found little similarity among these criteria [19]. The clinical practicability of each set of criteria in different countries outside the origin country was also not clear.
The Medication Safety Review Clinic in Taiwanese Elders (MSRC-Taiwan) [20] was an interventional study to determine the feasibility and effectiveness of multidisciplinary team evaluations in detecting and solving DRPs among geriatric outpatients prescribed multiple medications. The aim of the present study is to compare the practicability of six sets of PIM criteria published in different regions in the MSRC-Taiwan sample by using secondary data analysis. Additionally, we also compare the percentage of PIMs considered as DRPs from experts' review.
Methods
We analysed baseline data from the MSRC-Taiwan study conducted at two hospitals in Taipei, Taiwan. The study was approved by the institutional review board at the National Taiwan University Hospital (NTUH) in October 2007. The details of the study design have been described elsewhere [20].
We enrolled 193 elderly adults (aged ≥65 years) who had either: (i) been prescribed eight or more chronic medications (drugs prescribed for ≥28 days); or (ii) visited three or more different physicians during a 3 month screening period. Each participant visited the clinic four times. Drug-related problems were reported after the first visit. The research team of geriatricians contacted the prescribing physicians within 14 days and presented mutually agreed-upon interventions to patients at the week 3 visit (n = 173). Unless immediate action was necessary to protect patient safety, all drug-level interventions were implemented at the next scheduled visit to the prescriber (with reminder notes attached to the medical records), and not to the MSRCs. The participants were asked to come back to the MSRCs at week 12 (n = 149) and week 24 (n = 139) to determine the problem-solving rates and the changes in the baseline characteristics after interventions.
The MSRCs only reviewed oral prescription medications and dietary supplements taken regularly for at least 28 days. Short-term and as needed medications were excluded, because the prescribing period might end short of the next scheduled clinic visit to the prescriber for administering recommended interventions. Topical, local or inhaled medications were not included owing to their minimal systemic effects.
To determine whether each medication/dietary supplement was problematic and to identify correlates of DRPs, a comprehensive geriatric assessment was performed for each participant. Information was collected on demographics, chronic medical conditions (including chronic obstructive pulmonary diseases, dementia, urinary incontinence and 24 other medical conditions), physical functioning {the Nagi Index [21] and the Instrumental Activities of Daily Living (IADLs) [22]}, cognition {the Mini-Mental State Examination (MMSE) [23]}, mental health {the Geriatric Depression Scale-15 items (GDS-15) [24]}, fall and dizziness history, and utilization of healthcare resources in the past 6 months (clinics, emergency department visits and hospitalizations).
Initially, DRPs were identified after considering indication, dosage, adherence, interactions, adverse reactions and therapeutic effects of each medication. A set of NTUH-modified PIM criteria based on the 2003 Beers criteria was also used to measure the appropriateness of use of medications. However, the modified list only included statements rated as ‘high severity’ from the 2003 Beers criteria so as to not overwhelm the prescribers. Furthermore, amiodarone was excluded from our modified list because of objections from cardiologists. When a PIM was identified, the research team would decide if it was considered a DRP, taking into account all relevant clinical data. Each DRP was recorded on a Pharmaceutical Care Network Europe (PCNE) Classification for Drug-Related Problems Version 5.1 sheet [1]. The advantages of this instrument are its hierarchical design and requirements to record outcomes of interventions [2]. As one medication may have more than one problem, each problem was recorded separately on a sheet.
Six out of the seven criteria described in our previous review [19], the 2003 version of the Beers criteria [4] (from the USA), the Rancourt (from Canada) [17], the Laroche (from France) [13], the Screen Tool of Older Person's Prescription (STOPP; from Ireland) [16], the Winit-Watjana (from Thailand) [15] and the Norwegian General Practice (NORGEP) criteria (from Norway) [14] were applied to detect PIMs in this data set. The McLeod list [25] was not used because some statements from the 1997 criteria were outdated, and many drugs listed were no longer used in clinical practice. For example, the use of β-blockers for heart failure was considered inappropriate in the McLeod criteria. However, β-blockers are indicated for heart failure patients in current guidelines. The number of statements in each set of criteria ranged from 34 (the Laroche criteria) to 111 (the Rancourt criteria).
For PIM analysis, only information on prescription medications was used. Dietary supplements were not used because only one set of criteria included ginkgo biloba and other complementary and alternative medicines as PIMs. The availability each medication listed in all six sets of criteria was investigated in the medication database of the Bureau of National Health Insurance [26] and the NTUH formulary. Previous secondary data analysis on PIM uses often applied only statements independent of co-morbid conditions because detailed medical diagnoses were often lacking. We were able to apply all statements from each set of criteria because chronic medical conditions were comprehensively collected. Some criteria suggested simultaneous prescription of two medications/medication classes as PIMs. For example, concomitant use of warfarin and nonsteroidal anti-inflammatory drugs was discouraged in all but the Laroche criteria. When this type of drug–drug interaction was detected, both offending medications/medication classes were considered as PIMs.
As one participant might have more than one PIM, prevalence of PIM is presented as both drug level and person level. At drug level, prevalence is defined as the numbers of PIMs divided by the total numbers of medications prescribed for the entire cohort. At person level, prevalence is defined as the percentage of participants who were prescribed with at least one PIM.
Also, we approached our analysis from two complementary perspectives: person level and drug level. In person-level analysis, individuals with at least one PIM identified from each set of criteria were compared with those without. Bivariate analysis was performed using Student's unpaired t-test for continuous variables and χ2 test with Fisher's exact adjustment when appropriate for categorical variables. Stepwise multivariate logistic regression models were used to identify correlates of having at least one PIM at the person level after adjusting the demographic and health-related and drug-related characteristics. In drug-level analysis, the number and percentage of PIMs identified from each set of criteria were reported. In addition, the number and percentage of PIMs considered as DRPs by MSRC-Taiwan research team experts as well as the problem-solving rate were recorded. All tests were two-tailed, and significance (α) was set at P < 0.05. Data were analysed using the Stata version 8 statistical package (Stata, College Station, TX, USA).
Results
Only 50–89% of listed medications under six sets of published explicit criteria were available in Taiwan, with 27–67% available at the NTUH. Overall, availability (percentage) of medications listed under the Laroche criteria from France was the lowest (Table 1).
Table 1.
Basic characteristics of six sets of published explicit criteria for potentially inappropriate medications*
| NORGEP [14] | Laroche [13] | Rancourt [17] | Beers [4] | STOPP [16] | Winit-Watjana [15] | |
|---|---|---|---|---|---|---|
| Year of publication | 2009 | 2007 | 2004 | 2003 | 2008 | 2009 |
| Country | Norway | France | Canada | USA | Ireland | Thailand |
| Number of statements | 36 | 34 | 111 | 68 | 65 | 77 |
| 1. Specific drug names listed, n (%) | 22 | 113 | 74 | 103 | 28 | 54 |
| 2. Available drugs in Taiwan, n (%) | 15 (68) | 57 (50) | 60 (81) | 71 (69) | 25 (89) | 50 (93) |
| 3. Available drugs at NTUH, n (%) | 7 (32) | 30 (27) | 39 (53) | 44 (43) | 21 (75) | 36 (67) |
Abbreviations: NORGEP, Norwegian General Practice criteria; NTUH, National Taiwan University Hospital; STOPP, the Screening Tool of Older Person's Prescriptions.
The order of each set of criteria was based on their prevalence in our study population, from low to high.
In person-level analysis, the mean age (±SD) of the participants (n = 193) was 76.2 ± 6.2 years, and half of them were men. The mean number of chronic conditions and chronic medications were 9 ± 2.6 and 8.9 ± 3.1, respectively, demonstrating the high medical complexity in this group. Despite these complicated chronic conditions, the functional status (Nagi Index 25; IADL score 29) and mental status (MMSE score 27) were relatively preserved (Table 2).
Table 2.
Baseline characteristics of the entire study cohort by presence of potentially inappropriate medications (PIMs) with six criteria
| Total | +PIMs with NORGEP [14] | +PIMs with Laroche [13] | +PIMs with Rancourt [17] | +PIMs with Beers [4] | +PIMs with STOPP [16] | +PIMs with Winit-Watjana [15] | |
|---|---|---|---|---|---|---|---|
| n = 193 | n = 47 (24%) | n = 95 (49%) | n = 103 (53%) | n = 106 (55%) | n = 121 (63%) | n = 140 (73%) | |
| n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | |
| Demographics | |||||||
| 1. Age | 76.2 (6.2) | 78.4 (6.7)** | 76.7 (6.3) | 78.3 (5.9)*** | 76.8 (6.2) | 77 (6.3)* | 76.6 (6.4) |
| 2. Sex (male) | 103 (53.4) | 25 (53.2) | 54 (56.8) | 51 (49.5) | 64 (60.4)* | 67 (55.4) | 83 (59.3)* |
| 3. Education (>9 years) | 96 (49.7) | 27 (57.5) | 48 (50.5) | 51 (49.5) | 54 (50.9) | 62 (51.2) | 74 (52.9) |
| Health-related characteristics | |||||||
| 4. Number of chronic conditions | 9.0 (2.6) | 9.8 (2.4)* | 9.6 (2.6)*** | 9.4 (2.3)* | 9.7 (2.6)*** | 9.6 (2.5)*** | 9.5 (2.6)*** |
| 5. Number of chronic medications | 8.9 (3.1) | 10.2 (3.2)*** | 10 (0.3)*** | 9.6 (3.2)*** | 9.8 (3.1)*** | 9.6 (3)*** | 9.4 (3.1)*** |
| 6. Number of ED visits in 6 months | 0.17 (0.4) | 0.21 (0.4) | 0.22 (0.4) | 0.16 (0.4) | 0.24 (0.4)* | 0.22 (0.4)* | 0.19 (0.4) |
| 7. Nagi Index Score | 24.9 (4) | 24.3 (4.1) | 24.8 (4.3) | 24.6 (4.4) | 24.7 (4.3) | 24.7 (4) | 24.9 (4) |
| 8. IADL Score | 29.3 (4.6) | 28.8 (5) | 29 (5.2) | 29 (4.8) | 29.4 (4.5) | 29.4 (4.1) | 29.5 (4.3) |
| 9. MMSE Score | 27.3 (3.7) | 27.1 (4.2) | 26.9 (4.3) | 26.9 (4.1) | 27.1 (3.8) | 27.1 (3.7) | 27.2 (3.7) |
| 10. GDS-15 Score | 1.3 (3.3) | 1.4 (3.3) | 2 (3.9)** | 1.7 (3.7) | 1.7 (3.6) | 1.5 (3.4) | 1.5 (3.5) |
| 11. Dizziness (Yes) | 69 (35.8) | 18 (38.3) | 37 (39.5) | 46 (44.7)** | 42 (39.6) | 48 (39.7) | 54 (38.6) |
| 12. Falls (Yes) | 29 (15) | 8 (17) | 20 (21.1)* | 17 (16.5) | 25 (23.6)*** | 27 (22.3)*** | 25 (17.9) |
Comparisons were made between subjects with at least one PIM and subjects without a PIM. Abbreviations: ED, emergency department; GDS, geriatric depression scale; IADL, instrument activity of daily life; MMSE, minimal mental state examination; NORGEP, Norwegian General Practice criteria; STOPP, the Screening Tool of Older Person's Prescriptions.
P < 0.05
P < 0.01
P < 0.001.
The proportion of patients who had at least one PIM varied from 24% (the NORGEP criteria) to 73% (the Winit-Watjana criteria; Figure 1). For those prescribed with PIMs, most used only one or two listed drugs. When more than three PIMs were found for one patient, often drug–drug interactions were involved, because both drugs with interactions were considered as PIMs (data not shown). Among these criteria, the increase of PIM prevalence was concordant with the number of statements and the availability of PIMs in the local market, except for the Rancourt criteria (Figure 2).
Figure 1.
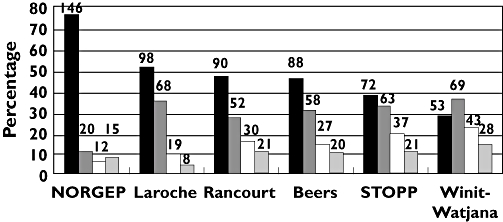
Prevalence of potentially inappropriate medications from six sets of explicit criteria (total n = 193). Numbers of patients are shown above the bars. Abbreviations: NORGEP, Norwegian General Practice criteria; STOPP, the Screening Tool of Older Person's Prescriptions. No PIM (▪); 1 PIM ( ); 2 PIMs (□); >3 PIMS (
); 2 PIMs (□); >3 PIMS ( )
)
Figure 2.
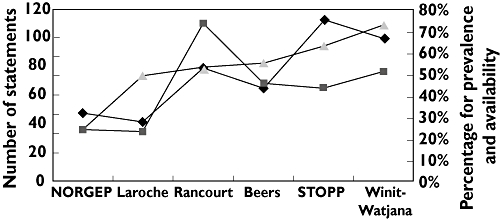
Person-level analysis of prevalence, number of statements and availability (percentage) of potentially inappropriate medications in National Taiwan University Hospital (NTUH) based on six sets of criteria. Abbreviations: NORGEP, Norwegian General Practice criteria; STOPP, the Screening Tool of Older Person's Prescriptions. Statements ( ); Availability (%) (
); Availability (%) ( ); Prevalence (%) (
); Prevalence (%) ( )
)
In the bivariate analysis, the common characteristics associated with having at least one PIM in all criteria were a high number of chronic conditions and a high number of chronic medications. Other factors associated with higher likelihood of having at least one PIM included age, sex, frequency of emergency department visits, the Nagi Index score, the GDS score, and history of dizziness or falls (Table 2).
Correlates of having at least one PIM varied significantly for the six sets of criteria in multivariate logistic regressions. A higher number of chronic medications was associated with higher likelihood of PIM use based on five of the six sets of criteria. Correlates independently associated with PIM use based on three sets of criteria included advanced age and history of falls. A higher number of chronic conditions, higher IADL score and higher number of emergency department visits were independent correlates based on two sets of criteria. Higher Nagi Index, higher GDS score, having dizziness and being male were significant when only one particular set of criteria was applied (Table 3).
Table 3.
Multivariate logistic regression for having at least one drug as potentially inappropriate medication with six sets of criteria
| NORGEP [14] | Laroche [13] | Rancourt [17] | Beers [4] | STOPP [16] | Winit-Watjana [15] | |
|---|---|---|---|---|---|---|
| Odds ratio (95% confidence interval) | Odds ratio (95% confidence interval) | Odds ratio (95% confidence interval) | Odds ratio (95% confidence interval) | Odds ratio (95% confidence interval) | Odds ratio (95% confidence interval) | |
| Demographics | ||||||
| 1. Age | 1.1 (1–1.1*) | 1.1 (1.1–1.2***) | 1.1 (1–1.1*) | |||
| 2. Sex (male) | 2.8 (1.4–5.7**) | |||||
| Health-related characteristics | ||||||
| 3. Number of chronic conditions | 1.2 (1–1.4*) | 1.4 (1.2–1.6***) | ||||
| 4. Number of chronic medications | 1.2 (1.1–1.4**) | 1.4 (1.2–1.6***) | 1.2 (1.1–1.3**) | 1.4 (1.2–1.6***) | 1.2 (1–1.4*) | |
| 5. Number of ED visits in 6 months | 2.9 (1.1–7.2*) | 2.9 (1.1–8.2*) | ||||
| 6. Nagi Index Score | 1.1 (1–1.2*) | |||||
| 7. IADL Score | 1.1 (1–1.2*) | 1.1 (1–1.2*) | ||||
| 8. GDS-15 Score | 1.2 (1–1.3*) | |||||
| 9. Dizziness (Yes) | 2.0 (1–3.8*) | |||||
| 10. Falls (Yes) | 3.1 (1.1–8.2*) | 8.3 (2.6–27**) | 18.7 (3.6–96.5***) |
Only variables retained in the stepwise selection model are reported. Abbreviations: ED, emergency department; GDS, geriatric depression scale; IADL, instrument activity of daily life; NORGEP, Norwegian General Practice criteria; STOPP, the Screening Tool of Older Person's Prescriptions.
P < 0.05
P < 0.01
P < 0.001.
The top three offending drugs from each set criteria varied significantly (Table 4). However, the top three drugs were most similar among the Beers (doxazocin, alprazolam and dipyridamole), the Rancourt (alprazolam, atovastatin and dipyridamole) and the Winit-Watjana criteria (alprazolam, aspirin and doxazocin).
Table 4.
The leading three potentially inappropriate medications identified among geriatric outpatients prescribed with multiple medications (total number of patients = 193)
| NORGEP [14] | Laroche [13] | Rancourt [17] | Beers [4] | STOPP [16] | Winit-Watjana [15] | |
|---|---|---|---|---|---|---|
| First ranking, n (%) | Aminophylline | Zolpidem | Alprazolam | Doxazocin | Aspirin | Alprazolam |
| 17 (8.8) | 17 (8.8) | 18 (9.3) | 24 (12.4) | 24 (12.4) | 34 (17.6) | |
| Second ranking, n (%) | Zolpiclone | Dipyridamole | Atovastatin | Alprazolam | Amlodipine | Aspirin |
| 11 (5.7) | 14 (7.3) | 15 (7.8) | 15 (7.8) | 20 (10.4) | 24 (12.4) | |
| Third ranking, n (%) | Celecoxib | Pentoxifylline | Dipyridamole | Dipyridamole | Furosemide | Doxazocin |
| 8 (4.1) | 13 (6.7) | 14 (7.3) | 14 (7.3) | 16 (8.3) | 24 (12.4) |
The ranking of each set of criteria was based on their prevalence in our study population, from low to high. Abbreviations: NORGEP, Norwegian General Practice criteria; STOPP, the Screening Tool of Older Person's Prescriptions.
From drug-level analysis, the prevalence of PIM ranged from 5.6 (the NORGEP criteria) to 14.8% (the Winit-Watjana criteria; Table 5). Approximately 31% (the STOPP criteria) to 42% (the NORGEP criteria) of PIMs identified were considered as DRPs by the medication review team experts. Approximately 60–70% of the DRPs had outcomes available at the week 24 visits, with the problem-solving rate ranging from 72 to 84% (Table 5).
Table 5.
The relationships between potentially inappropriate medications (PIMs) and drug-related problems (DRPs) among geriatric outpatients prescribed with multiple medications (total number of patients = 193; total number of medications = 1713)
| NORGEP [14] | Laroche [13] | Rancourt [17] | Beers [4] | STOPP [16] | Winit-Watjana [15] | |
|---|---|---|---|---|---|---|
| Number of medications considered as PIMs | n = 96 (5.6) | n = 132 (7.7) | n = 185 (10.8) | n = 177 (10.3) | n = 199 (11.6) | n = 254 (14.8) |
| Reported as DRP (%) | 40 (41.7) | 51 (38.6) | 73 (39.5) | 69 (39) | 61 (30.7) | 86 (33.9) |
| DRP follow up in 24 weeks (%) | 29 (72.5) | 36 (70.6) | 45 (61.6) | 43 (62.3) | 36 (59) | 59 (68.6) |
| Problem-solving rate* (%) | 22 (75.9) | 26 (72.2) | 38 (84.4) | 31 (72.1) | 30 (83.3) | 46 (78) |
(Problem totally solved + problem partly solved/follow-up numbers). The ranking of each set of criteria was based on their prevalence in our study population, from low to high. Abbreviations: NORGEP, Norwegian General Practice criteria; STOPP, the Screening Tool of Older Person's Prescriptions.
Discussion
We found that the prevalence of PIM varied significantly with the applied instruments. Criteria with a higher number of statements and a higher percentage of local market/institution drug availability tended to detect more PIMs. Furthermore, having greater number of chronic medications was associated with higher likelihood of having at least one PIM at person level. Only 30–40% of identified PIMs were considered as DRPs by medication review team experts. Approximately 70–85% of the PIM problems could be solved in 24 weeks.
To the best of our knowledge, this is the first study in which more than two sets of PIM criteria were applied to a single cohort. Among the six sets of explicit criteria applied, prevalence of PIMs from previous research was available for the Beers (18–42%) [7, 9, 10, 27], the STOPP (21–25%) [10], the Rancourt (55%) [17] and the Laroche criteria (22%) [28]. Our prevalence estimates were significantly higher (at least 50%) than those reported from the Beers, the STOPP and the Laroche criteria because we enrolled participants prescribed with very high numbers of medications, a known risk factor for PIMs. Nevertheless, our prevalence according to the Rancourt criteria was similar to that from a nursing home population [17].
When applying PIM criteria developed from other countries, availability of medications in the local market might have a strong impact on PIM prevalence estimates. Our findings also indicated that significant portions of PIMs listed under the six sets of criteria were not available in Taiwan. Several authors cited drug availability issues as a major determinant for creating country-specific PIM criteria [13, 16, 29]. Furthermore, there was no consensus regarding which drugs should be included in some medication classes, such as anticholinergics or muscle relaxants. We found it difficult to compare our prevalence estimates with other studies because of these methodological differences. We suggest that authors attach their detailed PIM lists as appendices to increase the comparability across international studies.
The number of statements also had a positive correlation with prevalence of PIM, except in the Rancourt criteria. According to the Rancourt criteria, some drugs can potentially be inappropriate for different reasons. Each reason is treated as one statement. For example, use of triazolam is discouraged when a single dose >0.25 mg, a daily dose >1.25 mg or a prescribing duration >30 days are detected (three statements) [17]. When significant amounts of these drugs are not available at the NTUH, the prevalence might be greatly underestimated.
Our previous review paper found few similarities in PIM listed under the six sets of published criteria [19]. Significant variations of the top three offending medications identified from each set of criteria in the MSRC data set are expected. In PIMs independent of co-morbidities, alprazolam (the Beers, Rancourt and Winit-Watjana criteria) and dipyridamole (the Beers, Rancourt and Laroche criteria) were among the top three offending medications. This result was similar to previous studies which showed the most frequent Beers PIMs were benzodiazepine and dipyridamole [11, 30]. In PIMs considering chronic conditions, aspirin prescribed for patients with peptic ulcer was a common PIM (roughly 12%) when the STOPP and Winit-Watjana criteria were applied. In contrast, only 1% of older adults in a Irish national representative cohort was prescribed aspirin without concomitant use of histamine H2 receptor antagonists or proton pump inhibitors with the STOPP criteria [31]. The difference was probably from lack of reimbursement of these two medication classes for gastrointestinal protection purpose from the Taiwanese National Health Insurance.
Consistent with previous studies, a higher number of medications was a strong determinant of PIMs based on five of the six sets of criteria [11, 30, 32]. In contrast, the number of chronic conditions was no longer significant for most criteria when the number of medications was adjusted in multivariate analysis. Older adults prescribed with multiple drugs should receive frequent medication reviews to avoid use of PIMs [30]. Older age was associated with a higher prevalence of PIM in our study. Both positive [30] and negative associations [27] have been reported in the literature. The association between falls and PIMs was found with three of the six sets of criteria in our study. Elderly patients prescribed benzodiazepine or anticholinergic medications have an increased risk of falling [33, 34]. These medications are commonly listed PIMs in most sets of criteria. For those PIM correlates identified for only one or two sets of criteria, the existing literature often have inconsistent findings. For example, use of PIMs from the Beers and the STOPP criteria were associated with a higher number of emergency department visits in our analysis. The relationship between PIM use and utilization of healthcare resources was not supported in a previous review [12].
The MSRC-Taiwan study was designed to detect and solve DRPs for geriatric outpatients with multiple medications. The 6 month overall problem-solving rate for all DRPs was approximately 90% (data available on request). When performing this secondary data analysis, only approximately 40% of the PIMs from the six sets of criteria were reported as DRPs, and their problem-solving rates were 72–84%. In the MSRCs, only high-severity Beers drugs were considered as PIMs and amiodarone was excluded. Even when Beers drugs were detected, it was left to the discretion of the research team whether to report them as DRPs. Some medications listed under the other five sets of explicit criteria were not considered as PIMs in the Beers criteria. However, significant portions of them were still considered as DRPs from expert reviews, such as zolpiclone and zolpidem. A previous study also showed that the agreement between explicit criteria and expert assessment of PIMs was low [35]. Many prescribers were not familiar with PIM criteria, which may explain the lower problem-solving rate compared with other aspects of DRPs, such as duplication of medication.
Clinicians have limited time during their busy practice. To increase the applicability of PIM criteria to routine practice, time efficiency is an important issue to consider. We found that criteria with fewer drug–disease and drug–drug interaction statements (e.g. Laroche criteria) or fewer total statements were usually less time consuming (e.g. the NORGEP criteria). The sets of criteria with concerns on inappropriate dose, duration and duplication of medication often needed more time to administer (e.g. the Rancourt criteria). Several sets of criteria considered entire medication categories (such as long-acting benzodiazepines) as PIMs. Sets of criteria (e.g. the Laroche criteria) specifying the medications available in each medication category were easier to apply than those that did not (e.g. the STOPP criteria).
Our study had several limitations. First, we may have underestimated the prevalence of PIM because several medications available in Taiwan with similar pharmacological properties to certain PIMs were not included in the six sets of criteria. Second, individual drugs included in several medication classes, such as anticholinergics, may have been different from those used in other studies. Third, the causal relationship of PIM correlates could not be established with a cross-sectional study. Finally, findings from two hospitals can be generalized to only a certain degree.
In conclusion, the prevalence of PIM varied significantly when different criteria were applied. The number of statements and availability of PIMs in the local market were major determinants of PIM prevalence according to six different sets of criteria. Caution should be exercised in applying PIM criteria developed in other countries when medication availability in the local market is limited. Country-specific PIM criteria may be more efficient in indentifying PIMs in routine practice. Further prospective clinical studies are needed to address the impacts of PIMs on elderly patients' healthcare outcomes.
Acknowledgments
The study was sponsored by the ‘Medication Safety Review Clinic in Taiwanese Elders’ project from the Department of Health, Taiwan.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.PCNE Working Group on Drug-Related Problems. Pharmaceutical Care Network, E. 2006. PCNE articles, sorted by primary authors. Available at http://www.pcne.org/sig/drp/drug-related-problems.php (last accessed 20 May 2011)
- 2.van Mil JW, Westerlund LO, Hersberger KE, Schaefer MA. Drug-related problem classification systems. Ann Pharmacother. 2004;38:859–67. doi: 10.1345/aph.1D182. [DOI] [PubMed] [Google Scholar]
- 3.O'Mahony D, Gallagher PF. Inappropriate prescribing in the older population: need for new criteria. Age Ageing. 2008;37:138–41. doi: 10.1093/ageing/afm189. [DOI] [PubMed] [Google Scholar]
- 4.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–24. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 5.Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135:642–6. doi: 10.7326/0003-4819-135-8_part_2-200110161-00002. [DOI] [PubMed] [Google Scholar]
- 6.Pugh MJ, Hanlon JT, Zeber JE, Bierman A, Cornell J, Berlowitz DR. Assessing potentially inappropriate prescribing in the elderly Veterans Affairs population using the HEDIS 2006 quality measure. J Manag Care Pharm. 2006;12:537–45. doi: 10.18553/jmcp.2006.12.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey IM, De Wilde S, Harris T, Victor C, Richards N, Hilton SR, Cook DG. What factors predict potentially inappropriate primary care prescribing in older people? Analysis of UK primary care patient record database. Drugs Aging. 2008;25:693–706. doi: 10.2165/00002512-200825080-00006. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman IH, Langenberg P, Baumgarten M, Orwig D, Byrns PJ, Simoni-Wastila L, Magaziner J. Inappropriate drug use and risk of transition to nursing homes among community-dwelling older adults. Med Care. 2006;44:722–30. doi: 10.1097/01.mlr.0000215849.15769.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fick DM, Mion LC, Beers MH, Waller JL. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health. 2008;31:42–51. doi: 10.1002/nur.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan C, O'Mahony D, Kennedy J, Weedle P, Byrne S. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol. 2009;68:936–47. doi: 10.1111/j.1365-2125.2009.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai HY, Hwang SJ, Chen YC, Chen TJ, Lin MH, Chen LK. Prevalence of the prescribing of potentially inappropriate medications at ambulatory care visits by elderly patients covered by the Taiwanese National Health Insurance program. Clin Ther. 2009;31:1859–70. doi: 10.1016/j.clinthera.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, Hanlon JT. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370:173–84. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 13.Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol. 2007;63:725–31. doi: 10.1007/s00228-007-0324-2. [DOI] [PubMed] [Google Scholar]
- 14.Rognstad S, Brekke M, Fetveit A, Spigset O, Wyller TB, Straand J. The Norwegian General Practice (NORGEP) criteria for assessing potentially inappropriate prescriptions to elderly patients. A modified Delphi study. Scand J Prim Health Care. 2009;27:153–9. doi: 10.1080/02813430902992215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winit-Watjana W, Sakulrat P, Kespichayawattana J. Criteria for high-risk medication use in Thai older patients. Arch Gerontol Geriat. 2008;47:35–51. doi: 10.1016/j.archger.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert Doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46:72–83. doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- 17.Rancourt C, Moisan J, Baillargeon L, Verreault R, Laurin D, Gregoire JP. Potentially inappropriate prescriptions for older patients in long-term care. BMC Geriatr. 2004;4:9. doi: 10.1186/1471-2318-4-9. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bongue B, Naudin F, Laroche ML, Galteau MM, Guy C, Gueguen R, Convers JP, Colvez A, Maarouf N. Trends of the potentially inappropriate medication consumption over 10 years in older adults in the East of France. Pharmacoepidemiol Drug Saf. 2009;18:1125–33. doi: 10.1002/pds.1762. [DOI] [PubMed] [Google Scholar]
- 19.Chang CB, Chan DC. Comparison of published explicit criteria for potentially inappropriate medications in older adults. Drugs Aging. 2010;27:947–57. doi: 10.2165/11584850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Chan DC, Chen JH, Kuo HK, We CJ, Lu IS, Chiu LS, Wu SC. Drug-related problems (DRPs) identified from geriatric medication safety review clinics. Arch Gerontol Geriatr. 2011 doi: 10.1016/j.archger.2011.02.005. doi: 10. 1016/j. archger.2011.02.005 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Nagi SZ. A study in the evaluation of disability and rehabilitation potential: concepts, methods, and procedures. Am J Public Health Nations Health. 1964;54:1568–79. doi: 10.2105/ajph.54.9.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu CY, Lu CH, Yu S, Yang YY. Correlations between scores on Chinese versions of long and short forms of the Geriatric Depression Scale among elderly Chinese. Psychol Rep. 1998;82:211–4. doi: 10.2466/pr0.1998.82.1.211. [DOI] [PubMed] [Google Scholar]
- 25.McLeod PJ, Huang AR, Tamblyn RM, Gayton DC. Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ. 1997;156:385–91. [PMC free article] [PubMed] [Google Scholar]
- 26.Database of medications covered by National Health Insurance, Taiwan. Available at http://www.nhi.gov.tw/query/query1.aspx?menu = 18&menu_id = 683&webdata_id = 3468&WD_ID = 756 (last accessed 20 May 2011)
- 27.Fialova D, Topinkova E, Gambassi G, Finne-Soveri H, Jonsson PV, Carpenter I, Schroll M, Onder G, Sorbye LW, Wagner C, Reissigova J, Bernabei R. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293:1348–58. doi: 10.1001/jama.293.11.1348. [DOI] [PubMed] [Google Scholar]
- 28.Kolzsch M, Kopke K, Fischer T, Hofmann W, Kuhnert R, Bolbrinker J, Kuhlmey A, Drager D, Kreutz R. Prescribing of inappropriate medication in nursing home residents in Germany according to a French consensus list: a cross-sectional cohort study. Pharmacoepidemiol Drug Saf. 2011;20:12–9. doi: 10.1002/pds.2005. [DOI] [PubMed] [Google Scholar]
- 29.Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107:543–51. doi: 10.3238/arztebl.2010.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aparasu RR, Mort JR. Inappropriate prescribing for the elderly: beers criteria-based review. Ann Pharmacother. 2000;34:338–46. doi: 10.1345/aph.19006. [DOI] [PubMed] [Google Scholar]
- 31.Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69:543–52. doi: 10.1111/j.1365-2125.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert SM, Colombi A, Hanlon J. Potentially inappropriate medications and risk of hospitalization in retirees: analysis of a US retiree health claims database. Drugs Aging. 2010;27:407–15. doi: 10.2165/11315990-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CM, Chen MJ, Tsai CY, Ho LH, Hsieh HL, Chau YL, Liu CY. Medical conditions and medications as risk factors of falls in the inpatient older people: a case-control study. Int J Geriatr Psychiatry. 2011;26:602–7. doi: 10.1002/gps.2569. [DOI] [PubMed] [Google Scholar]
- 34.Agashivala N, Wu WCK. Effects of potentially inappropriate psychoactive medications on falls in US nursing home residents analysis of the 2004 National Nursing Home Survey Database. Drug Aging. 2009;26:853–60. doi: 10.2165/11316800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Steinman MA, Rosenthal GE, Landefeld CS, Bertenthal D, Kaboli PJ. Agreement between drugs-to-avoid criteria and expert assessments of problematic prescribing. Arch Intern Med. 2009;169:1326–32. doi: 10.1001/archinternmed.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]


