Abstract
AIMS
To assess the level of paracetamol off label prescribing in the community and the potential for paracetamol under or overdosing.
METHODS
The Scottish Practice Team Information (PTI) database containing prescribing data for approximately 35 839 children aged (0–12 years) was analysed for paracetamol prescriptions for the year 2006. Off label prescribing was defined as prescribing outside the BNFc age and dose recommendations.
RESULTS
Two thousand seven hundred and sixty-one children aged 0–12 years were issued with 4423 prescriptions for paracetamol. (1446 males). Children 1–5 years (1329, 42.2%) accounted for 48.9% (2164) of all paracetamol prescriptions. Eighteen per cent (793) of individual prescriptions were off label and after accounting for repeat prescriptions 625 (22.75%) individuals were exposed to off label prescriptions. A further 15% (668) of prescriptions contained insufficient dosage data to determine their status, 13.3% (368) being underdosed and 4.4% (121) overdosed at least once during the study year. In total 11.3% (502) of all prescriptions were classified as underdose, 2.9% (127) as overdose and 15% (667) had no dosage instructions. Age was significantly related to non recommended dosage (χ2 test, P < 0.001). Children 1–3 months old were at highest risk of being overdosed; 27% of prescriptions recommended actual or potential overdosage and 25% (354) of children aged 6–12 years were prescribed an actual or potential underdose. Overall 57.2% of all prescriptions failed to comply with current BNFc recommendations.
CONCLUSION
Paracetamol off label prescribing is common in primary care, with relatively high levels of potential overdosing in the youngest children and potential underdosing in the oldest children.
Keywords: children, off label, overdose, paracetamol, underdose
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Paracetamol is the most frequently prescribed analgesic and antipyretic in children.
The adherence to paracetamol dosage recommendation in children is poor, with paracetamol prescriptions alone accounting for 30% of off label prescribing as a result of dose violation.
WHAT THIS STUDY ADDS
Our study has identified that approximately a fifth of paracetamol prescriptions issued to children by their GP are off label, with the most common reason being either too small or too high a dose.
Both overdosing in young children and underdosing in older children together with their associated risks of toxicity or under treatment occur relatively frequently.
Introduction
Paracetamol or N-acetyl-p-aminophenol (APAP) is the single most frequently used agent for the management of fever and pain [1, 2], with 84% of all infants in the UK being administered paracetamol at least once during their first 6 months of life [3]. The number and age of the children prescribed paracetamol imposes a significant responsibility on the prescriber to ensure that the drug is used safely and effectively, which in turn requires careful selection of an appropriate dosage regimen. However the optimal use of paracetamol requires the prescriber to take account of both a child's age and/or weight which in turn gives rise to multiple dosage recommendations in formularies such as the British National Formulary for Children (BNFc). This resulting complexity in dosage recommendations further increases the likelihood of off label paracetamol prescribing [4]. Paediatric off label prescribing is common, with reports that approximately 33% of all prescriptions issued to children aged 0–18 years are off label and that 26% of all children aged 0–16 years are issued with an off label prescription in primary care [5, 6]. Paracetamol prescriptions alone are reported to account for 30% of all paediatric off label prescribing, due primarily to violations of dosage regimens [4]. The likely outcome of failing to adhere to paracetamol dosage recommendations and issuing an off label prescription is a possible underdose or overdose, resulting in possible treatment failure or toxicity, respectively. The evidence for the toxicity of unintended therapeutic paracetamol overdose in children, including hepatotoxicity, is now well recognized [7]. We therefore sought to describe the pattern of paediatric paracetamol prescribing in primary care in Scotland.
Patients and methods
Paracetamol prescribing data for children aged 0–12 years was extracted from the Practice Team Information (PTI) database for the period 1 January−31 December 2006. The PTI database, which is representative of the urban/rural and ethnic mix of Scotland, contained prescribing information for over 35 839 children registered with 40 general practices located throughout Scotland [8].
Paracetamol daily dose was defined as the total amount of paracetamol prescribed per day in mg day−1 The clarity of the dosage instructions for each prescription was categorized as specific, range, missing or unclear. Specific was defined as an exact dose of paracetamol with a clear dosing frequency, range as an exact minimum and maximum amount with clear dosing frequency, missing as the dose and or the dosing frequency not indicated and unclear when instructions such as ‘take as required’ were given without specific dose and or frequency instructions.
Overdosing was defined as any prescription in which the prescribed daily dose exceeded the highest dose recommendation in the BNFc for severe conditions, and potential overdosing when the prescriber stated a dose range the upper dose of which could result in a dose exceeding the maximum recommended in the BNFc. Where the maximum recommended dose of paracetamol was weight dependent, the weight of the child was calculated from the BNFc weight charts for children aged 0–1 year and from the following formula for children aged 1–12 years: weight = 3(age) + 7 was used [9] (see Table 1 for the maximum licensed BNFc dose recommendations for oral paracetamol).
Table 1.
Licensed dose recommendations for oral paracetamol for pain, pyrexia with discomfort
| Age | Dose | Possible maximum |
|---|---|---|
| Neonate 28–32 weeks | 10–15 mg kg−1, 8−12 hourly | 30 mg kg−1 |
| Neonate over 32 weeks | 10–15 mg kg−1, 6–8 hourly | 60 mg kg−1 |
| 1–3 months | 30–60 mg, 4–6 hourly | 240 mg |
| 3–12 months | 60–120 mg, 4–6 hourly | 480 mg |
| 1–6 years | 120–250 mg, 4–6 hourly | 1 g |
| 6–12 years | 250–500 mg, 4–6 hourly | 2 g |
| 12–18 years | 500 mg, 4–6 hourly | 4 g |
Underdosing was defined as a daily dose less than the minimum recommended by the BNFc, and potential underdosing when the prescriber recommended a dose range the maximum dose of which was the minimum dose recommended by the BNFc for a child of that age. Dosage was defined as unpredictable when the dose or frequency were not given, or when a statement such as ‘take as required’, ‘take as on the bottle’ or ‘take as directed’ was given. All prescriptions were assessed against dosage recommendations in the BNFc (2010) [10].
Prescriptions were also classified as non-combined when paracetamol was prescribed alone and combined when coprescribed with ibuprofen or codeine.
Descriptive statistics and chi-square (χ2) for trends were analysed using the SPSS 17.0 (Chicago, Illinois). P values of 0.05 or less were considered statistically significant for any associations.
Results
For the study period the PTI database contained prescribing information for 35 839 children (18 448 male) aged between 0 and 12 years. A total of 4423 paracetamol prescriptions were issued to 7.7% of the paediatric population (2761 children, 52.4% males) with almost half (48.9%, 1350 children) issued to children under 5 years of age. The majority of prescriptions (98.3%, 4347) were for oral paracetamol and the remainder (1.7%, 76) for rectal paracetamol. Therefore only prescriptions for oral paracetamol were considered for further analysis.
The majority of prescriptions (52.9%, 2319) were specific for dosage and frequency, while 15.2% (673) were classified as unpredictable (no clear dosage guidance recorded). A total of 17.9% (793) of prescriptions were outside BNFc recommendations, and 15.1% contained insufficient data to enable categorization. Prescriptions outside BNFc recommendations were issued to 22.7% (626 children) of all children prescribed paracetamol.
Under or overdosing (Tables 1 and 2, Figures 1 and 2)
Table 2.
Number (%) of paracetamol prescriptions classified as underdose, potential underdose, overdose and potential overdose according to age
| Age group | Number of prescriptions | Underdose | Potential underdose | Overdose | Potential overdose |
|---|---|---|---|---|---|
| 1–3 months | 229 | 8 (3.5) | 0 | 50 (21.8) | 11 (4.8) |
| 4–11 months | 628 | 20 (3.2) | 24 (3.8) | 20 (3.2) | 10 (1.6) |
| 1–5 years | 2164 | 203 (9.4) | 182 (8.4) | 38 (1.8) | 7 (0.3) |
| 6–12 years | 1398 | 271 (19.4) | 83 (5.9) | 19 (1.4) | 21 (1.5) |
Figure 1.
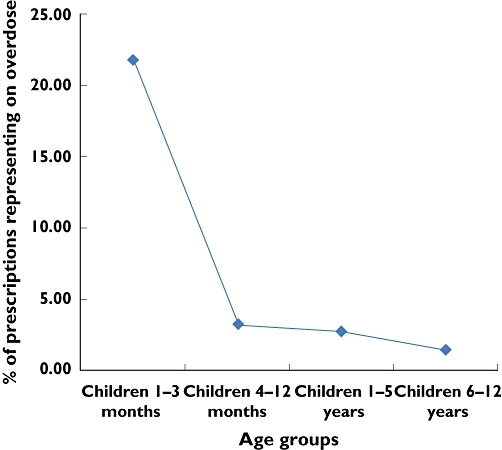
Percentages of prescriptions recommending a paracetamol overdose by age (χ2 for linear trend = 149.68, P < 0.001)
Figure 2.
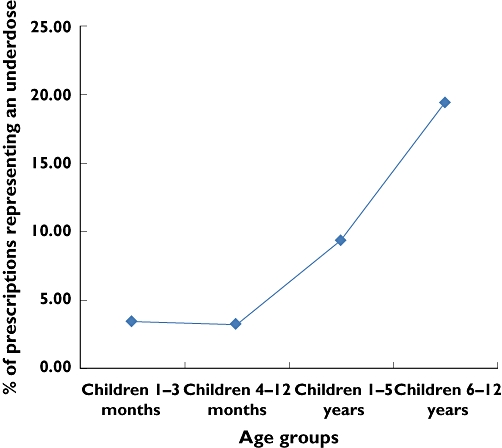
Percentages of prescriptions recommending an underdose by age (χ2 for linear trend = 134.73, P < 0.001)
Analysis of prescriptions in terms of age and dosage outcomes, revealed that 11.3% (502), 6.5% (289) and 2.9% (127) of children prescribed paracetamol were exposed to an underdose, potential underdose or overdose, respectively. Children aged 1–3 months were at the highest risk of being overdosed or potentially overdosed, 21.8% and 4.8%, respectively, while older children (6–12 years old) were at the highest risk of being underdosed or potentially underdosed, 19.4% and 5.9%, respectively. Prescriber compliance with dosage recommendations was best for children aged 4–12 months, in whom 73.9% (464) complied with the BNFc recommendations. For children aged 1–5 years, 67.4% (1459) of prescriptions complied with the BNFc recommendations, 1.8% recommended a potential overdosing regimen and 9.4% a potential underdosing regimen. The likelihood of being issued with a prescription recommending a potential overdose decreased significantly with increasing age (χ2 for linear trend = 149.68, P < 0.001), while underdosing increased significantly with age (chi-square for linear trend = 134.73, P < 0.001) (Figures 1 and 2).
The majority of prescriptions for paracetamol only and for paracetamol and ibuprofen were compliant with BNFc dosing recommendations, 63% (2788 of 4423) and 73.9% (386 of 522), respectively. Only four prescriptions for paracetamol and codeine, all of which were off label, were issued to the study population.
Discussion
Our study has identified that approximately a fifth of paracetamol prescriptions issued to children by their GP are off label, with the most common reason being either too small or too high a dose. A further 15% of prescriptions contained no dosage instructions at all.
The widespread use of paracetamol by parents and carers before seeking medical advice is common place [3, 11, 12] and increases the risk of toxicity when a child is further prescribed paracetamol by a physician. Therefore the health care team has an important role in ameliorating the potential dangers associated with use. In this study more than 15% of prescriptions contained no minimum or maximum dosing instructions, and approximately a third of these only stated ‘to be taken as required’. It is recognized that up to 50% of parents do not understand official dosage recommendations for medicines such as paracetamol, and absence of dosing instructions can leave parents in a state of confusion, or strengthen the public perception that paracetamol is harmless [13]. Both factors may contribute to the occurrence of paracetamol overdose, toxicity and deaths [7, 14].
Our study indicated that approximately 1 in 10 prescriptions prescribed a potential underdose with the risk of underdosing increasing with age. Unfortunately the risk associated with underdosing of paracetamol and possible treatment failure has not received the same attention as the risks associated with overdosing, possibly reflecting the perception that this practice is safe. However, considering the current demands for cost effective therapy, a prescribing habit that could lead to treatment failure is of potential concern. Furthermore underdosing was the most frequent reason for a paracetamol prescription being off label, a situation similar to that reported by Ekins-Daukes et al. for a wide range of paediatric medicines [6].
Almost 3% of paracetamol prescriptions recommended an overdose, with over a fifth of these issued to the youngest children aged 1–3 months. Although reports of confirmed hepatotoxicity caused by paracetamol overdose in children are uncommon, isolated case reports of paracetamol associated liver toxicity continue to emerge [7, 14–17], almost half of which occurred in children less than 2 years of age [7]. While the results of this study suggest significant levels of paracetamol prescribing which could result in potential overdose to children, we were unable to assess the actual paracetamol dose administered by the parent or carer. It is therefore not possible to determine whether an overdose was actually administered.
In conclusion, this is the first study to describe the patterns of paracetamol prescribing by primary care physicians in the community. Both overdosing in young children and underdosing in older children together with their associated risks of toxicity or under treatment occur relatively frequently. The reasons for these prescribing patterns are unclear. However it is possible that in the absence of reports relating to underdosing and treatment failure, primary care physicians have been sensitised by earlier reports of paracetamol overdosing and hepatotoxicity.
Acknowledgments
This work was carried out under the auspices of the Scottish Medicines for Children Network (ScotMSCN), a Centre for mounting clinical trials and addressing the knowledge gaps in support of the effective and safe use of medicines in children.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Arana A, Morton NS, Hansen TG. Treatment with paracetamol in infants. Acta Anaesthesiol Scand. 2001;45:20–9. doi: 10.1034/j.1399-6576.2001.450104.x. [DOI] [PubMed] [Google Scholar]
- 2.Gehri M, Guignard E, Djahnine SR, Cotting JQ, Yersin C, Di Paolo ER, Krahenbuhl JD, Pannatier A. When fever, paracetamol? Theory and practice in a paediatric outpatient clinic. Pharm World Sci. 2005;27:254–7. doi: 10.1007/s11096-004-4771-x. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins N, Golding J. A survey of the administration of drugs to young infants. The Alspac Survey Team. Avon Longitudinal Study of Pregnancy and Childhood. Br J Clin Pharmacol. 1995;40:79–82. doi: 10.1111/j.1365-2125.1995.tb04539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy S, Peden V. Unlicensed and off label analgesic use in paediatric pain management. Pediatric Anesthesia. 2001;11:431–6. doi: 10.1046/j.1460-9592.2001.00697.x. [DOI] [PubMed] [Google Scholar]
- 5.Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164:552–8. doi: 10.1007/s00431-005-1698-8. [DOI] [PubMed] [Google Scholar]
- 6.Ekins-Daukes S, Helms PJ, Simpson CR, Taylor MW, McLay JS. Off-label prescribing to children in primary care: retrospective observational study. Eur J Clin Pharmacol. 2004;60:349–53. doi: 10.1007/s00228-004-0752-1. [DOI] [PubMed] [Google Scholar]
- 7.Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: hepatoxicity after multiple doses in children. J Pediatr. 1998;132:22–7. doi: 10.1016/s0022-3476(98)70479-2. [DOI] [PubMed] [Google Scholar]
- 8.Practice team Information (PTI) statistics. Information and Services Division, Scottish Government. Available at http//www.isdscotland.org/isd/1044.html (last accessed September 2010)
- 9.Luscombe M, Owens B. Weight estimation in resuscitation: is the current formula still valid? Arch Dis Child. 2007;92:412–5. doi: 10.1136/adc.2006.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paediatric Formulary Committee. BNF for Children. London: BMJ Publishing Group, RPS Publishing, and RCPCH Publications; 2010. [Google Scholar]
- 11.Deligne J, Grimaldi L, Jonville-Bera AP, Giraudeau B, Blum-Boisgard C, Autret-Leca E. Antipyretic drug use in children in French office based medical practice. Pharmacoepidemiol Drug Saf. 2007;16:812–7. doi: 10.1002/pds.1422. [DOI] [PubMed] [Google Scholar]
- 12.Betz MG, Grunfeld AF. ‘Fever phobia’ in the emergency department: a survey of children's caregivers. Eur J Emerg Med. 2006;13:129–33. doi: 10.1097/01.mej.0000194401.15335.c7. [DOI] [PubMed] [Google Scholar]
- 13.Heubi JE, Bien JP. Acetaminophen use in children: more is not better. J Pediatr. 1997;130:175–7. [PubMed] [Google Scholar]
- 14.Ekins-Daukes S, Helms PJ, Taylor MW, McLay JS. Off-label prescribing to children: attitudes and experience of general practitioners. Br J Clin Pharmacol. 2005;60:145–9. doi: 10.1111/j.1365-2125.2005.02397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearns GL, Leeder JS, Wasserman GS. Acetaminophen overdose with therapeutic intent. J Pediatr. 1998;132:5–8. doi: 10.1016/s0022-3476(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 16.Rivera-Penera T, Gugig R, Davis J, McDiarmid S, Vargas J, Rosenthal P, Berquist W, Heyman MB, Ament ME. Outcome of acetaminophen overdose in pediatric patients and factors contributing to hepatotoxicity. J Pediatr. 1997;130:300–4. doi: 10.1016/s0022-3476(97)70359-7. [DOI] [PubMed] [Google Scholar]
- 17.Kubic A, Burda AM, Bockewitz E, Wahl M. Hepatotoxicity in an infant following supratherapeutic dosing of acetaminophen for twenty-four hours. Semin Diagn Pathol. 2009;26:7–9. doi: 10.1053/j.semdp.2008.12.010. [DOI] [PubMed] [Google Scholar]


