Abstract
To determine the effectiveness and safety of once-daily combination therapy with amlodipine, valsartan and hydrochlorothiazide for reducing ambulatory blood pressure (ABP) in patients with moderate to severe hypertension, a multicenter, double-blind study was performed (N=2271) that included ABP monitoring in a 283-patient subset. After a single-blind, placebo run-in period, patients were randomized to receive amlodipine/valsartan/hydrochlorothiazide (10/320/25 mg), valsartan/hydrochlorothiazide (320/25 mg), amlodipine/valsartan (10/320 mg) or amlodipine/hydrochlorothiazide (10/25 mg) each morning for 8 weeks. Efficacy assessments included change from baseline in 24-h, daytime and night time mean ambulatory systolic BP (SBP) and diastolic BP (DBP). Statistically significant and clinically relevant reductions from baseline in all these parameters occurred in all treatment groups (P<0.0001, all comparisons versus baseline). At week 8, least squares mean reductions from baseline in 24-h, daytime and night time mean ambulatory SBP/DBP were 30.3/19.7, 31.2/20.5 and 28.0/17.8 mm Hg, respectively, with amlodipine/valsartan/hydrochlorothiazide; corresponding reductions with dual therapies ranged from 18.8–24.1/11.7–15.5, 19.0–25.1/12.0–16.0 and 18.3–22.6/11.1–14.3 mm Hg (P⩽0.01, all comparisons of triple versus dual therapy). Treatment with amlodipine/valsartan/hydrochlorothiazide maintained full 24-h effectiveness, including during the morning hours; all hourly mean ambulatory SBP and mean ambulatory DBP measurements were ⩽130/85 mm Hg at end point. Amlodipine/valsartan/hydrochlorothiazide combination therapy was well tolerated. Once-daily treatment with amlodipine/valsartan/hydrochlorothiazide (10/320/25 mg) reduces ABP to a significantly greater extent than component-based dual therapy and maintains its effectiveness over the entire 24-h dosing period.
Keywords: triple therapy, amlodipine, valsartan, hydrochlorothiazide
Subject terms: Hypertension, Combination drug therapy
Introduction
The blood pressure (BP) surge that occurs between 6 AM and noon1 has been associated with a peak in the incidence of cardiovascular events and sudden death.2 This surge in BP (∼10–30 mm Hg in systolic BP (SBP) and 7–23 mm Hg in diastolic BP (DBP))2 coincides with an increase in pulse rate and sympathetic tone and activation of the renin-angiotensin-aldosterone system.3, 4, 5, 6 In turn, activation of the renin-angiotensin-aldosterone system results in increased levels of aldosterone and angiotensin II, a potent vasoconstrictor that modulates vasomotor tone, cell growth and extracellular matrix deposition.7 Several studies have shown that among patients who appear to have well-controlled morning BP, as assessed by office measurements, ∼60% have poorly controlled morning BP when assessed by ambulatory BP monitoring (ABPM).8, 9
It is well established that the majority of patients with hypertension require two or more antihypertensive agents from complementary classes to achieve BP control,10, 11, 12, 13 and the results from several recent studies indicate that ∼23–54% of patients with hypertension require three or more agents.10, 14, 15, 16, 17 Results were recently reported for the first large-scale, randomized, double-blind clinical trial designed to compare the effectiveness and safety of once-daily triple therapy with the calcium channel blocker amlodipine (Aml), the angiotensin receptor antagonist valsartan (Val) and the thiazide diuretic hydrochlorothiazide (HCTZ) (10/320/25 mg) versus component-based dual therapy with Val/HCTZ, Aml/Val or Aml/HCTZ for the treatment of moderate to severe hypertension.18 The results of this study showed that triple therapy with Aml/Val/HCTZ was well tolerated and was significantly more effective in reducing mean sitting SBP (MSSBP) and mean sitting DBP (MSDBP) and in providing BP control than component-based dual therapy. The current article reports the results of ABPM in a subgroup of patients who participated in this triple-therapy study. The objective was to determine the effectiveness of triple therapy for controlling BP throughout the 24-h interval.
Methods
This study was a randomized, double-blind, parallel-group, active-control trial conducted in 15 countries. The study design, patient selection criteria and disposition of all patients enrolled were reported in detail by Calhoun et al.18
Study treatment
The study included an antihypertensive washout period and single-blind, placebo run-in period of up to 4 weeks, followed by an 8-week, double-blind treatment period (Figure 1). At the end of the placebo run-in period, patients were randomly assigned (1:1:1:1) to receive triple therapy with Aml/Val/HCTZ (10/320/25 mg) or dual therapy with Val/HCTZ (320/25 mg), Aml/Val (10/320 mg) or Aml/HCTZ (10/25 mg). Randomization was achieved using a validated, interactive, voice-response system. As shown in Figure 1, the study design included a two-step dose-escalation period in the triple-therapy arm and a single-step dose-escalation period in each of the dual-therapy arms over the first 2 weeks, followed by 6 weeks of treatment at full dosage administered once-daily at 8 AM. On study visit days, patients were instructed not to take their study medication until assessments were completed.
Figure 1.
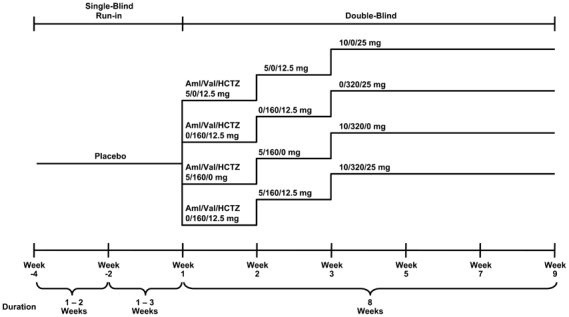
Study design.18 Aml, amlodipine; HCTZ, hydrochlorothiazide; Val, valsartan.
Patients
Patients 18–85 years of age with moderate or severe hypertension (grade 2 or 3 or stage 2;19, 20 MSSBP ⩾145 and <200 mm Hg and MSDBP ⩾100 and <120 mm Hg) were eligible to participate. Patients were excluded if, at screening, they were receiving four or more antihypertensive agents; three antihypertensive agents and had an MSSBP/MSDBP ⩾140/90 mm Hg; two antihypertensive agents and had an MSSBP/MSDBP ⩾180/110 mm Hg; or no antihypertensive agents and had an MSSBP/MSDBP <140/90 mm Hg. Other key exclusion criteria included significant cardiovascular, hepatic, and renal disease and concomitant type 1 diabetes or uncontrolled type 2 diabetes, as previously described.18
Patients meeting the screening criteria were immediately randomly assigned to receive treatment if MSSBP was ⩾180 mm Hg or MSDBP was ⩾110 mm Hg. The remaining patients were randomly assigned to receive treatment after a placebo run-in period of up to 4 weeks if MSSBP was ⩾145 mm Hg and MSDBP was ⩾100 mm Hg. Patients were removed from the study if they experienced MSSBP ⩾200 mm Hg or MSDBP ⩾120 mm Hg at any time during the study.
Efficacy assessments
The MSSBP and MSDBP measurements were obtained at each study visit, as reported by Calhoun et al.18 Per protocol, 24-h ABPM was conducted at baseline before randomization (week 1) and after 8 weeks of double-blind treatment (week 9) in a subset of patients enrolled in the study. Patients were fitted on the non-dominant arm with a Spacelabs 90207 ABPM device (Spacelabs Healthcare Supplies, Issaquah, WA, USA) between 0700 hours and 1000 hours on the first day of each monitoring period, and the device was calibrated to within ±7 mm Hg against the mean of three DBP readings.21 Pressure cuffs were set to inflate every 20 min over a 24-h time period and to deflate at a rate of 8 mm Hg per 2 heartbeats. The ABPM device was removed on the second or third day of each period after a minimum of 24 h, and the ABPM data were downloaded and evaluated on site using study-specific ABPM software (Medifacts International, Rockville, MD, USA). If the ABPM data did not meet pre-specified quality control criteria, the entire ABPM procedure could be repeated at the discretion of the study investigator.
Statistical analyses
The primary efficacy variables were change from baseline in MSSBP and MSDBP at week 9, as described by Calhoun et al.18 Secondary end points reported herein included the change from baseline in 24-h, daytime (0600 hours to 2200 hours) and night time (2200 hours to 0600 hours) mean ambulatory SBP (MASBP) and mean ambulatory DBP (MADBP). Mean 24-h ABPM data from the intent-to-treat population were analyzed using an analysis of covariance model for repeated measures with treatment, region and postdosing hour as factors, baseline 24-h mean ABPM results as a covariate, and treatment by postdosing-hour interactions. Daytime and night time ABPM data from the intent-to-treat population were analyzed using an analysis of covariance model for repeated measures with treatment, region and time (daytime, night time) as factors, baseline 24-h mean ABPM results as a covariate, and treatment-by-time interactions. The mean changes from baseline in MASBP and MADBP (with 95% confidence intervals) were assessed at week 9. The differences in the mean changes from baseline in MASBP and MADBP between the triple-therapy arm and the dual-therapy arms were estimated using least squares mean data. MASBP and MADBP at each hour were summarized at baseline and end point for each treatment group.
Further, post hoc analyses of morning (0600 hours to 2400 hours) MASBP and MADBP were individually performed using an analysis of covariance model with treatment and region as factors and baseline 24-h mean ABPM results as a covariate.
Multiplicity adjustment for P-values from the analyses of primary efficacy variables was described by Calhoun et al.18 No multiplicity adjustment was made for P-values from the analyses of ABPM data.
Additionally, post hoc summary statistics were performed to assess the mean reductions in 24-h ambulatory BP (ABP) in patients grouped according to the severity of hypertension at baseline as assessed by clinic SBP (⩾140 and <160 mm Hg; ⩾160 and <200 mm Hg; ⩾180 and <200 mm Hg).
Results
Baseline characteristics of the patients undergoing ABPM
Of the 4285 patients enrolled, 2271 were assigned to double-blind treatment and 2060 (90.7%) completed treatment,18 including all 283 patients who underwent 24-h ABPM. The demographic and baseline characteristics of the subgroup of patients undergoing ABPM were similar between treatment arms (Table 1), and similar to those of the study population as a whole.18 Of the patients undergoing ABPM, baseline MSSBP/MSDBP was 165.2/105.2 mm Hg and MASBP/MADBP was 148.2/93.4 mm Hg.
Table 1.
Baseline demographics and patient characteristics for the subgroup of patients undergoing 24-h ambulatory BP monitoring
| Characteristic | Aml/HCTZ (10/25 mg) (n=76) | Aml/Val (10/320 mg) (n=71) | Val/HCTZ (320/25 mg) (n=69) | Aml/Val/HCTZ 10/320/25 mg (n=67) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 42 (55.3) | 40 (56.3) | 40 (58.0) | 42 (62.7) |
| Age, mean (s.d.), years | 55.5 (8.9) | 53.6 (9.3) | 53.0 (8.5) | 54.1 (9.9) |
| Age group, n (%) | ||||
| ⩾65 years | 16 (21.1) | 9 (12.7) | 5 (7.2) | 10 (14.9) |
| Race, n (%) | ||||
| White | 60 (78.9) | 54 (76.1) | 56 (81.2) | 54 (80.6) |
| Black | 14 (18.4) | 12 (16.9) | 10 (14.5) | 10 (14.9) |
| Other | 2 (2.6) | 5 (7.0) | 3 (4.4) | 3 (4.5) |
| Ethnicity, n (%) | ||||
| Hispanic/Latino | 13 (17.1) | 8 (11.3) | 13 (18.8) | 9 (13.4) |
| Non-Hispanic/Latino | 63 (82.9) | 63 (88.7) | 56 (81.2) | 58 (86.6) |
| BMI, mean (s.d.), kg m −2 | 30.9 (4.7) | 30.8 (4.7) | 32.0 (5.6) | 31.2 (5.2) |
| Sitting BP, mean (s.d.), mm Hg | ||||
| SBP | 164.6 (13.5) | 166.3 (13.5) | 164.4 (12.2) | 165.6 (13.3) |
| DBP | 105.5 (4.4) | 105.0 (4.4) | 104.9 (5.0) | 105.4 (3.9) |
| 24-h ambulatory BP, mean (s.d.), mm Hg | ||||
| SBP | 147.3 (13.1) | 149.7 (14.2) | 146.4 (13.5) | 149.6 (13.4) |
| DBP | 93.4 (9.4) | 93.1 (8.1) | 92.8 (9.1) | 94.4 (10.0) |
| Severity of baseline systolic HTN, n (%) | ||||
| MSSBP ⩾140 and <160 mm Hg | 33 (43.4) | 26 (36.6) | 26 (37.7) | 31 (46.3) |
| MSSBP ⩾160 and <200 mm Hg | 43 (56.6) | 45 (63.4) | 43 (62.3) | 36 (53.7) |
| MSSBP ⩾180 and <200 mm Hg | 12 (15.8) | 15 (12.1) | 7 (10.1) | 11 (16.4) |
| Baseline SBP, mean (s.d.), mm Hg | ||||
| MSSBP ⩾140 and <160 mm Hg | 153.1 (4.1) | 152.9 (4.5) | 151.6 (4.2) | 154.1 (4.4) |
| MSSBP ⩾160 and <200 mm Hg | 173.4 (11.4) | 174.1 (10.4) | 172.1 (8.3) | 175.5 (9.9) |
| MSSBP ⩾180 and <200 mm Hg | 189.2 (6.3) | 186.9 (6.3) | 185.4 (5.1) | 187.5 (5.1) |
Abbreviations: Aml, amlodipine; BMI, body mass index; BP, blood pressure; DBP, diastolic BP; HCTZ, hydrochlorothiazide; HTN, hypertension; MSSBP, mean sitting SBP; SBP, systolic BP; s.d., standard deviation; Val, valsartan.
Changes from baseline in MASBP and MADBP
All four treatments resulted in clinically relevant and statistically significant reductions from baseline in least squares MASBP and MADBP over the 24-h dosing period and during the daytime and night time hours (Figure 2; P<0.0001 versus baseline for all comparisons). However, the improvements in 24-h, daytime and night time ABP were greatest in patients receiving Aml/Val/HCTZ (P⩽0.01 for all comparisons). Among the patients receiving triple therapy, the 24-h MASBP decreased by 30.3 mm Hg (95% confidence interval: −31.7, −28.8) and the 24-h MADBP decreased by 19.7 mm Hg (95% confidence interval: −20.7, −18.7). Consistent results were observed for the daytime and night time hours.
Figure 2.
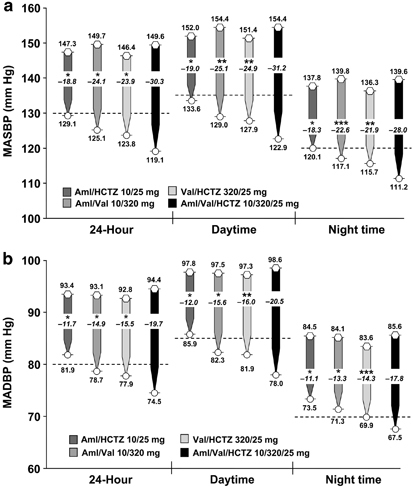
Least squares mean change from baseline in 24-h, daytime and night time ambulatory (a) SBP and (b) DBP. Hexagons represent baseline values and circles represent end point values. Dotted lines indicate the upper end of the European Society of Hypertension/European Society of Cardiology target ranges for MASBP/MADBP (24 h: 125–130/80 mm Hg; daytime: 130–135/85 mm Hg; night time: 120/70 mm Hg).20 *P⩽0.0001 versus Aml/Val/HCTZ; **P⩽0.001 versus Aml/Val/HCTZ; ***P⩽0.01 versus Aml/Val/HCTZ. Aml, amlodipine; ESH/ESC, European Society of Hypertension/European Society of Cardiology; HCTZ, hydrochlorothiazide; MADBP, mean ambulatory diastolic blood pressure; MASBP, mean ambulatory systolic blood pressure; Val, valsartan.
The absolute 24-h, daytime and night time MASBP/MADBP levels at study end point were lowest in the triple-therapy group (119/75, 123/78 and 111/68 mm Hg, respectively).
Hourly ABP over the 24-h dosing period
Mean hourly ABPM data obtained at baseline and at week 9 are presented in Figure 3. At baseline, MASBP levels were >130 mm Hg and MADBP levels were >80 mm Hg at all time points throughout the 24-h dosing period in all treatment groups. At the end of the study (week 9), all (100%) of the hourly MASBP levels were ⩽130 mm Hg in the Aml/Val/HCTZ group. In comparison, 91.7, 83.3 and 41.7% of the hourly MASBP levels were <130 mm Hg in the Val/HCTZ, Aml/Val and Aml/HCTZ groups, respectively. All hourly end point MADBP levels were <85 mm Hg in the triple-therapy group and 87.5% (21 of 24) of the hourly levels were <80 mm Hg.
Figure 3.
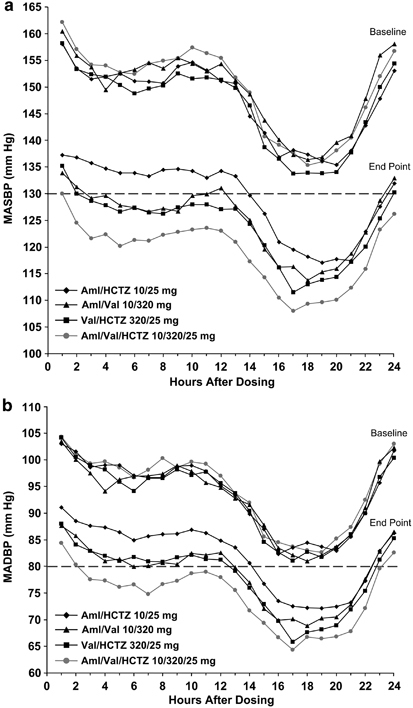
Hourly MASBP (a) and MADBP (b) at baseline and end point over the 24-h dosing interval. Dotted lines indicate the upper end of the European Society of Hypertension/European Society of Cardiology target range for mean 24-h MASBP/MADBP (125–130/80 mm Hg).20 Aml, amlodipine; ESH/ESC, European Society of Hypertension/European Society of Cardiology; HCTZ, hydrochlorothiazide; MADBP, mean ambulatory diastolic blood pressure; MASBP, mean ambulatory systolic blood pressure; Val, valsartan.
Changes from baseline in early morning MASBP and MADBP
Statistically significant greater reductions from baseline in morning MASBP and MADBP were observed in patients receiving triple therapy (30.2/20.6 mm Hg) compared with Val/HCTZ (24.5/15.7 mm Hg; P<0.01), Val/Aml (24.6/15.4 mm Hg; P<0.01) and HCTZ/Aml (19.7/12.4 mm Hg; P<0.0001). MASBP/MADBP between 0600 hours and 1200 hours was 124/80 mm Hg in the triple-therapy group.
24-h ABP by severity of hypertension at baseline
Reductions in 24-h MASBP were greater across all treatment groups in the subgroups of patients with baseline MSSBP ⩾160 and <200 mm Hg or baseline MSSBP ⩾180 and <200 mm Hg, compared with the reductions in patients with baseline MSSBP ⩾140 and <160 mm Hg (Figure 4). In patients with baseline MSSBP ⩾160 and <200 mm Hg or and patients with baseline MSSBP ⩾180 and <200 mm Hg, decreases in MASBP were greater with Aml/Val/HCTZ (34.2 and 37.0 mm Hg, respectively) than with dual therapy (range: 21.0–26.4 and 22.5–31.2 mm Hg, respectively). Similarly, decreases in MASBP in patients with baseline MSSBP ⩾140 and <160 mm Hg were also greater with triple therapy than with dual therapy.
Figure 4.
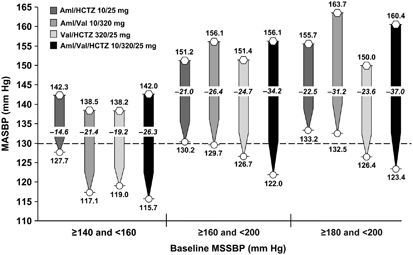
Change from baseline in 24-h MASBP according to the severity of hypertension at baseline. Hexagons represent baseline values and circles represent end point values. Dotted line indicates the upper end of the European Society of Hypertension/European Society of Cardiology target range for mean 24-h MASBP (125–130 mm Hg).20 Aml, amlodipine; ESH/ESC, European Society of Hypertension/European Society of Cardiology; HCTZ, hydrochlorothiazide; MASBP, mean ambulatory systolic blood pressure; MSSBP, mean sitting systolic blood pressure; Val, valsartan.
Discussion
This is the first large-scale, controlled trial to prospectively study the effects of once-daily triple therapy with Aml/Val/HCTZ versus component-based dual therapy for controlling ABP throughout the 24-h interval. The ABPM data from this subgroup of patients corroborate the clinic BP findings from the entire study population.18 Overall, the ABPM data show that treatment with Aml/Val/HCTZ lowered MASBP/MADBP by ∼30/20 mm Hg throughout the 24-h period, which was significantly better than the reductions achieved in patients receiving dual therapy. An even greater improvement in MASBP (reductions of 37 mm Hg) was observed among triple-therapy patients with severe systolic hypertension at baseline (MSSBP ⩾180 and <200 mm Hg).
The results from the current study further show that the reductions in ABP are relatively uniform throughout the 24-h dosing period. Notably, at end point, hourly MASBP/MADBP levels among patients receiving Aml/Val/HCTZ remained ⩽130/85 mm Hg for every hour of the 24-h dosing period, including the early morning hours. MASBP/MADBP between 0600 hours and 1200 hours, the hours coinciding with the morning surge in BP, was 124/80 mm Hg in the triple-therapy group. The ABP levels achieved with triple therapy are clinically relevant considering the 24-h, daytime and night time means were lower than the lower end of the European Society of Hypertension/European Society of Cardiology SBP/DBP target goal ranges for ABP (125–130/80, 130–135/85 and 120/70 mm Hg, respectively).20
The results from this ABPM subgroup analysis, in conjunction with the results from the primary efficacy analysis,18 have important implications for the management of patients with moderate to severe hypertension. The consistent reductions in ABP observed in this study throughout the 24-h dosing interval are important, because ABP has been shown to be a strong predictor of cardiovascular morbidity and mortality.22, 23 Moreover, ABPM over 24 h is the most accurate method of assessing the effectiveness of antihypertensive therapy24 and can characterize BP during the early morning hours, when cardiovascular and cerebrovascular events are most likely to occur.2 At the same time, however, there is a lack of evidence that controlling the morning BP surge translates into a reduction in cardiovascular events.25 Nonetheless, attaining 24-h BP control is a goal of antihypertensive therapy. In this regard, several studies (including the recently completed effects of force-titrated valsartan/hydrochlorothiazide versus amlodipine/hydrochlorothiazide on ambulatory blood pressure in patients with stage 2 hypertension (EVALUATE) study26) have shown that dual therapy with angiotensin receptor blockers in combination with a non-renin-angiotensin-aldosterone system antihypertensive agent is effective for controlling ABP throughout the day, including the high-risk hours coinciding with the morning surge in BP.27, 28, 29, 30, 31, 32 The results from this study show that additional improvements in ABP can be achieved throughout the day, including the morning hours, with angiotensin receptor blocker-based triple therapy that incorporates agents with complementary mechanisms of action.
Our study excluded individuals who were on four or more antihypertensive agents. This exclusion may have led to a greater percentage of patients responding to triple therapy as well as dual therapy in our study. However, for ethical reasons, it was not considered appropriate to enroll patients who were in need of four or more antihypertensive agents because the trial only allowed for a maximum of three drugs per patient. As with all clinical trials, study entry criteria can limit extrapolation of results to a broader patient population.
Conclusion
In patients with moderate to severe hypertension, once-daily therapy with Aml/Val/HCTZ (10/320/25 mg) reduces ABP throughout the 24-h dosing interval, including the overall 24-h, daytime and night time periods, to a significantly greater extent than component-based dual therapy. Aml/Val/HCTZ lowers MASBP/MADBP by ∼30/20 mm Hg throughout the day in these patients with even greater reduction observed in those patients with more severe systolic hypertension. Notably, triple therapy with Aml/Val/HCTZ effectively controls MASBP to ⩽130 mm Hg for every hour throughout the day, including early morning hours.
Acknowledgements
We acknowledge Fran Karo, PhD and Michael S McNamara, MS, of Oxford PharmaGenesis, Inc., for their editorial support in the development of this manuscript. This study was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Competing interests
Yves Lacourcière and David Calhoun received research funding from Novartis Pharmaceuticals Corporation for the current study. Robert Glazer, Joseph Yen and Nora Crikelair are employees of Novartis Pharmaceuticals Corporation.
Footnotes
Disclaimer
The principal investigator and the co-authors made the decision to submit the manuscript for publication and take responsibility for the content.
This study is registered as clinical trial number NCT00327587.
References
- 1.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795–797. doi: 10.1016/S0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 2.Gosse P, Schumacher H. Early morning blood pressure surge. J Clin Hypertens (Greenwich) 2006;8:584–589. doi: 10.1111/j.1524-6175.2006.04773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leppäluoto J, Ruskoaho H. Atrial natriuretic peptide, renin activity, aldosterone, urine volume and electrolytes during a 24-h sleep-wake cycle in man. Acta Physiol Scand. 1990;139:47–53. doi: 10.1111/j.1748-1716.1990.tb08896.x. [DOI] [PubMed] [Google Scholar]
- 4.Veerman DP, Imholz BP, Wieling W, Wesseling KH, van Montfrans GA. Circadian profile of systemic hemodynamics. Hypertension. 1995;26:55–59. doi: 10.1161/01.HYP.26.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Sica DA. What are the influences of salt, potassium, the sympathetic nervous system, and the renin-angiotensin system on the circadian variation in blood pressure? Blood Press Monit. 1999;4(Suppl 2):S9–S16. [PubMed] [Google Scholar]
- 6.Lamarre-Cliche M, de Champlain J, Lacourcière Y, Poirier L, Karas M, Larochelle P. Effects of circadian rhythms, posture, and medication on renin-aldosterone interrelations in essential hypertensives. Am J Hypertens. 2005;18:56–64. doi: 10.1016/j.amjhyper.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens. 1999;12:205S–213S. doi: 10.1016/S0895-7061(99)00103-X. [DOI] [PubMed] [Google Scholar]
- 8.Redon J, Roca-Cusachs A, Mora-Macia J. Uncontrolled early morning blood pressure in medicated patients: the ACAMPA study. Analysis of the control of blood pressure using ambulatory blood pressure monitoring. Blood Press Monit. 2002;7:111–116. doi: 10.1097/00126097-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Polónia J, Alcantâra P, Amado P, Silva JA, Nazaré J, Braz-Nogueira J. Lack of adequate blood pressure control in the morning and evening periods in medicated hypertensive patients considered to be controlled in the office. Rev Port Cardiol. 2005;24:1059–1072. [PubMed] [Google Scholar]
- 10.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens (Greenwich) 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 11.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 12.Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073–2082. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 13.Milani RV. Reaching for aggressive blood pressure goals: role of angiotensin receptor blockade in combination therapy. Am J Manag Care. 2005;11(7 Suppl):S220–S227. [PubMed] [Google Scholar]
- 14.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 15.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 16.Bangalore S, Messerli FH, Cohen JD, Bacher PH, Sleight P, Mancia G. Verapamil-sustained release-based treatment strategy is equivalent to atenolol-based treatment strategy at reducing cardiovascular events in patients with prior myocardial infarction: an INternational VErapamil SR-Trandolapril (INVEST) substudy. Am Heart J. 2008;156:241–247. doi: 10.1016/j.ahj.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun DA, Lacourcière Y, Chiang YT, Glazer RD. Triple antihypertensive therapy with amlodipine, valsartan, and hydrochlorothiazide: a randomized clinical trial. Hypertension. 2009;54:32–39. doi: 10.1161/HYPERTENSIONAHA.109.131300. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL., Jr Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G. 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 21.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ. 2004;328:634–640. doi: 10.1136/bmj.328.7440.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ommen ES, Lipkowitz MS. The role of ambulatory BP monitoring in clinical care. Geriatrics. 2007;62:11–14. [PubMed] [Google Scholar]
- 23.Conen D, Bamberg F. Noninvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2008;26:1290–1299. doi: 10.1097/HJH.0b013e3282f97854. [DOI] [PubMed] [Google Scholar]
- 24.Staessen JA, Beilin L, Parati G, Waeber B, White W. Task force IV: clinical use of ambulatory blood pressure monitoring. Participants of the 1999 Consensus Conference on ambulatory blood pressure monitoring. Blood Press Monit. 1999;4:319–331. doi: 10.1097/00126097-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 25.White WB. Importance of blood pressure control over a 24-hour period. J Manag Care Pharm. 2007;13(8 Suppl B):34–39. doi: 10.18553/jmcp.2007.13.s8-b.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacourcière Y, Wright JT, Jr, Samuel R, Zappe D, Purkayastha D, Black HR. Effects of force-titrated valsartan/hydrochlorothiazide versus amlodipine/hydrochlorothiazide on ambulatory blood pressure in patients with stage 2 hypertension: the EVALUATE study. Blood Press Monit. 2009;14:112–120. doi: 10.1097/MBP.0b013e32832a9da7. [DOI] [PubMed] [Google Scholar]
- 27.Lacourcière Y, Gil-Extremera B, Mueller O, Byrne M, Williams L. Efficacy and tolerability of fixed-dose combinations of telmisartan plus HCTZ compared with losartan plus HCTZ in patients with essential hypertension. Int J Clin Pract. 2003;57:273–279. [PubMed] [Google Scholar]
- 28.Lacourcière Y, Neutel JM, Schumacher H. Comparison of fixed-dose combinations of telmisartan/hydrochlorothiazide 40/12.5 mg and 80/12.5 mg and a fixed-dose combination of losartan/hydrochlorothiazide 50/12.5 mg in mild to moderate essential hypertension: pooled analysis of two multicenter, prospective, randomized, open-label, blinded-end point (PROBE) trials. Clin Ther. 2005;27:1795–1805. doi: 10.1016/j.clinthera.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Neutel JM, Smith D. Ambulatory blood pressure comparison of the anti-hypertensive efficacy of fixed combinations of irbesartan/hydrochlorothiazide and losartan/hydrochlorothiazide in patients with mild-to-moderate hypertension. J Int Med Res. 2005;33:620–631. doi: 10.1177/147323000503300603. [DOI] [PubMed] [Google Scholar]
- 30.Neldam S, Edwards C. Telmisartan plus HCTZ vs. amlodipine plus HCTZ in older patients with systolic hypertension: results from a large ambulatory blood pressure monitoring study. Am J Geriatr Cardiol. 2006;15:151–160. doi: 10.1111/j.1076-7460.2006.05219.x. [DOI] [PubMed] [Google Scholar]
- 31.Minami J, Abe C, Akashiba A, Takahashi T, Kameda T, Ishimitsu T. Long-term efficacy of combination therapy with losartan and low-dose hydrochlorothiazide in patients with uncontrolled hypertension. Int Heart J. 2007;48:177–186. doi: 10.1536/ihj.48.177. [DOI] [PubMed] [Google Scholar]
- 32.Sharma AM, Davidson J, Koval S, Lacourcière Y. Telmisartan/hydrochlorothiazide versus valsartan/hydrochlorothiazide in obese hypertensive patients with type 2 diabetes: the SMOOTH study. Cardiovasc Diabetol. 2007;6:28. doi: 10.1186/1475-2840-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]


