Abstract
Rationale: The opportunistic pathogen Pseudomonas aeruginosa causes both acute and chronic lung infections and is particularly problematic in patients with cystic fibrosis and those undergoing mechanical ventilation. Decreased lung function contributes significantly to morbidity and mortality during P. aeruginosa infection, and damage inflicted by P. aeruginosa virulence factors contributes to lung function decline.
Objectives: We sought to describe direct contribution of a bacterial phospholipase C/sphingomyelinase, PlcHR, to alteration of host lung physiology and characterize a potential therapeutic for protection of lung function.
Methods: We infected C57Bl/6 mice with P. aeruginosa wild-type or isogenic plcHR deletion strains and measured lung function using computer-controlled ventilators. For in vivo testing, miltefosine was delivered intraperitoneally 1 hour after infection. Infection and respiratory endpoints were at 24 hours after infection.
Measurements and Main Results: P. aeruginosa wild-type infection caused significant lung function impairment, whereas the effects of a ΔplcHR strain infection were much less severe. Surfactometry analysis of bronchoalveolar lavage fluid indicated that PlcHR decreased pulmonary surfactant function. Miltefosine has structural similarity to the PC and sphingomyelin substrates of PlcHR, and we found that it inhibits the cleavage of these choline-containing lipids in vitro. Miltefosine administration after P. aeruginosa infection limited the negative effects of PlcHR activity on lung function.
Conclusions: We have directly linked production of a single virulence factor in P. aeruginosa with effects on lung function, and demonstrated that the inhibitor miltefosine protects lung function from PlcHR-dependent surfactant dysfunction.
Keywords: Pseudomonas, surfactant, phospholipase, respiratory mechanics
At a Glance Commentary
Scientific Knowledge on the Subject
Although studies have examined the role of many Pseudomonas aeruginosa virulence factors during infection, none have demonstrated direct effects on pulmonary physiology, which manifests because there are no therapies designed to explicitly protect lung function during bacterial infection.
What This Study Adds to the Field
Here we show that a single extracellular bacterial protein with phospholipase C/sphingomyelinase (PC-PLC/SMase) activity alters respiratory physiology during infection and we have identified a small molecule that inhibits this PC-PLC/SMase activity and demonstrated that it can protect lung function during infection in the mouse. Conservation of this specific PC-PLC/SMase family in other respiratory pathogens, including Mycobacterium tuberculosis, suggests that this inhibitor could have broad efficacy.
Pseudomonas aeruginosa is a common, gram-negative opportunistic pathogen responsible for 8–16% of nosocomial infections (1). Chronic P. aeruginosa infections are common in the lungs of patients with cystic fibrosis (CF) and are associated with over 80% of the morbidity and mortality in this population (2–4). Chronic P. aeruginosa infections are also seen in patients undergoing mechanical ventilation, and in people with chronic obstructive pulmonary disease (5, 6). Acute P. aeruginosa pneumonia is associated with high mortality (7, 8).
Numerous P. aeruginosa virulence factors impact the growth, survival, and immune evasion of P. aeruginosa in vivo, and contribute to host inflammation and tissue damage during P. aeruginosa lung infections (9). One secreted virulence factor in particular, PlcHR, has been linked to declines in lung function and increase in frequency of exacerbation in patients with CF (10, 11). The preferred substrates for PlcH activity are phosphatidylcholine (PC) and sphingomyelin (12), lipids that make up the bulk of cellular membranes and pulmonary surfactant. Many reports detail the potential detrimental effects of PlcH PC-phospholipase C/sphingomyelinase (PC-PLC/SMase) activity during infection (13), but have not examined pulmonary function.
PlcH may influence patient status and disease course through its effects on pulmonary surfactant, a fluid largely composed of dipalmitoylphosphatidylcholine (14). P. aeruginosa phospholipases from culture supernatants are sufficient for surfactant PC degradation (15), and we have shown that P. aeruginosa PlcH is necessary for the efficient breakdown of lung surfactant PC (16). Pulmonary surfactant lowers the surface tension of the airway–surface liquid and surfactant dysfunction has been observed in patients with CF with chronic P. aeruginosa infections (17–19) and in individuals with P. aeruginosa and other bacterial pneumonias (20, 21). Decreased surfactant activity has been proposed to be a contributing factor to lung disease in CF (22) and other respiratory illnesses including pneumonia (20, 23) and chronic bacterial infections (24). Lung surfactant homeostasis in disease is complex, and multiple host and pathogen factors have the potential to alter the production, function, or levels of lung surfactant components during P. aeruginosa infections (25–29).
Given the potential for contribution of PlcHR to pulmonary surfactant function, we hypothesized that isogenic plcHR mutants would cause less damage to the pulmonary surfactant system and therefore result in improved lung function. Currently, there are no therapies directed exclusively at protection of surfactant function during infection. We predicted that a small molecule inhibitor of PlcHR would be a promising therapeutic agent during P. aeruginosa lung infections directed at protection of respiratory function. Some of the results of these studies have been previously reported in the form of abstracts (30, 31).
Methods
Strains, Growth Conditions, and Strain Construction
P. aeruginosa strain PAO1 and the isogenic ΔplcHR deletion strain, and Escherichia coli strains, were maintained on Lysogeny Broth medium. When necessary, gentamicin was added to 10 μg/ml for E. coli, and 50 μg/ml for P. aeruginosa. The plcHR deletion in our PAO1 strain was made via the pMQ30 plasmid by recombination as described previously (32, 33) using the following primers: plcH-GOI-F 5′-CGACGATACTGTCCCAACCT-3′, plcH-SOE-R 5′catgatcggcgatGGTACCcagctcctcgtcggCAAGCGTCACCAAAACTTCA-3′, plcH-SOE-F 5′-ccgacgaggagctgGGTACCatcgccgatcatgGTTTTATTTCCCGGGTGGTT-3′, and plcH-GOI-R 5′-CAGCAGTTGTTCGTCGACAT-3′. Similar to results we reported previously with a similar deletion mutant, the ΔplcHR deletion produced no choline-induced nitrophenolphosporylcholine (NPPC) hydrolysis activity (16).
Sputum RNA Isolation and Quantitative Reverse-Transcriptase Polymerase Chain Reaction
Sputum samples from patients with CF over the age of 12 with a history of infection by P. aeruginosa were collected in accordance with the ethical guidelines of the Committee for the Protection of Human Subjects at Dartmouth College (Hanover, NH). Immediately after collection, two volumes of RNA-protect bacterial agent (Qiagen, Valencia, CA) were added to maintain RNA integrity and the samples were kept at −80°C until analysis. On the day of RNA isolation, samples were thawed and liquefied with progressively smaller needles for processing, 40–80 μl of 2% dithiothreitol was added, and the sample was then incubated on ice for 5 minutes and spun down at 13,000 × rpm for 3 minutes.
RNA extraction was done via a phenol chloroform extraction protocol adapted from Mamessier and coworkers (34) with minor modifications as described next. The recovered RNA was treated with RQ1 DNase (Promega, Madison, WI) to remove contaminating DNA, and DNases were removed by RNeasy kit (Qiagen). cDNA synthesis was accomplished using Superscript III (Invitrogen) and 5′-NSNSNSNSNS-3′ primers in a 20-μl reaction. Quantitative analysis of plcH and ppiD levels was determined using Taqman duplex polymerase chain reaction to find the ratios of plcH/ppiD. Primers and probes used for plcH expression were as follows: forward primer 5′-GGCTCCAGGAGCAGAACAAC-3′; reverse primer 5′-TGCCAGAGGTCCGAATCG-3′; probe 5′-6FAM-AACGCCCTCGCCTGGTTCAGGA-MGBNFQ-3′. Primers and probes used for ppiD expression were as follows: forward primer 5′-CGGGCACCGGTTTCG-3′; reverse primer 5′-AAGTCGCGGGTCTGCTTCT-3′; probe 5′-VIC-CACCGACAACGAATTGCAGTCCTTCG-MGBNFQ-3′.
As controls, ratios of plcH/ppiD were also obtained from wild-type (WT) P. aeruginosa grown in media in which plcH mRNA levels were induced or at basal levels. The induced cultures were grown in 3-(N-morpholino)propanesulfonic acid medium with 2-mM sodium pyruvate, 8-μM ferric chloride, and 3% (vol/vol) bovine lung surfactant (Survanta), whereas uninduced cells had 20-mM pyruvate and no surfactant.
Purification of PlcHR
Purifications of active PlcHR and the catalytic mutant PlcHR T178A were done according to the methods of Vasil and colleagues and was determined to be LPS-free (35, 36).
Hemolysis and NPPC Hydrolysis Assays
Purified PlcHR was diluted to 10 μg/ml in phosphate-buffered saline (PBS) and miltefosine (Sigma) was added to generate the specific final concentrations. Washed sheep erythrocytes were added to the hemolysis reaction and incubated at 37°C. Intact erythrocytes were pelleted and hemoglobin in the supernatants was measured by absorbance at 540 nm. Hemolysis was reported as percent hemolysis with the hemolysis caused by PlcHR in the absence of miltefosine set at 100%. PLC activity measurement by NPPC hydrolysis was conducted as previously reported by our group (16).
Mouse Lung Infection
The protocol for animal infection and respiratory physiology measurements was approved by the Institutional Animal Care and Use Committee of the University of Vermont (Burlington, VT), in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines. All surgeries were performed under pentobarbital anesthesia, ventilated paralyzed animals were monitored for distress using ECG, and all efforts were made to minimize animal suffering.
Overnight LB cultures of P. aeruginosa were measured by OD600, pelleted, washed twice with PBS, and resuspended to give 5 × 107 viable P. aeruginosa in 40 μl. Actual inoculum was determined by serial dilution of the input bacterial suspension on Pseudomonas isolation agar (Difco). Adult male C57Bl/6J mice, 8–12 weeks old (Jackson Laboratories, Detroit, MI), were inoculated with 40 μl (5 × 107 CFU) of P. aeruginosa PAO1 or isogenic ΔplcHR via oropharyngeal aspiration following brief anesthesia with isoflourane (16, 37). When appropriate, miltefosine was delivered via intraperitoneal injection 1 hour after infection at a total dose of 10 mg/kg. At 24 hours after infection, mice were anesthetized with intraperitoneal sodium pentobarbital, tracheas were cannulated, and bronchoalveolar lavage fluid (BALF) collected. Lungs and spleens were excised and immediately placed into 1 ml of cold PBS followed by homogenization. Viable bacterial counts in lungs and spleens were determined by plating serial dilutions of organ homogenate onto Pseudomonas isolation agar plates followed by incubation at 37°C for 24 hours. White blood cell counts in the BALF were done using an Advia automated cell counter (Siemens, Berlin, Germany) and cell type was done by manual examination of hematoxylin and eosin–stained slides. To measure lung weights, excised lungs were blotted three times and weighed in plastic weigh boats. For histology lungs were inflation-fixed at 20 cm H2O pressure with buffered formalin for 24 hours, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Cell-free protein content in the BALF was determined by Bradford assay with bovine serum albumin as the standard.
Mouse Ventilation and Lung Physiology
For mouse ventilation studies, mice were anesthetized with pentobarbital; tracheas were cannulated; they were placed on the ventilator and paralyzed with intraperitoneal pancuronium bromide (0.5 mg/kg); and heart rate was monitored by ECG to ensure proper anesthesia. Ventilation, pressure–volume (P-V) measurements, and respiratory impedance were conducted as previously described (38–40). A full description of the ventilation techniques and analysis can be found in the online supplement.
For direct instillation of purified PlcHR via the cannula, the dose of 50 ng PlcHR per mouse was based on the intranasal instillation used by Wieland and colleagues (41), which yields a high but nonlethal dose. PlcHR caused dose-dependent effects on lung function (data not shown); the dose chosen caused surfactant dysfunction at 1.5 hours after instillation, which is comparable with that observed after infection with WT P. aeruginosa for 24 hours.
Surfactant Activity
For surfactant analysis, lavage was conducted from mice with sterile normal saline. Lavage was fractionated (22), extracted, and inorganic phosphate content of the lipid fraction was measured (42). The large aggregate fractions were brought to 1 mg/ml phospholipid concentration in capillary surfactant buffer and analyzed using a capillary surfactometer (Calmia Biomedical, Toronto, ON, Canada) according to Lema and colleagues (15). Each sample (from one mouse) was measured on the capillary surfactometer six times, and the average from each mouse (n > 6 per group) was averaged to generate the mean reported in the results section.
For in vitro surfactometry with PlcHR, samples of Survanta were diluted to 2 mg/ml phospholipid in capillary surfactant buffer, to which miltefosine and purified PlcHR in capillary surfactant buffer were added. Additions of miltefosine and PlcHR to subsequent samples were staged 4 to 5 minutes apart to account for the 120-second surfactometer run, and the loading and placement of the capillary for the next sample.
Results
plcH mRNA Levels in CF Sputum
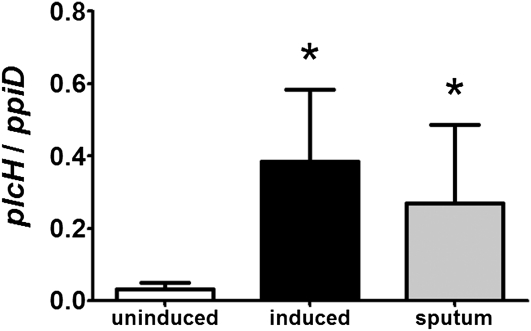
In the mouse lung, we found that induction of plcH mRNA levels is dependent on the GbdR transcription factor (16), which is activated by glycine betaine and dimethylglycine, two catabolic intermediates in the pathway for the catabolism of choline-containing lipids (33). Similar regulation of plcH transcription was also observed in lung surfactant (33). Using the ratio of plcH to ppiD, a control transcript that remains at constant levels in all conditions tested to indicate levels of plcH expression, we determined if plcH is also highly expressed in the sputum of patients with CF who are chronically infected with P. aeruginosa. Total RNA from the sputum of nine patients with CF infected with P. aeruginosa was isolated, and plcH and ppiD mRNA levels were measured by quantitative Taqman reverse-transcriptase polymerase chain reaction. For comparison, total P. aeruginosa RNA was extracted from multiple independent cultures grown on different days in a defined medium with pyruvate as the sole source of carbon, in which no PlcH activity is detected, or in medium with PC-rich lung surfactant, which induces plcH expression in a GbdR-dependent manner (16). There was a 12-fold increase in plcH expression in the induced controls compared with the uninduced controls (P = 0.01) (Figure 1). The high ratio of plcH/ppiD levels seen in the sputum samples compared with the uninduced controls is similar to the induced controls indicating expression of plcH in CF sputum (P = 0.01). These results suggest that plcH is expressed in chronic CF infections, and this finding is consistent with a previous study by Hollsing and coworkers (43) that showed detection of serum antibodies to PlcH in all 62 of the patients with CF infected with P. aeruginosa analyzed.
Figure 1.
Real-time quantification of relative plcH mRNA levels in Pseudomonas aeruginosa RNA isolated from cystic fibrosis sputum. The plcH/ppiD mRNA ratios from uninduced (pyruvate-grown) and induced (surfactant-grown) P. aeruginosa were determined in 4-hour cultures from four independent experiments and averaged together. Ratios of plcH to ppiD in sputum samples represent the average from nine patients. Error bars represent the SD. *P < 0.05.
Purified PlcHR Directly Affects Lung Function
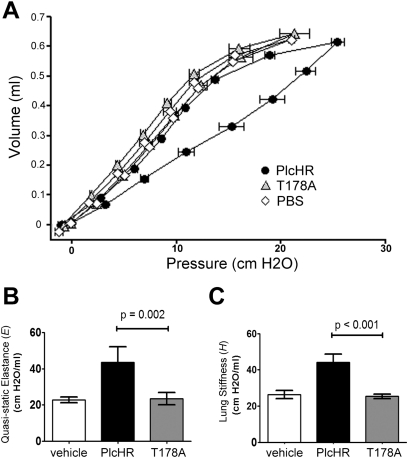
Because of correlations between decreased lung function and poor prognosis in patients infected with P. aeruginosa (44), and the evidence that plcH is robustly expressed during chronic infections (Figure 1), we explored the effects of PlcH specifically on lung function using purified PlcH protein. PlcH is purified from culture supernatants in complex with PlcR (35), and thus PlcHR was used in these experiments. PlcHR (50 ng as described in the Methods section) was administered intratracheally to anesthetized mice on a ventilator, and lung function was assessed by measuring the P-V relationship and impedance using forced oscillations on ventilated mice.
Single P-V loops are comprised of an inspiratory limb and an expiratory limb. The curves shown represent the average of data from multiple animals measured at the same time point after treatment. Here we have focused our attention on the inspiratory limb in the range of normal ventilation because this region of the P-V curve is most sensitive to changes in surfactant function (45). Buffer alone (PBS) did not affect lung function as shown by the similarities of the inspiratory curve, which is always the right limb of the P-V loop, and the expiratory curve. Buffer-treated control mice had P-V loops similar to those of untreated mice (data not shown). PlcHR instillation into the lung led to a striking increase in hysteresis or widening between the two arms of the P-V loop showing perturbation of the inspiratory phase (Figure 2A). To determine if the effects of PlcHR on lung function were dependent on catalytic activity, equivalent amounts of catalytically inactive PlcH variant (T178A) (35) were added as PlcH(T178A)R, and lung function was measured. Catalytically inactive PlcH(T178A)R did not lead to P-V loop changes relative to the buffer control (Figure 2A).
Figure 2.
Intratracheal instillation of purified PlcHR resulted in a loss of lung function. (A) Pressure–volume (P-V) loops were measured in vehicle-treated (phosphate-buffered saline [PBS], white diamonds), PlcHR-treated (black circles), and catalytically inactive PlcHR T178A-treated (gray triangles) animals. Both PlcHR additions were 50 ng of protein in 50 μl of PBS. The quasistatic pressure is plotted against volume and the symbols represent mean ± SEM. Y-error bars have been removed for clarity. (B) Quasistatic elastance (E) calculated from the inspiratory limb of the P-V curves in (A). (C) Lung stiffness (H) calculated from respiratory impedance measurements based on the constant phase model. Means in (B) and (C) are shown ± 1 SD from a representative experiment of five mice per group. Two independent experiments with five animals per infected group showed similar results. Tests of significance conducted using a one-way analysis of variance with a Bonferroni multiple comparison test.
The P-V loop profile observed on instillation of PlcHR can be indicative of decreased activity of the pulmonary surfactant, which is largely composed of PC lipids that are known substrates of PlcHR (46). The shape of the P-V curve was indicative of surfactant dysfunction. In humans, compliance (C, L/cm H2O) measurements are often used to assess surfactant function. Because of the differences between humans and mice, particularly in the methods used to quantify lung function, we have chosen to report quasistatic elastance (E) which is the mathematical inverse of compliance. E, and by direct relation C, is the physiologically most relevant metric influenced by pulmonary surfactant. E is measured from the slope of the P-V inspiratory limb, which is increased in the setting of surfactant dysfunction and distal lung closure (27, 47). Because we calculated E from the range of points defining the middle to end of tidal volume, our reported E emphasizes any pathology altering lung physiology in the range of normal breathing. Treatment with catalytically active PlcHR caused a large increase in E (Figure 2B), supporting a direct effect on surfactant function.
The parameter E, as measured previously, is a largely time-independent metric because it is measured after a 1-second pause in volume adjustment to allow for tissue rheology effects to dampen. An important missing component of E, therefore, is the time dependence of the P-V relationship. The parameter H, termed here “lung stiffness/elastance,” is a time-resolved metric derived from measures of impedance fitted to the constant phase model and is a sensitive measure of distal lung closure (40, 48, 49). Thus, E and H are complimentary measurements of the relationship between pressure and volume in the lung. The nearly twofold increase in H on instillation of active PlcHR, but not the catalytically inactive variant (Figure 2C), indicated significant changes in distal airway closure to levels that were comparable with those observed in mouse models of acute asthma or lung injury (39, 48). Dysfunction of pulmonary surfactant and resultant airway closure is the physiologic phenomenon that best explains the correspondence of data from E, H, and P-V curve shape.
The Role of PlcHR in P. aeruginosa Alteration of Mouse Lung Function
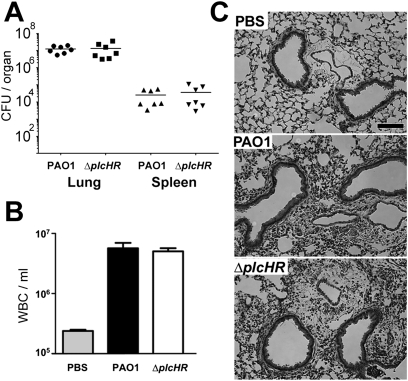
To determine the role of PlcHR in P. aeruginosa mouse lung infections, a low-dose inoculum (1 × 106 CFU) of WT and ∆plcHR P. aeruginosa PAO1 was administered into the lungs of mice by oropharyngeal aspiration. PlcHR was found to be important for colonization because the WT was present at approximately 40-fold greater levels than the isogenic ∆plcHR strain after 24 hours (see Figure E1 in the online supplement). The defects in virulence in mutants lacking plcH were consistent data for other mouse models of infection (50, 51). To specifically examine the effects of PlcHR on lung function during P. aeruginosa infection of the mouse lung, we developed a lung infection regime in which WT and ΔplcHR strains were present in the lung at equivalent CFU to avoid differences in bacterial load or inflammation, which could indirectly affect lung physiology. We found that animals infected with either WT or the ∆plcHR mutant, at a dose of 5 × 107 CFU per mouse, had no detectable differences in bacterial burden or inflammation at either 6 (see Figure E2) or 24 hours (Figure 3A). In addition, levels of infiltrating white blood cells and lung histology were similar in WT and ΔplcHR-infected mice (Figures 3B and 3C), suggesting that there were no significant differences in airway inflammation. Furthermore, cell-free protein as measured in the BALF showed no difference in protein content of the BALF between the two infections (1.11 ± 0.18 mg/ml for WT infection compared with 1.06 ± 0.23 mg/ml in ΔplcHR infection; P = 0.68) suggesting there were no detectable differences in epithelial barrier function at this time point. At 24 hours, both P. aeruginosa WT and ∆plcHR caused systemic bacteremia, and there were no significant differences in spleen CFU (Figure 3A) or blood bacterial burden (not shown) between strains.
Figure 3.
Deletion of plcHR did not alter bacterial load or inflammation at high infective dose. (A) Bacterial burden from whole homogenized lung and spleen. CFU counts from individual mice plotted with mean noted by the horizontal lines. Means are not significantly different. (B) WBC infiltration into the BALF as measured by automated counter (Advia). Mean ± SEM plotted for 7 mice/group, and were not significantly different. (C) Hematoxylin and eosin stained lung sections from control and infected animals. Scale bar = 100 μm. Histology is from a representative experiment with 4 mice per group and is representative of two independent experiments. Statistical significance for bacterial burden calculated using a two-tailed t test.
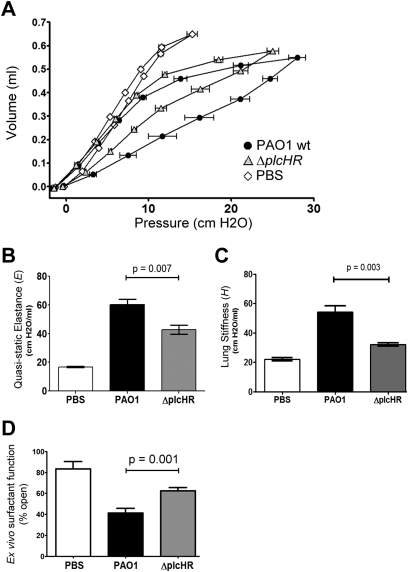
On qualitative examination, the mice infected with ΔplcHR seemed to have less respiratory distress than mice infected with WT PAO1. This observation suggested that some aspect of respiratory physiology was different between the two groups. Based on the data that showed that purified PlcHR induced lung closure in vivo (Figure 2), we predicted that a ΔplcHR mutant would have a decreased impact on lung mechanics during infection compared with the WT. P-V and impedance measurements were performed as described previously on mice infected with either WT P. aeruginosa PAO1 or the isogenic ∆plcHR strain, both at the higher dose. WT infected animals showed extremely pathologic lung physiology as assessed by hysteresis of the P-V curve and decrease in the slope of the inspiratory limb (Figures 4A and 4B). Infection with the ΔplcHR strain resulted in lung physiology that is abnormal compared with mock-infected mice (PBS instillation), but resulted in better lung function compared with animals infected with WT P. aeruginosa, using measures of P-V hysteresis (Figure 4A), E (Figure 4B), and H (Figure 4C). Based on these data, for the same amount of breathing effort, the ΔplcHR-infected mice were able to open approximately twice as much lung for gas exchange.
Figure 4.
Deletion of plcHR alters respiratory mechanics during infection. (A) Pressure–volume (P-V) loops measured in sham-treated (white diamonds) and Pseudomonas aeruginosa–infected mice (black circles for wild-type PAO1 infected; gray triangles for ΔplcHR infected). The quasistatic pressure is plotted against volume and the symbols represent mean ± SEM. Y-error bars have been removed for clarity. (B) Quasistatic elastance (E) calculated from the inspiratory limb of the P-V curves in (D). (C) Lung stiffness (H) calculated from respiratory impedance measurements based on the constant phase model. (D) Capillary surfactometry was used to assess the surfactant function of the large aggregate fraction of mouse bronchoalveolar lavage fluid. Higher values of percent open indicate better surfactant function. The black bars and gray bars represent surfactant function from mice infected by wild-type and ΔplcHR P. aeruginosa, respectively. Data for A–C are from the same representative experiment with seven mice per group in the infected groups and four mice per group in the control (phosphate-buffered saline [PBS]) group. Data are representative of two independent experiments. Data for D are from one representative experiment, where three independent experiments with at least five animals per infected group show similar results. Mean plotted ± SEM for a minimum of six mice as described in the Methods section. Tests of significance conducted using a one-way analysis of variance with a Bonferroni multiple comparison post-test.
To verify that this difference in lung function was caused by the loss of the plcHR genes and not an unlinked mutation, we used two methods of genetic complementation. Both of these methods show alterations in lung function proportional to the complementation of PlcH enzymatic activity (see Figure E3). Another possibility was that extravascular fluid accumulation was different in the WT versus ΔplcHR infections, because of the known lethal effect of PlcHR on endothelial cells and on angiogenesis (35), and that this resulted in altered lung function. To test this hypothesis we measured wet lung weights from mice who were uninfected, infected with PAO1, and infected with ΔplcHR and saw no statistically significant difference in wet weights between animals infected with PAO1 and ΔplcHR (P > 0.10). For mice who were uninfected, infected with PAO1, and infected with ΔplcHR, the wet weights of the excised lungs were 0.198 ± 0.009, 0.272 ± 0.006, and 0.259 ± 0.007, respectively.
Direct Measurement of Lung Surfactant Activity from WT Mice and Mice Infected with ∆plcHR
The lung physiology measurements from our instillation and infection experiments suggested that PlcHR could alter pulmonary surfactant function in vivo. To test this hypothesis, we analyzed surfactant recovered from lung lavage of WT mice or mice infected with ∆plcHR 24 hours after infection. Capillary surfactometry measures the ability of surfactant to maintain an open passage through a glass capillary that compares to the size of a respiratory bronchiole (0.22 mm). Using this technique, surfactant function is reported as the percent of time the capillary remains open, with a percentage open time of 100% representing fully functional surfactant, and an open time of 0% representing completely nonfunctional surfactant. To measure surfactant function, we prepared the large aggregate (LA) fraction of pulmonary surfactant from lavage samples and directly measured surfactant function using a capillary surfactometer. Our LA preparations from healthy mice show slightly more than 80% open time (Figure 4D). The WT infected mice showed compromised surfactant function (∼ 40% open time), but infection with ΔplcHR led to much less of an effect on pulmonary surfactant function (∼ 60% open time) compared with WT infection (P = 0.0105) (Figure 4D). Surfactant function can be influenced by total surfactant phospholipid levels, the distribution between small aggregate (SA) and large aggregate (LA) fractions, or levels of particular phospholipids. We determined that neither the total lavaged phospholipid content nor the large aggregate (LA) to small aggregate (SA) ratio was different when comparing surfactant from ΔplcHR with WT mice infected with P. aeruginosa (data not shown). Although it is not surprising that there was still some perturbation of surfactant function in the animals infected with ΔplcHR, these data indicated that the preservation of pulmonary function in mice infected with ΔplcHR was predominately caused by direct alteration of surfactant function. These data are consistent with the findings of Lema and coworkers (15), which showed that P. aeruginosa supernatants directly decrease the PC fraction of lung surfactant.
Miltefosine Inhibits PlcHR Activity and PlcHR Disruption of Surfactant Function In Vitro
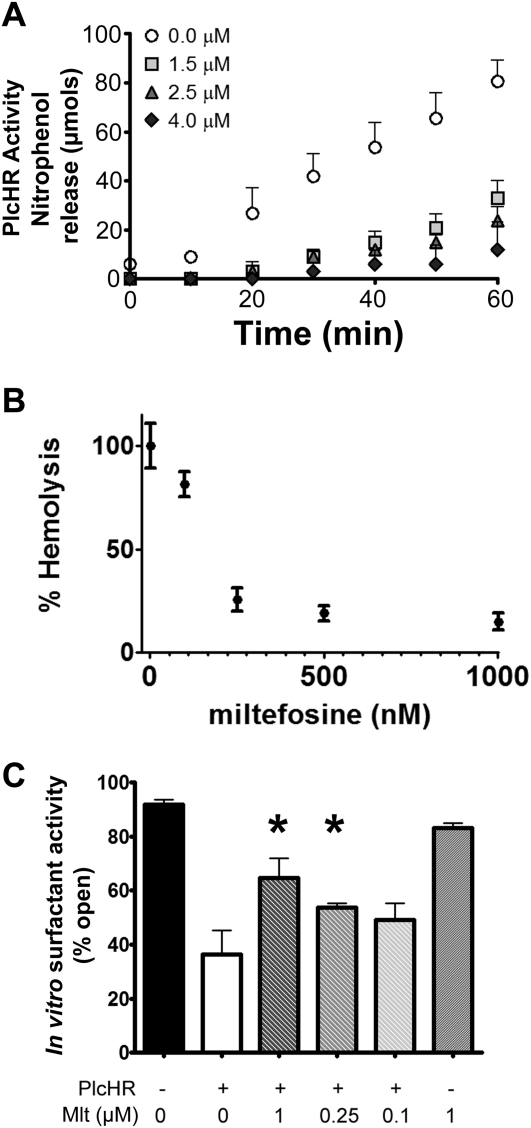
In search of potential inhibitors of PlcH, we tested whether the ether-linked phosphocholine compound, miltefosine (hexadecylphosphocholine), which is already in use clinically in the treatment of leishmaniasis (52, 53), could block PlcH activity toward the colorimetric substrate, NPPC. Miltefosine effectively inhibited NPPC cleavage at 1.5 μM when purified PlcHR was used (Figure 5A). PlcHR is also capable of hemolysis of sheep erythrocytes because of its ability to cleave sphingomyelin (54, 55), which is the only choline phospholipid in the outer leaflet of sheep erythrocytes. Miltefosine prevented hemolysis by 10 μg/ml PlcHR at concentrations down to 250 nM (Figure 5B).
Figure 5.
Miltefosine inhibited PlcHR hemolysis, enzymatic activity, and surfactant dysfunction in vitro. (A) Miltefosine inhibited nitrophenolphosporylcholine hydrolysis by purified PlcHR, measured by nitrophenol release followed as a change in absorbance at 410 nm. The legend indicates the concentration of miltefosine in the reaction (micromolar). Means for parts A and B are from three replicates and correspond to at least three independent experiments conducted on different days. Error bars indicate SD. (B) Miltefosine inhibited PlcHR-dependent hemolysis of sheep erythrocytes in a concentration-dependent manner. (C) Capillary surfactometry was used to assess the surfactant function of purified bovine surfactant (Survanta) after treatment with 10 mg/ml PlcHR with or without the labeled doses of miltefosine. Higher values of percent open indicate better surfactant function. Mean plotted ± SEM for six samples as described in the Methods section. Data are representative of two independent experiments and significance conducted using a one-way analysis of variance with a Bonferroni multiple comparison test. *P < 0.05.
Because we had linked loss of PlcH expression to surfactant dysfunction in vivo, we measured the ability of miltefosine to protect a bovine surfactant preparation (Survanta) from PlcHR-dependent degradation and loss of function in vitro using capillary surfactometry. As shown in Figure 5C, miltefosine was able to protect surfactant function from the disruptive activity of 10 μg/ml PlcHR in vitro in a dose-dependent manner at concentrations down to 250 nM. Miltefosine alone did not alter surfactant activity (Figure 5C).
Miltefosine Protects Lung Function in a Mouse Model of P. aeruginosa Pneumonia
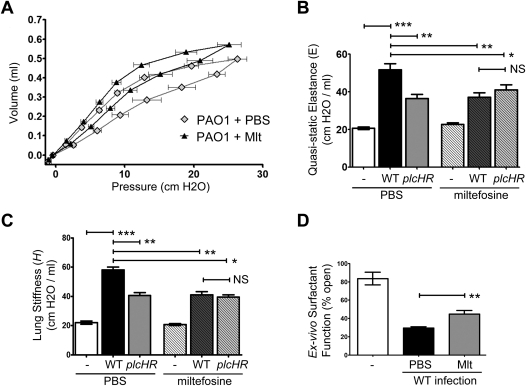
Based on our in vitro inhibition results, we hypothesized that miltefosine might provide therapeutic benefit in our mouse lung infection model by inhibiting PlcH activity. To test this hypothesis, we infected mice with WT PAO1 P. aeruginosa via oropharyngeal aspiration as described previously. One hour after infection, either miltefosine (10 mg/kg) or vehicle control (PBS) was injected intraperitoneally. Lung function was assessed at 24 hours after infection. We made the qualitative observation that the mice treated with miltefosine were in less respiratory distress than their untreated counterparts, similar to observations with the mice infected with ΔplcHR relative to mice infected with WT animals. We therefore examined the lung physiology of miltefosine-treated versus mock-treated animals.
Treatment with miltefosine resulted in significant protection of lung function as evidenced by measures of P-V, E, and H in the setting of infection (Figures 6A–6C). Importantly, this dramatic protection of lung function was apparent in the absence of any measureable changes in bacterial burden or gross inflammation (see Figure E4). Injection of miltefosine or PBS into uninfected mice had no detectable effect on lung physiology (Figures 6B and 6C, fourth bar in each panel).
Figure 6.
Miltefosine protects against PlcHR-dependent alteration in lung physiology during Pseudomonas aeruginosa infection. (A) Pressure–volume (P-V) loops measured in mice infected for 24 hours with PAO1 that received injection 1 hour after infection of phosphate-buffered saline (PBS) (vehicle, gray diamonds) or 10 mg/kg miltefosine (black triangles). The quasistatic pressure is plotted against volume and the symbols represent mean ± SEM for six mice per group. Y-error bars have been removed for clarity. (B) Quasistatic elastance (E) calculated from the inspiratory limb of the P-V curves in (A). (C) Lung stiffness (H) calculated from respiratory impedance measurements based on the constant phase model. Means in (B) and (C) are shown ± 1 SD from a representative experiment of six mice per group for PAO1-infected mice, and five mice per group for ΔplcHR infected mice. (D) Capillary surfactometry was used to assess the surfactant function of the large aggregate fraction of mouse bronchoalveolar lavage fluid. Higher values of percent open indicate better surfactant function. Bars represent surfactant function from mice infected with wild-type (WT) P. aeruginosa that received injection of PBS (black) or 10 mg/kg miltefosine (gray). Mean plotted ± SEM for a minimum of six mice as described in the Methods section. Data are representative of two independent experiments. For all figure parts, three independent experiments with at least five animals per infected group show similar results. Test for significance done using one-way analysis of variance with a Bonferroni multiple comparison post-test (B–D). *P < 0.05, **P < 0.01, ***P < 0.001.
To determine if miltefosine functioned primarily through inhibition of PlcHR-mediated surfactant dysfunction in vivo, miltefosine effects on lung physiology in mice infected with a ΔplcHR strain was measured. In support of PlcH inhibition being the primary mechanism governing miltefosine's beneficial effects, miltefosine provided no protection of lung function in mice infected with a ΔplcHR strain of P. aeruginosa (Figures 6B and 6C, compare third and sixth bars in each panel).
Based on our hypothesis that miltefosine functions via inhibition of PlcHR enzyme activity, we predicted that miltefosine treatment should protect surfactant function during infection. We demonstrated that treatment of infected mice with miltefosine results in improved surfactant function compared with the vehicle control (P = 0.0402) (Figure 6D). The level of protection of the surfactant system is comparable with that seen when comparing WT with ΔplcHR infection (Figure 4D).
Discussion
We have demonstrated that PlcH, together with its nonenzymatic chaperone PlcR, causes dysfunction of mammalian pulmonary surfactant during an experimental model of P. aeruginosa lung infection. Treatment of infected mice with injected miltefosine was able to abrogate PlcH-dependent surfactant dysfunction to a degree similar to that seen in infections with a ΔplcHR mutant. The impact of miltefosine protection from PlcHR activity was best demonstrated by the failure of miltefosine to provide physiologic benefit to mice infected with a ΔplcHR strain (Figures 6B and 6C). Direct measurement of ex vivo surfactant function from infected animals also demonstrated that much of the protection of pulmonary function could be attributed to preservation of surfactant function by miltefosine (Figures 4 and 6). This study provides the first direct evidence that miltefosine treatment, and PlcHR inhibition in general, may have beneficial effects for patients with P. aeruginosa lung infections. Orthologs of PlcHR are present in a number of important lung pathogens including the CF pathogens Burkholderia cepacia and Burkholderia cenocepacia, and the select agents Burkholderia pseudomallei and Burkholderia mallei (36). Orthologs are also found in the lung pathogens Mycobacterium tuberculosis, Francisella tularensis, and Bordetella pertussis (36). Interestingly, M. tuberculosis strains may carry as many as four distinct genes encoding orthologs of PlcH (36). Thus, miltefosine or a derivative compound may be beneficial in protecting lung function during P. aeruginosa infections and during infection with other bacterial pathogens expressing PlcH-like proteins. Further work is needed to address the potential protective effect of miltefosine and inhibition of these homologous virulence determinants during infection with other bacterial pathogens.
We identified miltefosine as an inhibitor of PlcH hemolysis during a directed screen of a small set of PC-like analogs. Miltefosine is currently used as a therapeutic to treat leishmaniasis in several countries (56). The mechanism by which miltefosine protects against leishmania infection is not yet known but some evidence suggests that it affects phosphoplipid metabolism in the parasite (57). Miltefosine has been shown to have some antibacterial activity in vitro (58, 59), but in vivo efficacy against bacteria has not yet been reported. Our study demonstrates that although miltefosine is not effective as a traditional antibacterial compound in vivo (see Figure E4), it is capable of dramatically preserving lung function during P. aeruginosa infection (Figure 6A). Thus, although we are not decreasing bacterial load or inflammation in the high-dose model of infection, respiratory failure caused by surfactant dysfunction is a life-threatening symptom of bacterial lung infection. The absence of PlcHR or with miltefosine treatment in PlcHR-treated animals results in a 50% increase in the respiratory volume of the lung at pressures generated during tidal breathing. For the same amount of breathing effort, therefore, the mice treated with miltefosine are able to open more lung for gas exchange. This magnitude of change would have profound clinical significance in humans in terms of ventilator settings, time on the ventilator, and oxygenation. The inoculum of P. aeruginosa given to these mice is approximately the LD50 dose at 48 hours. Given miltefosine's effects we did examine whether plcHR deletion or miltefosine treatment improved mouse survival, but neither the deletion nor the treatment altered animal survival (data not shown). This is not particularly surprising because the model leads to severe bacteremia and mice likely die from systemic shock and multiorgan failure rather than respiratory failure.
It is important to note that P. aeruginosa possesses activities in addition to those of PlcH that can alter lung surfactant. Specifically in relation to surfactant lipids, Mallampalli and colleagues have shown an alteration of surfactant levels during a chronic infection model with mucoid P. aeruginosa that seems to depend on cell–cell contact, but the effector has not been reported (25, 27). ExoU, a phospholipase A2, also plays a role in P. aeruginosa lung infection models (60). ExoU is not predicted to play a role in surfactant dysfunction because it is a type III secreted effector that is delivered directly from the bacterium to the host cell cytoplasm where it is activated in the presence of mammalian cytoplasmic SOD1 (61). Furthermore, the P. aeruginosa strain (i.e., PAO1) used in these studies does not have a gene encoding ExoU. Schmalzer and colleagues (61) have postulated extracellular activation of ExoU by SOD1 in the context of necrotic cells, but this has not been demonstrated to date. Other P. aeruginosa phospholipases have not been linked to its virulence in humans or in animal models. Although other virulence determinants likely play important roles during P. aeruginosa infection of the lung, this study demonstrates that PlcHR is a major contributor to inactivation of surfactant and subsequent airway closure during P. aeruginosa lung infection.
PlcH has many other effects on the host that may be relevant to lung disease in addition to its effects on lung function (Figure 1) and lung colonization in low-dose infections (see Figure E1). PlcHR has been shown to alter host innate immune responses in a number of ways (41, 62, 63). Most recently, PlcH has been shown to be selectively proapoptotic to endothelial cells, and to inhibit angiogenesis in vitro and in vivo (35). Mutants lacking plcH produce more localized foci of damage and inflammatory cell congregation suggesting that PlcH production somehow aids in bacterial dispersion in a rat lung chronic infection model (64). PlcH exhibits SMase activity (46), and the product of this activity, ceramide, has been implicated in poor bacterial clearance in CF (65, 66). Bacterial pneumonia is a multifactorial disease and we have noted that addition of purified bovine surfactant or purified mouse large aggregate fractions results in no improvement in our model. This could represent alternate PlcH functions or it may be representative of results from surfactant replacement in human adults with surfactant dysfunction, which rarely demonstrates significant improvement (67). Our data indicate that miltefosine blocks cleavage of both PC-containing surfactant (Figure 5C) and sphingomyelin-rich sheep erythrocytes (Figure 5B) (54, 55), suggesting that miltefosine is an important inhibitor of both PC and sphingomyelin cleavage by PlcH. Future experiments will determine the value of miltefosine, or other PlcH inhibitors yet to be described, in protecting against the other important roles of PlcH during infections of the lung.
In addition to its inhibition of PlcH activity, miltefosine may also have other effects on the host. Although we cannot completely rule out a role for alteration of the host immune system in our current model, we detected no change in immune cell infiltration, immune cell composition, or gross histologic differences (see Figure E4, and data not shown). Miltefosine has also been demonstrated to alter lipid metabolism in mammalian cells. Of particular interest for this study, miltefosine can inhibit PC synthesis by inhibiting the proper localization of the cytidylyltransferase (68, 69), and seems to alter the sphingomyelin/ceramide ratio by inhibiting synthesis of sphingomyelin (70, 71). The lipid synthesis effects described previously were seen at higher doses of miltefosine than those required for PlcH inhibition. In our study, a single dose of miltefosine injected intraperitoneally did not result in any measurable alteration of surfactant function in healthy animals (Figures 6B and 6C). This is supported by the in vitro inhibition of PlcHR-induced surfactant dysfunction by miltefosine, where effects on de novo surfactant synthesis do not play a role (Figure 5).
Our report represents the first in vivo demonstration of a role for PlcHR in surfactant dysfunction during a mouse model of pneumonia. These data are consistent with previous reports on the effects of P. aeruginosa (15) or commercially available PLC from Clostridium perfringins (29) on surfactant activity in vitro. Additionally, this is the first P. aeruginosa virulence factor that has been directly linked to an alteration of pulmonary physiology unrelated to changes to inflammation and bacterial burden. Thus, PlcHR represents a potential therapeutic target to protect against respiratory failure in patients with acute and chronic P. aeruginosa infections. We have identified a PlcHR inhibitor miltefosine that is already being used in humans, and demonstrated its efficacy for protection of mouse lung physiology during P. aeruginosa infection. Future clinical studies will examine the impact of miltefosine on lung compliance in the context of bacterial infections. These findings could have substantial translational impact for patients infected with P. aeruginosa.
Supplementary Material
Acknowledgments
The authors thank Dr. Charles G. Irvin (University of Vermont) and Dr. Alix Ashare (Dartmouth Medical School) for critical reading of this manuscript. They also thank Dr. Jason H.T. Bates (University of Vermont) and Adel Malek (Dartmouth Medical School) for their helpful discussion. They thank Adriana Vasil for her efforts and skill in the production and purification of PlcHR and the PlcH (Ala178Thr)R mutant proteins.
Footnotes
Author contributions: Data acquisition and analysis, M.J.W., M.J.G., S.R., and J.L.A.; experimental design and interpretation, M.J.W., L.K.A.L., G.B.A., M.L.V., L.W.L., and D.A.H.; and drafting of manuscript for important intellectual content, M.J.W., L.K.A.L., M.L.V., and D.A.H.
Supported by National Institutes of Health grants P20-RR018787 (D.A.H.), P20-RR021905 (M.J.W.), and P20-RR15557 (L.W.L. and L.K.A.L.) from the National Center for Research Resources; R01AI091702 (D.A.H.); by the Ruth Kirchstein NRSA institutional fellowship awarded to the Department of Microbiology and Immunology, Dartmouth Medical School (grant T32 AI07519, supporting M.J.W.); by the Cystic Fibrosis Foundation Research Development Program (D.A.H. and M.J.W.); by a Cystic Fibrosis Foundation Postdoctoral Fellowship (M.J.W.); and by a National Institutes of Health NHLBI grant HL062608 to M.L.V. (University of Colorado School of Medicine).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201103-0374OC on May 11, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Van Delden C, Iglewski BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 1998;4:551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, Ramsey BW, Clausen CR. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 1998;27:158–163 [DOI] [PubMed] [Google Scholar]
- 3.Govan JR, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 1996;60:539–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajan S, Saiman L. Pulmonary infections in patients with cystic fibrosis. Semin Respir Infect 2002;17:47–56 [DOI] [PubMed] [Google Scholar]
- 5.Craven DE. Preventing ventilator-associated pneumonia in adults: sowing seeds of change. Chest 2006;130:251–260 [DOI] [PubMed] [Google Scholar]
- 6.Foglia E, Meier MD, Elward A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin Microbiol Rev 2007;20:409–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rello J, Rodriguez R, Jubert P, Alvarez B. Severe community-acquired pneumonia in the elderly: epidemiology and prognosis. Study Group for Severe Community-Acquired Pneumonia. Clin Infect Dis 1996;23:723–728 [DOI] [PubMed] [Google Scholar]
- 8.Restrepo MI, Anzueto A. The role of gram-negative bacteria in healthcare-associated pneumonia. Semin Respir Crit Care Med 2009;30:61–66 [DOI] [PubMed] [Google Scholar]
- 9.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 2005;171:1209–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanotte P, Mereghetti L, Lejeune B, Massicot P, Quentin R. Pseudomonas aeruginosa and cystic fibrosis: correlation between exoenzyme production and patient's clinical state. Pediatr Pulmonol 2003;36:405–412 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen D, Emond MJ, Mayer-Hamblett N, Saiman L, Marshall BC, Burns JL. Clinical response to azithromycin in cystic fibrosis correlates with in vitro effects on Pseudomonas aeruginosa phenotypes. Pediatr Pulmonol 2007;42:533–541 [DOI] [PubMed] [Google Scholar]
- 12.Lopez DJ, Collado MI, Ibarguren M, Vasil AI, Vasil ML, Goni FM, Alonso A. Multiple phospholipid substrates of phospholipase c/sphingomyelinase HR2 from Pseudomonas aeruginosa. Chem Phys Lipids 2011;164:78–82 [DOI] [PubMed] [Google Scholar]
- 13.Wiener-Kronish JP, Sakuma T, Kudoh I, Pittet JF, Frank D, Dobbs L, Vasil ML, Matthay MA. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol 1993;75:1661–1669 [DOI] [PubMed] [Google Scholar]
- 14.Caminiti SP, Young SL. The pulmonary surfactant system. Hosp Pract (Off Ed) 1991;26:87–90, 94–100 [DOI] [PubMed] [Google Scholar]
- 15.Lema G, Dryja D, Vargas I, Enhorning G. Pseudomonas aeruginosa from patients with cystic fibrosis affects function of pulmonary surfactant. Pediatr Res 2000;47:121–126 [DOI] [PubMed] [Google Scholar]
- 16.Wargo MJ, Ho TC, Gross MJ, Whittaker LA, Hogan DA. GbdR regulates Pseudomonas aeruginosa plcH and pchP transcription in response to choline catabolites. Infect Immun 2009;77:1103–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hull J, South M, Phelan P, Grimwood K. Surfactant composition in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 1997;156:161–165 [DOI] [PubMed] [Google Scholar]
- 18.Meyer KC, Sharma A, Brown R, Weatherly M, Moya FR, Lewandoski J, Zimmerman JJ. Function and composition of pulmonary surfactant and surfactant-derived fatty acid profiles are altered in young adults with cystic fibrosis. Chest 2000;118:164–174 [DOI] [PubMed] [Google Scholar]
- 19.Mander A, Langton-Hewer S, Bernhard W, Warner JO, Postle AD. Altered phospholipid composition and aggregate structure of lung surfactant is associated with impaired lung function in young children with respiratory infections. Am J Respir Cell Mol Biol 2002;27:714–721 [DOI] [PubMed] [Google Scholar]
- 20.Brogden KA. Changes in pulmonary surfactant during bacterial pneumonia. Antonie van Leeuwenhoek 1991;59:215–223 [DOI] [PubMed] [Google Scholar]
- 21.Baughman RP, Stein E, MacGee J, Rashkin M, Sahebjami H. Changes in fatty acids in phospholipids of the bronchoalveolar fluid in bacterial pneumonia and in adult respiratory distress syndrome. Clin Chem 1984;30:521–523 [PubMed] [Google Scholar]
- 22.Griese M, Birrer P, Demirsoy A. Pulmonary surfactant in cystic fibrosis. Eur Respir J 1997;10:1983–1988 [DOI] [PubMed] [Google Scholar]
- 23.Baughman RP, Sternberg RI, Hull W, Buchsbaum JA, Whitsett J. Decreased surfactant protein A in patients with bacterial pneumonia. Am Rev Respir Dis 1993;147:653–657 [DOI] [PubMed] [Google Scholar]
- 24.LeVine AM, Lotze A, Stanley S, Stroud C, O'Donnell R, Whitsett J, Pollack MM. Surfactant content in children with inflammatory lung disease. Crit Care Med 1996;24:1062–1067 [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Xu Z, Henderson FC, Ryan AJ, Yahr TL, Mallampalli RK. Chronic Pseudomonas aeruginosa infection reduces surfactant levels by inhibiting its biosynthesis. Cell Microbiol 2007;9:1062–1072 [DOI] [PubMed] [Google Scholar]
- 26.Hayashida S, Harrod KS, Whitsett JA. Regulation and function of CCSP during pulmonary Pseudomonas aeruginosa infection in vivo. Am J Physiol Lung Cell Mol Physiol 2000;279:L452–L459 [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Wu Y, Henderson F, McCoy DM, Salome RG, McGowan SE, Mallampalli RK. Adenoviral gene transfer of a mutant surfactant enzyme ameliorates Pseudomonas-induced lung injury. Gene Ther 2006;13:974–985 [DOI] [PubMed] [Google Scholar]
- 28.Attalah HL, Wu Y, Alaoui-El-Azher M, Thouron F, Koumanov K, Wolf C, Brochard L, Harf A, Delclaux C, Touqui L. Induction of type-IIa secretory phospholipase A2 in animal models of acute lung injury. Eur Respir J 2003;21:1040–1045 [DOI] [PubMed] [Google Scholar]
- 29.Holm BA, Keicher L, Liu MY, Sokolowski J, Enhorning G. Inhibition of pulmonary surfactant function by phospholipases. J Appl Physiol 1991;71:317–321 [DOI] [PubMed] [Google Scholar]
- 30.Wargo MJ, Allard JL, Lundblad LKA, Hogan DA, Whittaker LA. The role of Pseudomonas aeruginosa plcH in alteration of lung physiology in a mouse model of infection. Pediatr Pulmonol 2008;43:292–293 [Google Scholar]
- 31.Wargo MJ, Rajamani S, Allard JL, Hogan DA, Whittaker LA. A small molecule inhibitor of PlcH protects against surfactant dysfunction and resultant lung injury in mice. Pediatr Pulmonol 2009;44:299 [Google Scholar]
- 32.Schweizer HD. Small broad-host-range gentamycin resistance gene cassettes for site-specific insertion and deletion mutagenesis. Biotechniques 1993;15:831–834 [PubMed] [Google Scholar]
- 33.Wargo MJ, Szwergold BS, Hogan DA. Identification of two gene clusters and a transcriptional regulator required for Pseudomonas aeruginosa glycine betaine catabolism. J Bacteriol 2008;190:2690–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamessier E, Boniface S, Dupuy P, Reynaud-Gaubert M, Vervloet D, Magnan A. Study of cytokine gene expression in small cell samples: use in induced sputum. J Immunol Methods 2003;280:37–47 [DOI] [PubMed] [Google Scholar]
- 35.Vasil ML, Stonehouse MJ, Vasil AI, Wadsworth SJ, Goldfine H, Bolcome RE, III, Chan J. A complex extracellular sphingomyelinase of Pseudomonas aeruginosa inhibits angiogenesis by selective cytotoxicity to endothelial cells. PLoS Pathog 2009;5:e1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stonehouse MJ, Cota-Gomez A, Parker SK, Martin WE, Hankin JA, Murphy RC, Chen W, Lim KB, Hackett M, Vasil AI, et al. A novel class of microbial phosphocholine-specific phospholipases C. Mol Microbiol 2002;46:661–676 [DOI] [PubMed] [Google Scholar]
- 37.Amiel E, Lovewell RR, O'Toole GA, Hogan DA, Berwin B. Pseudomonas aeruginosa evasion of phagocytosis is mediated by loss of swimming motility and is independent of flagellum expression. Infect Immun 2010;78:2937–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen GB, Leclair T, Cloutier M, Thompson-Figueroa J, Bates JH. The response to recruitment worsens with progression of lung injury and fibrin accumulation in a mouse model of acid aspiration. Am J Physiol Lung Cell Mol Physiol 2007;292:L1580–L1589 [DOI] [PubMed] [Google Scholar]
- 39.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med 2007;175:768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med 2005;171:1363–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieland CW, Siegmund B, Senaldi G, Vasil ML, Dinarello CA, Fantuzzi G. Pulmonary inflammation induced by Pseudomonas aeruginosa lipopolysaccharide, phospholipase C, and exotoxin A: role of interferon regulatory factor 1. Infect Immun 2002;70:1352–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Arthur G. Improved procedures for the determination of lipid phosphorus by malachite green. J Lipid Res 1992;33:1233–1236 [PubMed] [Google Scholar]
- 43.Hollsing AE, Granstrom M, Vasil ML, Wretlind B, Strandvik B. Prospective study of serum antibodies to Pseudomonas aeruginosa exoproteins in cystic fibrosis. J Clin Microbiol 1987;25:1868–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003;168:918–951 [DOI] [PubMed] [Google Scholar]
- 45.Wagers S, Lundblad L, Moriya HT, Bates JH, Irvin CG. Nonlinearity of respiratory mechanics during bronchoconstriction in mice with airway inflammation. J Appl Physiol 2002;92:1802–1807 [DOI] [PubMed] [Google Scholar]
- 46.Berka RM, Vasil ML. Phospholipase C (heat-labile hemolysin) of Pseudomonas aeruginosa: purification and preliminary characterization. J Bacteriol 1982;152:239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kennedy JI., Jr High alveolar surface tension pulmonary edema: relationship to adult respiratory distress syndrome. J Thorac Cardiovasc Surg 1990;100:145–146 [PubMed] [Google Scholar]
- 48.Allen GB, Pavone LA, DiRocco JD, Bates JH, Nieman GF. Pulmonary impedance and alveolar instability during injurious ventilation in rats. J Appl Physiol 2005;99:723–730 [DOI] [PubMed] [Google Scholar]
- 49.Lundblad LK, Thompson-Figueroa J, Leclair T, Irvin CG, Bates JH. Thoracic gas volume measurements in paralyzed mice. Ann Biomed Eng 2004;32:1420–1427 [DOI] [PubMed] [Google Scholar]
- 50.Ostroff RM, Wretlind B, Vasil ML. Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect Immun 1989;57:1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. Common virulence factors for bacterial pathogenicity in plants and animals. Science 1995;268:1899–1902 [DOI] [PubMed] [Google Scholar]
- 52.Berman J. Current treatment approaches to leishmaniasis. Curr Opin Infect Dis 2003;16:397–401 [DOI] [PubMed] [Google Scholar]
- 53.Mishra J, Saxena A, Singh S. Chemotherapy of leishmaniasis: past, present and future. Curr Med Chem 2007;14:1153–1169 [DOI] [PubMed] [Google Scholar]
- 54.Pomerantsev AP, Kalnin KV, Osorio M, Leppla SH. Phosphatidylcholine-specific phospholipase C and sphingomyelinase activities in bacteria of the Bacillus cereus group. Infect Immun 2003;71:6591–6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montes LR, Lopez DJ, Sot J, Bagatolli LA, Stonehouse MJ, Vasil ML, Wu BX, Hannun YA, Goni FM, Alonso A. Ceramide-enriched membrane domains in red blood cells and the mechanism of sphingomyelinase-induced hot-cold hemolysis. Biochemistry 2008;47:11222–11230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mondal S, Bhattacharya P, Ali N. Current diagnosis and treatment of visceral leishmaniasis. Expert Rev Anti Infect Ther 2010;8:919–944 [DOI] [PubMed] [Google Scholar]
- 57.Rakotomanga M, Blanc S, Gaudin K, Chaminade P, Loiseau PM. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob Agents Chemother 2007;51:1425–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huelves L, Del Prado G, Gracia M, Rodriguez-Cerrato V, Ponte C. In vitro and in vivo activity of miltefosine against penicillin-sensitive and -resistant Streptococcus pneumoniae strains. J Chemother 2008;20:441–444 [DOI] [PubMed] [Google Scholar]
- 59.Llull D, Rivas L, Garcia E. In vitro bactericidal activity of the antiprotozoal drug miltefosine against Streptococcus pneumoniae and other pathogenic streptococci. Antimicrob Agents Chemother 2007;51:1844–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pankhaniya RR, Tamura M, Allmond LR, Moriyama K, Ajayi T, Wiener-Kronish JP, Sawa T. Pseudomonas aeruginosa causes acute lung injury via the catalytic activity of the patatin-like phospholipase domain of ExoU. Crit Care Med 2004;32:2293–2299 [DOI] [PubMed] [Google Scholar]
- 61.Schmalzer KM, Benson MA, Frank DW. Activation of ExoU phospholipase activity requires specific C-terminal regions. J Bacteriol 2010;192:1801–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terada LS, Johansen KA, Nowbar S, Vasil AI, Vasil ML. Pseudomonas aeruginosa hemolytic phospholipase C suppresses neutrophil respiratory burst activity. Infect Immun 1999;67:2371–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyers DJ, Berk RS. Characterization of phospholipase C from Pseudomonas aeruginosa as a potent inflammatory agent. Infect Immun 1990;58:659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graham LM, Vasil A, Vasil ML, Voelkel NF, Stenmark KR. Decreased pulmonary vasoreactivity in an animal model of chronic Pseudomonas pneumonia. Am Rev Respir Dis 1990;142:221–229 [DOI] [PubMed] [Google Scholar]
- 65.Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 2008;14:382–391 [DOI] [PubMed] [Google Scholar]
- 66.Yu H, Zeidan YH, Wu BX, Jenkins RW, Flotte TR, Hannun YA, Virella-Lowell I. Defective acid sphingomyelinase pathway with Pseudomonas aeruginosa infection in cystic fibrosis. Am J Respir Cell Mol Biol 2009;41:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med 2006;21:119–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jimenez-Lopez JM, Carrasco MP, Segovia JL, Marco C. Hexadecylphosphocholine inhibits phosphatidylcholine biosynthesis and the proliferation of Hepg2 cells. Eur J Biochem 2002;269:4649–4655 [DOI] [PubMed] [Google Scholar]
- 69.Geilen CC, Wieder T, Reutter W. Hexadecylphosphocholine inhibits translocation of ctp:Choline-phosphate cytidylyltransferase in madin-darby canine kidney cells. J Biol Chem 1992;267:6719–6724 [PubMed] [Google Scholar]
- 70.Wieder T, Orfanos CE, Geilen CC. Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hexadecylphosphocholine. J Biol Chem 1998;273:11025–11031 [DOI] [PubMed] [Google Scholar]
- 71.Marco C, Jimenez-Lopez JM, Rios-Marco P, Segovia JL, Carrasco MP. Hexadecylphosphocholine alters nonvesicular cholesterol traffic from the plasma membrane to the endoplasmic reticulum and inhibits the synthesis of sphingomyelin in Hepg2 cells. Int J Biochem Cell Biol 2009;41:1296–1303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.