Abstract
Rationale: Mechanical ventilation with O2-rich gas (MV-O2) offers life-saving treatment for respiratory failure, but also promotes lung injury. We previously reported that MV-O2 of newborn mice increased lung elastase activity, causing elastin degradation and redistribution of elastic fibers from septal tips to alveolar walls. These changes were associated with transforming growth factor (TGF)-β activation and increased apoptosis leading to defective alveolarization and lung growth arrest, as seen in neonatal chronic lung disease.
Objectives: To determine if intratracheal treatment of newborn mice with the serine elastase inhibitor elafin would prevent MV-O2–induced lung elastin degradation and the ensuing cascade of events causing lung growth arrest.
Methods: Five-day-old mice were treated via tracheotomy with recombinant human elafin or vehicle (lactated-Ringer solution), followed by MV with 40% O2 for 8–24 hours; control animals breathed 40% O2 without MV. At study's end, lungs were harvested to assess key variables noted below.
Measurements and Main Results: MV-O2 of vehicle-treated pups increased lung elastase and matrix metalloproteinase-9 activity when compared with unventilated control animals, causing elastin degradation (urine desmosine doubled), TGF-β activation (pSmad-2 tripled), and apoptosis (cleaved-caspase-3 increased 10-fold). Quantitative lung histology showed larger and fewer alveoli, greater inflammation, and scattered elastic fibers. Elafin blocked these MV-O2–induced changes.
Conclusions: Intratracheal elafin, by blocking lung protease activity, prevented MV-O2–induced elastin degradation, TGF-β activation, apoptosis, and dispersion of matrix elastin, and attenuated lung structural abnormalities noted in vehicle-treated mice after 24 hours of MV-O2. These findings suggest that elastin breakdown contributes to defective lung growth in response to MV-O2 and might be targeted therapeutically to prevent MV-O2–induced lung injury.
Keywords: elafin, elastin degradation, neonatal chronic lung disease, ventilator-induced lung injury, bronchopulmonary dysplasia
At a Glance Commentary
Scientific Knowledge on the Subject
Mechanical ventilation with O2-rich gas (MV-O2) offers life-saving treatment for respiratory failure, but also promotes lung injury. In neonates MV-O2 causes increased elastase activity and disordered elastin, leading to lung growth arrest. These features are recapitulated in newborn mice in which MV-O2 increases lung elastase activity, causing dysregulated elastin synthesis and assembly, transforming growth factor-β activation, apoptosis, and defective alveolarization.
What This Study Adds to the Field
This study shows that intratracheal treatment with the serine elastase inhibitor elafin, by blocking lung elastase and matrix metalloproteinase-9 activity, protects newborn mice from the adverse pulmonary effects of MV-O2. This study offers new insights on the pathogenesis and potential treatment of ventilator-induced lung injury, which is a major contributor to chronic lung disease in newborn infants, and to adult respiratory distress syndrome morbidity and mortality in older children and adults.
Mechanical ventilation with O2-rich gas (MV-O2) offers life-saving treatment for patients with respiratory failure. Such treatment, however, when prolonged can cause or aggravate lung injury, leading to chronic lung disease (CLD) in neonates whose lungs are incompletely developed, or to ventilator-induced lung injury (VILI) in older children and adults. Neonatal CLD, a variant of what Northway and coworkers (1) initially described as bronchopulmonary dysplasia, is characterized by failed formation of alveoli and lung microvessels, coupled with disordered lung elastin, resulting in structural and functional abnormalities that resemble pulmonary emphysema, as seen in adults with chronic obstructive pulmonary disease.
Elastin plays a critical role in mammalian lung development. A network of elastic fibers within the lung helps to provide structural integrity and distensibility to conducting airways, while enabling expansion and contraction of alveoli, pulsation of blood vessels, and elastic recoil of the surrounding matrix. Mutant mice that lack the elastin gene die soon after birth from cardiorespiratory failure linked to smooth muscle overgrowth in pulmonary arteries, defective airway branching, and loss of alveolar septation (2, 3).
It was reported that lungs of infants who died with CLD displayed thickened, tortuous, and irregularly distributed elastic fibers in the connective tissue matrix surrounding distal airspaces (4–6). These changes were associated with reduced secondary septation and fewer alveoli than in lungs of infants who died without CLD. Urinary excretion of desmosine, a biomarker of elastin degradation, increased during the first week of MV-O2 in infants with evolving CLD (7), suggesting that scattered elastin deposition reflects an aberrant matrix remodeling response to MV-O2. Breakdown of elastin in this disease has been attributed to inflammation linked to increased elastolytic activity in lung (8–10), resulting from infection or hyperoxia, conditions that often complicate the course of infants who are born very prematurely (11). Authentic animal models of CLD also exhibit lung inflammation and increased protease activity in response to lengthy MV-O2, resulting in lung growth arrest (12–16).
Consistent with the previously mentioned clinical and experimental findings, we discovered that newborn mice exposed to MV with 40% O2 (MV-O2) for up to 24 hours exhibited increased pulmonary elastase activity, causing lung elastin degradation and remodeling of the extracellular matrix (ECM) (17). These changes were associated with activation of transforming growth factor (TGF)-β and increased apoptosis, resulting in failed formation of alveoli and pulmonary capillaries, and scattered deposition of lung elastin (17–19). Based on previous studies showing that mice overexpressing the human serine elastase inhibitor elafin were protected against several forms of lung and cardiovascular injury, including hypoxia-induced pulmonary hypertension (20), myocardial infarction (21), viral myocarditis (22), and models of vascular injury (23, 24), we tested the hypothesis that intratracheal treatment of newborn mice with recombinant human elafin, given immediately before MV-O2 for 24 hours, would inhibit lung elastase activity and thereby prevent the adverse effects of MV on matrix elastin and lung growth. Elafin treatment prevented MV-O2–induced degradation of lung elastin, influx of inflammatory cells, TGF-β activation, apoptosis, and maldistribution of elastic fibers, and attenuated the defective formation of alveoli seen in the lungs of vehicle-treated control animals after MV-O2 for 24 hours. These findings, some of which were previously reported in the form of an abstract (25), point to a mechanistic link between increased elastolytic activity, inflammation, maladaptive TGF-β signaling, and lung growth arrest induced by prolonged MV with O2-rich gas.
Methods
Experimental Design
We used full-term 5-day-old CD-1 mice that weighed 3.4 ± 0.5 g. For each study, littermates were randomly assigned to one of three treatment groups: (1) intratracheal instillation of recombinant human elafin (Proteo-Biotech-AG, Kiel, Germany), 40 ng/g body weight (bw), in 10 μl/g bw of lactated-Ringer solution (L/R), followed by MV with 40% O2 (MV-O2); (2) L/R (vehicle) alone, 10 μl/g bw, followed by MV-O2; or (3) untreated, followed by 40% O2-breathing without MV.
Mice selected for MV underwent a tracheotomy after intramuscular ketamine (∼ 60 μg/g bw)/xylazine (∼ 12 μg/g bw), followed by MV-O2 at 180 breaths per minute (MicroVent 848; Harvard Apparatus, Holliston, MA) for either 8 or 24 hours. Tidal volumes averaged 7–8 μl/g bw. Routine care and physiologic monitoring (19) are briefly described in the online supplement. All surgical and animal care procedures and protocols were approved by Stanford University's Institutional Animal Care and Use Committee.
Serine Elastase and Matrix Metalloproteinase-9 Activity Assays
Lung tissue was stored at −80°C for later measurement of serine elastase activity using DQ-elastin substrate (16, 17, 24) (EnzChek; Invitrogen, Camarillo, CA), and matrix metalloproteinase (MMP)-9 activity by gelatin zymography (24), as detailed in the online supplement.
Postmortem Processing of Lungs for Quantitative Histology
Lungs were fixed intratracheally with buffered 4% paraformaldehyde overnight at 20 cm H2O (17). Fixed lungs were excised, their volumes measured by fluid displacement (26), and then paraffin-embedded for isotropic uniform random sectioning. Alveolar area was measured on random hematoxylin and eosin–stained 4-μm sections using the Bioquant image analysis system (R&M Biometrics, Nashville, TN); radial alveolar counts provided an index of alveolar number (19). Relative amount and distribution of insoluble lung elastin was assessed by quantitative image analysis of Hart's-stained tissue sections. The online supplement details isotropic uniform random sampling, morphometric analysis, and elastin quantification.
Assessment of TGF-β Activation and Apoptosis
To assess nuclear localization of pSmad-2, a marker of TGF-β activation, random tissue sections were pretreated for antigen retrieval (Dako, Carpinteria, CA), followed by blocking serum and application of primary antibody (rabbit anti-pSmad-2, 1:500; Cell Signaling, Boston, MA), with overnight incubation at 4°C. Immune complexes were visualized with the relevant peroxidase-coupled secondary antibody using the Vectastain Kit (Vector, Burlingame, CA). Apoptosis was detected by terminal uridine deoxynucleotidyl transferase dUTP terminal nick end labeling (TUNEL) assay using the ApopTag In Situ Apoptosis Detection kit (Chemicon, Temecula, CA) applied to paraformaldehyde-fixed sections. The Bioquant image analysis system (R&M Biometrics) was used to quantify stained nuclei/total nuclei in 10–15 ×400 fields in two random sections per animal.
Immunohistochemistry for Inflammatory Cells
Zinc-fixed (BD-Pharmingen, San Jose, CA) lung sections were incubated with blocking serum, then primary rat anti-F4/80 antibody (1:400; Abcam, Cambridge, MA) to stain monocytes, or rat anti-Ly-6G (1:200; eBioscience, San Diego, CA) to stain neutrophils. Sections were then incubated with biotinylated goat anti-rat antibody (1:200; Santa Cruz Biotech, Santa Cruz, CA), followed by streptavidin–horseradish peroxidase and diaminobenzidine (Dako). Monocytes–neutrophils were counted in 20 ×400 fields in two random sections per animal.
RNA Extraction and Quantitative Reverse Transcriptase Polymerase Chain Reaction for Cytokines and Chemokines
RNA was extracted from frozen lung samples, and quantitative reverse transcriptase polymerase chain reaction was performed using TaqMan primer/probe sets (ABI, Foster City, CA) on a CFX384 Real Time thermal cycler (Bio-Rad Labs, Hercules, CA), as previously described (19). ΔΔCt analysis was used to determine the expression level of each gene normalized to 18S using CFX384 analysis software (Bio-Rad).
Immunoblots for Cleaved Caspase-3
Lungs were frozen at −80°C for later protein extraction and immunoblot analysis of cleaved caspase-3 (17), as detailed in the online supplement.
Nuclear Extracts and Immunoblots for Nuclear Factor-κB–p65
Harvested lungs pretreated with protease inhibitor (cat #78410; Pierce Biotech, Rockford, IL) were homogenized in ice cold collection buffer supplied in a nuclear protein extraction kit (NE-Per Kit, cat #78833; Pierce Biotech). Nuclear extracts, obtained according to the manufacturer's instructions, were incubated overnight with nuclear factor (NF)-κB–p65 primary antibody (1:700, cat #sc-372; Santa Cruz Biotech).
ELISA for Active TGF-β
Lung tissue was homogenized in phosphate-buffered saline with added protease inhibitor (Pierce) and processed for analysis of TGF-β activity using an ELISA kit (MB100B; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Additional details are in the online supplement.
Urinary Desmosine
The 24-hour urine specimens were frozen for later radioimmunoassay of desmosine and creatinine concentrations, as previously reported (27).
Statistics
Data are mean ± SD. We used one-way analysis of variance and Bonferroni or Dunn multiple comparison test to compare results between groups. Datasets with marked variability were compared using the Kruskal-Wallis test with Dunn post hoc analysis (28). We used Prism-5 software (GraphPad, San Diego, CA) for statistical analysis. P less than 0.05 denoted significant differences.
Results
The purpose of this study was to determine if intratracheal instillation of the serine elastase inhibitor elafin would preserve matrix elastin and enable alveolar septation in lungs of newborn mice exposed to MV-O2 for up to 24 hours. We applied MV with 40% O2, rather than with air, based on earlier studies that showed a significant increase in lung elastase activity after 8 hours of MV with 40% O2, but not with air (17). We did studies of 8-hour duration to harvest lungs for measurement of elastase and MMP-9 activity, inflammatory cytokine and chemokine expression, and nuclear NF-κB–p65 protein. Lungs harvested at the end of 24-hour studies were used to assess all other end points. Pilot studies revealed that pulmonary responses to MV-O2 were virtually identical in 5-day-old untreated pups exposed to MV-O2 via tracheotomy for 24 hours when compared with 5-day-old mice treated via tracheotomy with L/R solution (see Figures E1–E6 in the online supplement).
Elafin Blocks Increased Lung Elastase and MMP-9 Activity, Thereby Preventing Elastin Degradation and Dispersion of Lung Elastin Induced by MV-O2
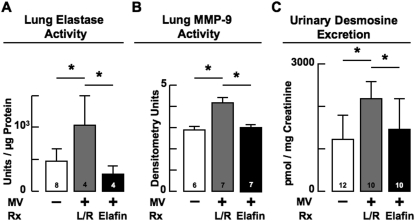
MV-O2 for 8 hours caused a doubling of serine elastase activity in lungs of vehicle-treated mice, an effect that was fully suppressed in pups treated with elafin (Figure 1A). Elafin treatment also resulted in suppression of the increased MMP-9 activity measured in lungs of vehicle-treated mice after 8 hours of MV-O2 (Figure 1B). Although elastase inhibitors are not known to suppress MMPs directly, they have been shown to block activation of the proform of these enzymes and to prevent inactivation of tissue inhibitors of MMPs (29).
Figure 1.
(A) Mechanical ventilation with O2-rich gas (MV-O2) for 8 hours increased serine elastase activity in lungs of 5-day-old lactated-Ringer solution (L/R)–treated mice (gray) compared with unventilated control mice that breathed 40% O2 for 8 hours (white). Treatment with elafin fully suppressed this effect (black). (B) MV-O2 for 8 hours increased matrix metalloproteinase (MMP)-9 activity in lungs of 5-day-old L/R-treated mice (gray) compared with unventilated control mice (white). Elafin treatment fully suppressed this MV-O2–induced effect (black). (C) MV-O2 for 24 hours increased urinary excretion of desmosine, a marker of elastin degradation, in 5-day-old L/R-treated mice (gray) compared with unventilated control mice (white). Elafin treatment suppressed this effect (black). Mean and SD. n = number noted at the base of each bar. *Significant difference, P < 0.05. Rx = treatment.
To determine if suppressing the increased elastase activity induced by MV-O2 prevented the breakdown of lung elastin, we assessed urinary excretion of desmosine, a surrogate marker of elastin degradation. Elafin treatment fully suppressed the twofold increase in cumulative urinary excretion of desmosine that was observed in vehicle-treated mice after 24 hours of MV-O2 (Figure 1C).
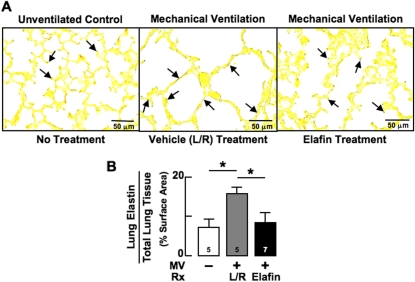
To see if blocking elastin breakdown helped to preserve the normal distribution of elastic fibers at the tips of secondary septa in lungs exposed to MV-O2 for 24 hours, we used quantitative image analysis to assess the amount and distribution of elastin in lung tissue sections treated with Hart's elastin stain. MV-O2 caused redistribution of elastin from the tips of secondary septa, resulting in elastic fibers being scattered throughout the walls of distal airspaces in vehicle-treated pups (Figure 2A). In contrast, lungs of elafin-treated mice exhibited a normal distribution of elastin at the septal tips, with considerably less dispersion of elastic fibers in alveolar walls after MV-O2 for 24 hours. Quantitative image analysis confirmed that lung content of elastin, expressed as a percent of lung tissue surface area, was similar in elafin-treated and unventilated control mice (Figure 2B). In contrast, lungs of vehicle-treated mice showed increased amounts of elastin that was fragmented and widely dispersed after MV-O2 for 24 hours.
Figure 2.
Mechanical ventilation with O2-rich gas (MV-O2) for 24 hours disperses lung elastin from septal tips to the walls of distal air spaces in 5-day-old mice. (A) Lung sections stained for elastin (arrows) showing elastin mainly at septal tips in the unventilated control lung (left), with elastic fibers dispersed along alveolar walls in the lactated-Ringer solution (L/R)–treated lung (middle) exposed to MV-O2 for 24 hours. Elafin treatment stabilized elastin at the septal tips (right). (Hart's stain; original magnification ×400.) (B) Summary data for quantitative image analysis of lung elastin showing that 24 hours of MV-O2 increased elastin deposition in L/R-treated lungs (gray), but not in elafin-treated lungs (black) compared with unventilated control lungs exposed to 40% O2 for 24 hours (white). Mean and SD. n = number noted at the base of each bar. *Significant difference, P < 0.05. Rx = treatment.
Elafin Inhibits the Inflammatory Response to MV-O2
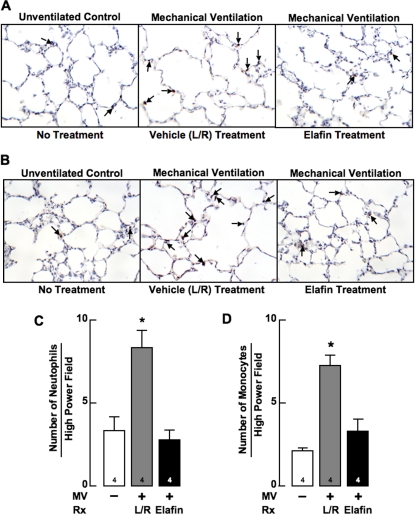
MV-O2 typically induces a leukocytic response in the lungs, which is amplified by the release of elastin degradation products (30). Elastic fiber fragments can recruit inflammatory cells to the lung, which in turn can generate increased elastase and MMP-9 activity, thereby contributing to further elastin breakdown (31). We therefore assessed the effect of elafin treatment on the number of neutrophils and monocytes that accumulated in the lungs of newborn mice after 24 hours of MV-O2. As noted in Figure 3, there was a threefold increase of both neutrophils and monocytes in the lungs of vehicle-treated pups exposed to 24 hours of MV-O2. Intrapulmonary elafin treatment blocked this response.
Figure 3.
Mechanical ventilation with O2-rich gas (MV-O2) for 24 hours increases lung inflammation in 6-day-old mice, an effect that treatment with elafin inhibits. Lung images (original magnification ×200) showing immunohistochemistry (IHC) for (A) neutrophils stained with rat anti-Ly-6G (arrows), and (B) monocytes–macrophages stained with rat anti-F4/80 antibody (arrows), in sections of lung obtained from an unventilated, untreated control mouse that breathed 40% O2 for 24 hours (left), a lactated-Ringer solution (L/R)–treated pup that had MV-O2 for 24 hours (middle), and an elafin-treated pup that had MV-O2 for 24 hours (right). Summary data showed a threefold increase of neutrophils (C) and a fourfold increase of monocytes–macrophages (D) in lungs of L/R-treated mice (gray) after MV-O2 for 24 hours compared with unventilated control mice that breathed 40% O2 for 24 hours (white). These effects were blocked in mice treated with elafin (black). Mean and SD. n = number noted at the base of each bar. *Significant difference versus control (no MV), P < 0.05. Rx = treatment.
To further assess elafin's inhibitory effects on the inflammatory response to MV-O2, we measured lung mRNA expression of various cytokines and chemokines after 8 hours of MV-O2. Elafin treatment fully suppressed the increased expression of IL-1β and interferon-γ–induced protein-10 (CXCL10) noted in lungs of vehicle-treated pups after MV-O2 for 8 hours, and also reduced levels of tumor necrosis factor-α and monocyte chemotactic protein-1 (CCL2) below levels of control and vehicle-treated pups (see Figure E8).
Because elafin has been reported to suppress activation of the inflammatory transcription factor NF-κB in endothelial cells and macrophages exposed to endotoxin and other atherogenic stimuli (32), we measured lung content of p65 protein, a subunit of the NF-κB transcription factor complex, which plays a key role in inflammatory and immune responses. We indeed discovered that elafin treatment markedly suppressed lung abundance of p65 after 8 hours of MV-O2 when compared with unventilated control animals and vehicle-treated pups (see Figure E9). Lung p65 protein did not increase in vehicle-treated pups that received MV-O2.
Elafin Inhibits MV-induced Up-regulation of Lung TGF-β Activity and Apoptosis
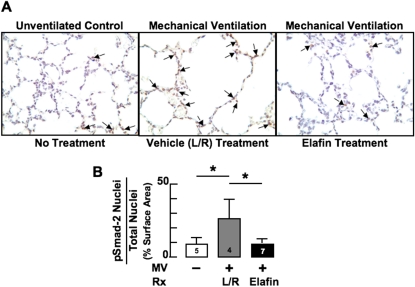
We previously found that MV, with or without associated hyperoxia, increased TGF-β signaling in the newborn lung (16, 18). A recent report indicates that degradation of elastic fibers can trigger the release of active TGF-β from the ECM, which in turn can contribute to overproduction of aberrant elastic fibers in the lung during postnatal development (33). Moreover, inflammatory cells that are recruited to the lung in response to degradation of matrix proteins can contribute to the increased TGF-β activity observed in the lungs after lengthy MV-O2 (34). We therefore assessed lung expression of pSmad-2, a marker of TGF-β activity, in newborn mice exposed to MV-O2 for 24 hours. The twofold to threefold increase in pSmad-2–expressing cells seen in lungs of vehicle-treated pups was completely suppressed in elafin-treated mice after 24 hours of MV. Consequently, lung abundance of pSmad-2–expressing cells was similar in mechanically ventilated pups treated with elafin compared with unventilated, untreated control mice that breathed 40% O2 for 24 hours (Figure 4).
Figure 4.
Mechanical ventilation with O2-rich gas (MV-O2) increases transforming growth factor (TGF)-β activation in lungs of 5-day-old mice, which treatment with elafin inhibits. (A) IHC for pSmad-2 protein in paraformaldehyde-fixed lung sections (original magnification ×400) showing increased nuclear staining (arrows), indicative of TGF-β activation, in lactated-Ringer solution (L/R)–treated mice exposed to MV-O2 for 24 hours (middle), compared with unventilated control mice that breathed 40% O2 for 24 hours (left). Elafin treatment prevented the increased TGF-β signaling caused by MV-O2 (right). (B) Summary data showing that MV-O2 for 24 hours caused a greater than twofold increase in pSmad-2 protein in lungs of L/R-treated mice (gray) compared with unventilated control mice (white). Elafin treatment blocked this effect (black). Mean and SD. n = number noted at the base of each bar. *Significant difference, P < 0.05. Rx = treatment.
To complement immunohistochemical assessment of pSmad-2 in peripheral lung as an index of TGF-β activation, which elafin inhibited during MV-O2, we used an ELISA assay to measure active TGF-β in whole lung homogenates obtained from the three groups of mice. Although the results were consistent with a suppressive effect of elafin on TGF-β signaling (see Figure E10), apparent differences in TGF-β activity between elafin-treated and control groups did not reach statistical significance (P = 0.08), perhaps related to small sample size (n = 4 per group), considerable variability in the control and vehicle-treated groups, and regional differences in TGF-β activity that may be obscured by measurements made on protein extracts of whole lung tissue.
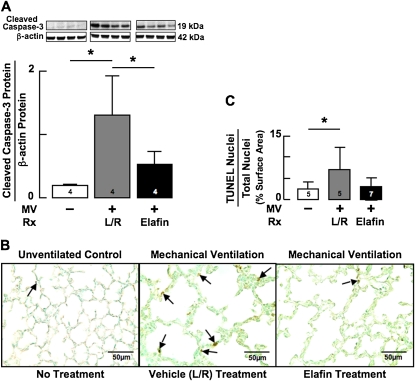
Because activation of TGF-β signaling can induce apoptosis of both alveolar epithelial cells (35) and microvascular endothelial cells (36), we assessed apoptosis in lungs of mice that had received 24 hours of MV-O2 with or without prior elafin treatment. Measurement of cleaved caspase-3 protein, a marker of apoptosis, showed nearly a 10-fold increase in lungs of vehicle-treated mice, whereas MV-O2 yielded no significant change of cleaved caspase-3 in lungs of elafin-treated pups (Figure 5A). Elafin inhibition of apoptosis was confirmed by quantitative IHC for TUNEL assay in lung sections from vehicle-treated mice compared with elafin-treated mice exposed to MV-O2 for 24 hours (Figures 5B and 5C).
Figure 5.
Mechanical ventilation with O2-rich gas (MV-O2) for 24 hours increases lung cell apoptosis in newborn mice, an effect that elafin treatment inhibits. (A) Immunoblots of cleaved caspase-3 protein, a marker of apoptosis, showing a nearly 10-fold increase in lungs of 5-day-old lactated-Ringer solution (L/R)–treated mice exposed to MV-O2 for 24 hours (gray) compared with unventilated control animals (white). Elafin treatment attenuated the MV-O2–induced increase in lung content of cleaved caspase-3 (black). (B) Terminal uridine deoxynucleotidyl transferase dUTP terminal nick end labeling (TUNEL) assay stained lung sections (original magnification ×400) showing abundant apoptotic cells (arrows) in 5-day-old L/R-treated mice after 24 hours of MV-O2 (middle), compared with unventilated control animals that breathed 40% O2 (left). Elafin treatment prevented the increased apoptosis (right). (C) Summary data showing that MV-O2 for 24 hours caused a threefold increase in TUNEL-stained cell nuclei relative to total cell nuclei in L/R-treated mice (gray) compared with unventilated control mice (white). Elafin treatment prevented the increased apoptosis (black). Mean and SD. n = number noted at the base of each bar. *Significant difference, P < 0.05. Rx = treatment.
Elafin Enables Lung Septation during MV-O2
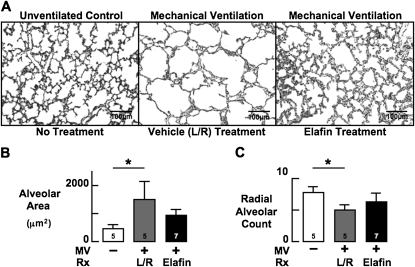
Previous studies showed that prolonged MV of newborn mice with either air or 40% O2 inhibits alveolar septation leading to lung growth arrest (18, 19). To determine if blocking lung elastase activity would help to preserve lung growth during MV-O2, we used quantitative morphometry to assess lung structure in elafin-treated mice compared with vehicle-treated mice after MV-O2 for 24 hours. Lung morphometry showed that distal airspace size was greater and radial alveolar counts were reduced after 24 hours of MV-O2 in vehicle-treated mice when compared with unventilated control animals (Figure 6). Elafin treatment attenuated these changes in lung structure.
Figure 6.
Mechanical ventilation with O2-rich gas (MV-O2) inhibits alveolar septation in newborn mice, an effect that elafin treatment inhibits. (A) Lung sections showing reduced alveolar septation and larger alveoli after MV-O2 for 24 hours in lungs of 5-day-old lactated-Ringer solution (L/R)–treated mice (middle) compared with unventilated control mice (left). Elafin treatment attenuated these structural changes (right). Lung volume was similar in the three groups (original magnification ×200). (B) Summary data showing that MV-O2 caused a threefold to fourfold increase in alveolar size in L/R-treated mice (gray) compared with unventilated control mice (white), but did not cause a significant increase of alveolar size in mice treated with elafin (black). (C) Summary data showing that MV-O2 caused a 30–40% decrease in radial alveolar count, an index of alveolar number, in lungs of L/R-treated mice (gray) compared with unventilated control mice (white), but did not significantly decrease radial alveolar count in lungs of mice treated with elafin (black). Mean and SD. n = number noted at the base of each bar. *Significant difference versus control (no MV), P < 0.05.
Discussion
This study shows for the first time that intratracheal instillation of the serine elastase inhibitor elafin, by blocking lung protease activity, can protect newborn mice from the adverse pulmonary effects of MV with O2-rich gas. Elafin delivered directly into the lungs via the airways fully suppressed the increased elastase and MMP-9 activity measured in the lungs of untreated pups after 8 hours of MV-O2. Inhibition of proteolytic activity prevented degradation and dispersion of lung elastin, and the increased TGF-β activation, apoptosis, and influx of neutrophils and monocytes noted in the lungs of vehicle-treated mice after 24 hours of MV-O2. Elafin treatment also attenuated the MV-induced emphysematous changes in alveolar structure seen in lungs of untreated mice after 24 hours of MV-O2. These findings offer new insights on the pathogenesis and potential treatment of CLD in neonates.
Elafin Inhibition of Lung Inflammation and Injury
Elafin is a low molecular weight (∼6 kD) inhibitor of human neutrophil elastase (NE) and proteinase-3. It is expressed in several human tissues, including skin and lung (37), where it is secreted by pulmonary epithelial cells and detected in bronchial secretions (38, 39). Elafin is secreted as a proform, termed “preelafin” (also known as “trappin-2”), which contains a transglutaminase domain that facilitates its binding to ECM proteins and helps to preserve its antiproteolytic function (40). Lung epithelial cell secretion of elafin increases in response to human NE (41). Elafin is not expressed in rodents (42), but transgenic mice engineered to express elafin in the lung were protected against injury induced by intratracheal injection of Pseudomonas aeruginosa (43). In another study, mice that were pretreated via the airways with recombinant pre-elafin exhibited less lung inflammation than did vehicle-treated control animals 6 hours after intranasal delivery of LPS (44). A more recent report by the same group of investigators described a beneficial effect of intranasal treatment with recombinant pre-elafin in reducing the acute lung inflammatory response after intranasal delivery of porcine pancreatic elastase, and in attenuating the emphysematous changes in lung structure seen after 2 weeks of repeated porcine pancreatic elastase doses coupled with recombinant pre-elafin treatment (45).
In our study using newborn mice, we injected recombinant human elafin directly into the lungs through a tracheotomy immediately before MV-O2. Our elafin dose of 40 ng/g bw in 10 μl/g bw was based on preliminary dose–response studies that showed complete suppression of the increased serine elastase activity measured in the lungs after 8 hours of MV-O2 (data not shown). These studies also demonstrated uniform distribution of elafin within the lungs after 24 hours of MV-O2, as assessed by IHC localization of elafin in lung sections taken from treated mice (Figure E7).
Other studies have explored the possible role of NE in the pathogenesis of lung injury associated with MV, with apparently conflicting results. One study examined the impact of the synthetic NE inhibitor sivelestat in protecting mice from VILI. Mice treated with sivelestat exhibited less lung injury, as assessed by neutrophil influx, cytokine release, apoptosis, and edema, than did untreated control animals after MV with high (20 ml/kg) tidal volumes for 4 hours (46). However, a human trial of sivelestat administered intravenously to mechanically ventilated patients with acute lung injury showed no apparent benefit in terms of mortality or MV-free days, although the report showed no evidence that the given treatment regimen blocked lung elastase activity or elastin degradation (47). In a study comparing the effects of MV in wild-type versus mutant mice that were deficient in NE (NE−/−), lung injury was greater after 3 hours of MV in mice that lacked NE, despite the presence of fewer neutrophils in their alveoli (48). Based on in vitro studies using neutrophils from wild-type and NE−/− mice, these investigators concluded that NE, through its capacity to cleave intercellular adhesion molecule-1, enables migration of neutrophils from the pulmonary circulation into alveoli, and that inhibiting this process promotes VILI.
In contrast to the NE knockout studies described previously, our study of elafin treatment did not abolish elastase activity in lung (Figure 1A), but merely inhibited the increased activity during MV-O2. Our finding that intratracheal treatment with elafin during MV-O2 not only reduced lung inflammation, but also reduced apoptosis and helped to preserve alveolar structure, suggests that increased elastase activity plays a prominent role in the pathogenesis of neonatal lung injury from MV-O2.
Inhibition of MV-O2-Evoked Elastase Activity Prevents Lung Elastin Degradation
Our previous study showed that MV-O2 caused serine elastase activity to increase four-fold in lungs of 4-day-old Balb/c mice, leading to increased synthesis of tropoelastin and dispersion of elastic fibers throughout the walls of distal air spaces (17). These changes occurred in the absence of apparent inflammation, which prompted speculation that lung parenchymal cells may have been the source of the increased elastase activity, notably smooth muscle cells or fibroblasts, in which elastase activity can be evoked (49–51).
In this study, using 5-day-old CD1 mice, MV-O2 caused a twofold to threefold increase of serine elastase activity and a similar increase in MMP-9 activity in lung, with a corresponding twofold to threefold increase in urinary excretion of desmosine, a biomarker of elastin degradation. These changes were accompanied by increased lung inflammation, as assessed by increased expression of proinflammatory cytokines and chemokines, and alveolar influx of neutrophils and monocytes during 24 hours of MV-O2. Such an inflammatory response has been well documented in newborn infants with evolving CLD (8) and in authentic animal models of this disease (15, 16). Elafin treatment of newborn mice blocked the increased lung elastolytic activity and desmosine excretion that occurred in response to MV-O2. Elastase inhibition was associated with diminished dispersion of elastic fibers and reduced apoptosis. Elastin degradation products have been shown to trigger lung inflammation and apoptosis, and thereby contribute to air space enlargement (30, 52). Inflammation can augment elastolysis and proteolysis. Thus, by inhibiting elastase activity, and thereby preserving elastin at septal tips where future alveoli are known to sprout, elafin seems to have facilitated lung growth during MV-O2.
Besides suppressing lung protease activity and preventing elastin fragmentation, elafin treatment inhibited activation of NF-κB, as assessed by a marked reduction of nuclear p65 protein in response to MV-O2. This finding is consistent with earlier reports citing elafin's attenuation of NF-κB–dependent inflammatory responses to LPS in cultured human endothelial cells, macrophages, and monocytes (32, 53). The fact that MV-O2 did not cause an increase of nuclear p65 in vehicle-treated mice suggests, however, that elafin inhibition of NF-κB activation is not likely the primary mechanism by which elafin thwarted the adverse pulmonary effects of MV-O2. It is possible, however, that inhibition of NF-κB had an indirect effect of suppressing lung inflammation in response to MV-O2, thereby contributing to elafin's lung protective role. Because NF-κB activity was assessed only at a single time point (8 h), we cannot exclude the possibility that its inhibition by elafin, either earlier or later than 8 hours, could have contributed to protecting the lung from MV-O2–induced injury. Our finding that MV-O2 did not increase NF-κB activation is consistent with a previous report, which showed that NF-κB activation did not occur in adult mice exposed to MV-O2 except when very high tidal volumes (30 ml/kg) and extreme hyperoxia (>95%) were applied (54). MV-O2 with modest tidal volumes (6 ml/kg) yielded no significant increase in lung content of phosphorylated NF-κB.
Impact of Elafin on MV-O2–induced Changes in TGF-β Signaling
Our previous study of preterm lambs showed that MV for 24 hours increased TGF-β in lung, resulting in dysregulated production of lung elastin and failed alveolar septation (18). A prior study with adult mice showed that increased elastase activity can mediate the release of growth factors from lung, among them TGF-β, which can increase tropoelastin production in lung fibroblasts (55). TGF-β has been shown to boost production of tropoelastin mRNA and soluble elastin protein in cultured neonatal lung fibroblasts (56). TGF-β also can induce endothelial cell apoptosis (57) and reduce expression of vascular endothelial growth factor receptor 2 expression in vascular endothelial cells (58). Other studies have shown that overexpression of TGF-β1 in newborn rodent lungs caused abnormal formation of alveoli and microvessels, as seen in CLD (59, 60).
In this study, it is likely that TGF-β inhibition in response to elafin treatment played a key role in preventing at least some of the adverse pulmonary effects of MV-O2, namely dysregulated elastin production and apoptosis, both of which can contribute to defective alveolar septation and lung growth. Thus, suppression of TGF-β activation may account, at least in part, for the beneficial effects of elafin treatment in stabilizing lung elastin and enabling alveolar septation in newborn mice during MV-O2.
Working Model of MV-O2–induced Lung Injury and the Impact of Elafin Treatment
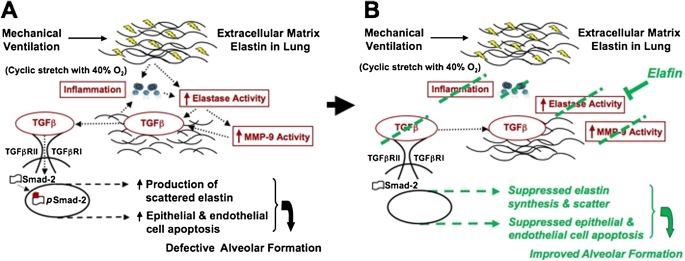
Figure 7 shows our working model, depicting how MV-O2 causes lung injury in newborn mice, and how intrapulmonary treatment with recombinant human elafin helps to protect against such injury. As illustrated in Figure 7A, cyclic stretch with O2-rich gas stimulates increased serine elastase activity in the lung, which leads to the breakdown and dispersion of matrix elastin. Fragmented elastic fibers, possibly associated with NF-κB activation, trigger an inflammatory response that further stimulates both elastase and MMP-9 activity. Increased proteolysis causes TGF-β release from the lung matrix, followed by TGF-β receptor coupling, phosphorylation, and nuclear transfer of pSmad-2. Inflammatory cells, both neutrophils and monocytes, are an additional source of active TGF-β. This series of events causes apoptosis of lung endothelial and epithelial cells, and matrix remodeling, culminating in failed alveolar septation and lung growth arrest. As depicted in Figure 7B, inhibition of serine elastase activity by elafin prevents the breakdown and increase in abnormally dispersed elastin, abrogates the inflammatory response, and blocks the activation and release of MMP-9. Inhibition of elastolytic activity also blocks activation and release of TGF-β from the ECM, which in turn protects against apoptosis and loss of alveolar septa, thereby enabling lung growth during MV-O2. This is a working model, the validity of which awaits further testing.
Figure 7.
Our working model, depicting how mechanical ventilation with O2-rich gas (MV-O2) causes lung injury in newborn mice (A), and how intratracheal instillation of recombinant human elafin helps to protect against such injury (B). As illustrated in (A), cyclic stretch with O2-rich gas stimulates increased serine elastase activity in the lung, which leads to the breakdown and dispersion of extracellular matrix elastin. Fragmented elastic fibers, perhaps coupled with nuclear factor-κB activation, triggers an inflammatory response that further stimulates both elastase activity and matrix metalloproteinase (MMP)-9 activity. Increased proteolysis causes transforming growth factor (TGF)-β release from the lung matrix, followed by TGF-β receptor coupling, phosphorylation, and nuclear transfer of pSmad-2. Inflammatory cells, both neutrophils and monocytes, are an additional source of active TGF-β. This series of events causes apoptosis of pulmonary endothelial and epithelial cells, culminating in failed alveolar septation. As depicted in (B), inhibition of serine elastase activity by elafin prevents elastin breakdown and the increase in abnormally dispersed elastic fibers, abrogates the inflammatory response (including suppression of nuclear factor-κB activity), and blocks activation and release of MMP-9. Inhibition of elastolytic activity also blocks activation and release of TGF-β from the extracellular matrix, which in turn protects against apoptosis and loss of alveolar septa, thereby enabling lung growth during MV-O2. The validity of this working model remains to be tested.
This model takes advantage of the fact that alveoli and lung capillaries form mainly after birth at term gestation in mice, enabling us to examine the impact of lengthy MV-O2 on lung growth and development, and to assess the potential benefit of novel treatment strategies. To help render this model relevant to conditions that prevail during development of CLD, we applied a ventilation strategy similar to the clinical approach adopted in many newborn intensive care units to treat respiratory failure in tiny infants. This approach uses relatively small tidal volumes, low inflation pressures, and modest concentrations of inspired O2 to maintain adequate respiratory gas exchange while minimizing the risk of lung injury.
There are, however, important differences between newborn mice that receive MV after birth at term gestation compared with premature infants and experimental models of CLD that have been created in premature baboons (13–15) and lambs (12, 16). These include a more fully developed respiratory drive, a more competent surfactant system, and the need for a tracheotomy rather than an endotracheal tube to deliver MV-O2 in newborn mice. Other limitations of this model include the challenge of maintaining newborn mice on MV for 24 hours (∼80% success rate) with limited monitoring capabilities; inability to apply more than 1 cm H2O positive end-expiratory pressure because of impaired cardiac output in the face of high heart rates (∼500 per min) and miniscule stroke volumes; and tiny lungs (∼50 mg), which limits the number of assays that can be done per animal.
Clinical Implications
Each year tens of thousands of intensive care patients incur acute lung injury caused by positive-pressure MV, typically associated with increased concentrations of inspired O2. MV-O2 treatment of patients with ARDS has been linked to pulmonary inflammation and increased elastolytic activity, resulting in breakdown of lung elastin and increased mortality (61–63). Consistent with this notion, a genome-wide expression analysis of blood samples taken from patients with ARDS showed a threefold decrease in the expression of peptidase inhibitor 3 (PI3, encoding elafin, a potent inhibitor of NE) during the acute phase compared with the recovery phase of the disease (64). A follow-up case-control study showed that polymorphisms in the PI3 (elafin) gene were linked to low circulating levels of elafin and increased risk of ARDS (65). These findings elicited speculation that endogenous elafin might help prevent or reduce the severity of ARDS.
In newborns whose lungs are not yet fully developed, a prolonged course of MV-O2 often leads to a chronic form of respiratory distress that has been called “the new bronchopulmonary dysplasia,” herein described as CLD. Most infants with this disease have a history of extreme prematurity and resultant risk of RDS, for which they receive surfactant treatment that enables adequate respiratory gas exchange in response to gentle MV using small tidal volumes and modest amounts of supplemental O2. Development of CLD in preterm infants has been linked to increased lung inflammation and elastase activity, leading to degradation of lung elastin, documented by increased urinary excretion of desmosine (7, 8). These findings prompted a clinical trial of α1-proteinase inhibitor (α1-PI) treatment to prevent CLD in high-risk, extremely premature infants (66). Although there was not a statistically significant difference in the incidence of CLD in infants treated intravenously with α1-PI compared with infants that received placebo, there was a trend toward less CLD with α1-PI treatment (P = 0.06), and a significant reduction in the incidence of pulmonary hemorrhage. Despite this report's intriguing results, no further clinical trials have been pursued to test the potential benefit of either α1-PI treatment or a more potent, specific elastase inhibitor, such as elafin, in protecting against the adverse effects of MV-O2. This study, which demonstrates the feasibility and efficacy of direct delivery of elafin into the lungs of newborn mice to combat VILI, supports consideration of such a trial.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL086631 (R.B.), HL086216 (K.P.), and HL082662 (M.N.); DFG Grant HI1315/3-1 (A.H.); and the Vera Moulton Wall Center for Pulmonary Vascular Disease at Stanford University.
Footnotes
Supported by National Institutes of Health Grants HL086631 (R.D.B.), HL086216 (K.P.), and HL082662 (M.R.N.); DFG Grant HI 1315/3–1 (A.H.); and the Vera Moulton Wall Center for Pulmonary Vascular Disease at Stanford University.
Author contributions: Conception and design, A.H., M.R., and R.D.B.; data acquisition, analysis, and interpretation, A.H., K.P., R.E., N.J., E.F.N., J.L.P., R.T., M.R.N., B.C.S., M.R., and R.D.B.; writing or substantial role in revising, A.H., M.R., and R.D.B.
Originally Published in Press as DOI: 10.1164/rccm.201012-2010OC on May 11, 2011
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease: bronchopulmonary dysplasia. N Engl J Med 1967;276:357–368 [DOI] [PubMed] [Google Scholar]
- 2.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature 1998;393:276–280 [DOI] [PubMed] [Google Scholar]
- 3.Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 2000;23:320–326 [DOI] [PubMed] [Google Scholar]
- 4.Hislop AA, Wigglesworth JS, Desai R, Aber V. The effects of preterm delivery and mechanical ventilation on human lung growth. Early Hum Dev 1987;15:147–164 [DOI] [PubMed] [Google Scholar]
- 5.Margraf LR, Tomashefski JF, Bruce MC, Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis 1991;143:391–400 [DOI] [PubMed] [Google Scholar]
- 6.Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics 2000;106:1452–1459 [DOI] [PubMed] [Google Scholar]
- 7.Bruce MC, Wedig KE, Jentoft N, Martin RJ, Cheng PW, Boat TF, Fanaroff AA. Altered urinary excretion of elastin cross-links in premature infants who develop bronchopulmonary dysplasia. Am Rev Respir Dis 1985;131:568–572 [DOI] [PubMed] [Google Scholar]
- 8.Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DKI, Gluck L. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. J Clin Invest 1983;72:656–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis 1984;130:817–821 [DOI] [PubMed] [Google Scholar]
- 10.Watterberg KL, Carmichael DF, Gerdes JS, Werner S, Backstrom C, Murphy S. Secretory leukocyte protease inhibitor and lung inflammation in developing bronchopulmonary dysplasia. J Pediatr 1994;125:264–269 [DOI] [PubMed] [Google Scholar]
- 11.Bruce MC, Schuyler M, Martin RJ, Starcher BC, Tomashefski JF, Wedig KE. Risk factors for the degradation of lung elastic fibers in the ventilated neonate. Am Rev Respir Dis 1992;146:204–212 [DOI] [PubMed] [Google Scholar]
- 12.Albertine KH, Kim BI, Kullama LK, Starcher BC, Cho SC, Carlton DP, Bland RD. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med 1999;159:945–958 [DOI] [PubMed] [Google Scholar]
- 13.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 1999;160:1333–1346 [DOI] [PubMed] [Google Scholar]
- 14.Altiok O, Yasumatsu R, Bingol-Karakoc G, Riese RJ, Stahlman MT, Dwyer W, Pierce RA, Bromme D, Weber E, Cataltepe S. Imbalance between cysteine proteases and inhibitors in a baboon model of bronchopulmonary dysplasia. Am J Respir Crit Care Med 2006;173:318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasumatsu R, Altiok O, Benarafa C, Yasumatsu C, Bingol-Karakoc G, Remold-O'donnell E, Cataltepe S. SERPINB1 upregulation is associated with in vivo complex formation with neutrophil elastase and cathepsin G in a baboon model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2006;291:L619–L627 [DOI] [PubMed] [Google Scholar]
- 16.Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, Kumar VH, Ryan RM, Swartz DD, Csiszar K, et al. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2007;292:L1370–L1384 [DOI] [PubMed] [Google Scholar]
- 17.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol 2008;294:L3–L14 [DOI] [PubMed] [Google Scholar]
- 18.Mokres LM, Parai K, Hilgendorff A, Ertsey R, Alvira CM, Rabinovitch M, Bland RD. Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol 2010;298:L23–L35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, Xu L, Shinwell ES, Zhang F, Beasley MA. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol 2007;293:L1099–L1110 [DOI] [PubMed] [Google Scholar]
- 20.Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 2002;105:516–521 [DOI] [PubMed] [Google Scholar]
- 21.Ohta K, Nakajima T, Cheah AY, Zaidi SH, Kaviani N, Dawood F, You XM, Liu P, Husain M, Rabinovitch M. Elafin-overexpressing mice have improved cardiac function after myocardial infarction. Am J Physiol Heart Circ Physiol 2004;287:H286–H292 [DOI] [PubMed] [Google Scholar]
- 22.Zaidi SH, Hui CC, Cheah AY, You XM, Husain M, Rabinovitch M. Targeted overexpression of elafin protects mice against cardiac dysfunction and mortality following viral myocarditis. J Clin Invest 1999;103:1211–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowan B, Baron O, Crack J, Coulber C, Wilson GJ, Rabinovitch M. Elafin, a serine elastase inhibitor, attenuates post-cardiac transplant coronary arteriopathy and reduces myocardial necrosis in rabbits afer heterotopic cardiac transplantation. J Clin Invest 1996;97:2452–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaidi SH, You XM, Ciura S, O'Blenes S, Husain M, Rabinovitch M. Suppressed smooth muscle proliferation and inflammatory cell invasion after arterial injury in elafin-overexpressing mice. J Clin Invest 2000;105:1687–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilgendorff A, Parai K, Ertsey R, Jain N, Starcher B, Rabinovitch M, Bland R. Adverse pulmonary effects of mechanical ventilation in newborn mice are prevented or attenuated by the serine elastase inhibitor elafin [abstract]. Am J Respir Crit Care Med 2010;181:A3893 [Google Scholar]
- 26.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 1970;26:57–60 [PubMed] [Google Scholar]
- 27.Starcher B, Green M, Scott M. Measurement of urinary desmosine as an indicator of acute pulmonary disease. Respiration 1995;62:252–257 [DOI] [PubMed] [Google Scholar]
- 28.Zar J. Biostatistical analysis. Upper Saddle River, NJ: Prentice Hall; 1998 [Google Scholar]
- 29.Itoh Y, Nagase H. Preferential inactivation of tissue inhibitor of metalloproteinases-1 that is bound to the precursor of matrix metalloproteinase 9 (progelatinase B) by human neutrophil elastase. J Biol Chem 1995;270:16518–16521 [DOI] [PubMed] [Google Scholar]
- 30.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 2006;116:753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol 2008;40:1101–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriksen PA, Hitt M, Xing Z, Wang J, Haslett C, Riemersma RA, Webb DJ, Kotelevtsev YV, Sallenave J-M. Adenoviral gene delivery of elafin and secretory leukocyte protease inhibitor attenuates NF-KB-dependent inflammatory responses of human endothelial cells and macrophages to atherogenic stimuli. J Immunol 2004;172:4535–4544 [DOI] [PubMed] [Google Scholar]
- 33.Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, von Melchner H, Davis EC, Rifkin DB. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity. J Cell Physiol 2009;219:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grotendorst GR, Smale G, Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol 1989;140:396–402 [DOI] [PubMed] [Google Scholar]
- 35.Alejandre-Alcazar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Perez J, Wygrecka M, Eul B, Kobrich S, Hesse M, et al. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2007;292:L537–L549 [DOI] [PubMed] [Google Scholar]
- 36.Lu Q, Patel B, Harrington EO, Rounds S. Transforming growth factor-{beta}1 causes pulmonary microvascular endothelial cell apoptosis via ALK5. Am J Physiol Lung Cell Mol Physiol 2009;296:L825–L838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nara K, Ito S, Ito T, Suzuki Y, Ghoneim MA, Tachibana S, Hirose S. Elastase inhibitor elafin is a new type of proteinase inhibitor which has a transglutaminase-mediated anchoring sequence termed “cementoin.” J Biochem 1994;115:441–448 [DOI] [PubMed] [Google Scholar]
- 38.Sallenave JM, Silva A, Marsden ME, Ryle AP. Secretion of mucus proteinase inhibitor and elafin by Clara cell and type II pneumocyte cell lines. Am J Respir Cell Mol Biol 1993;8:126–133 [DOI] [PubMed] [Google Scholar]
- 39.Sallenave JM, Silva A. Characterization and gene sequence of the precursor of elafin, an elastase-specific inhibitor in bronchial secretions. Am J Respir Cell Mol Biol 1993;8:439–445 [DOI] [PubMed] [Google Scholar]
- 40.Guyot N, Zani ML, Maurel MC, Dallet-Choisy S, Moreau T. Elafin and its precursor trappin-2 still inhibit neutrophil serine proteinases when they are covalently bound to extracellular matrix proteins by tissue transglutaminase. Biochemistry 2005;44:15610–15618 [DOI] [PubMed] [Google Scholar]
- 41.Reid PT, Marsden ME, Cunningham GA, Haslett C, Sallenave JM. Human neutrophil elastase regulates the expression and secretion of elafin (elastase-specific inhibitor) in type II alveolar epithelial cells. FEBS Lett 1999;457:33–37 [DOI] [PubMed] [Google Scholar]
- 42.Sallenave JM, Xing Z, Simpson AJ, Graham FL, Gauldie J. Adenovirus-mediated expression of an elastase-specific inhibitor (elafin): a comparison of different promoters. Gene Ther 1998;5:352–360 [DOI] [PubMed] [Google Scholar]
- 43.Simpson AJ, Wallace WA, Marsden ME, Govan JR, Porteous DJ, Haslett C, Sallenave JM. Adenoviral augmentation of elafin protects the lung against acute injury mediated by activated neutrophils and bacterial infection. J Immunol 2001;167:1778–1786 [DOI] [PubMed] [Google Scholar]
- 44.Vachon E, Bourbonnais Y, Bingle CD, Rowe SJ, Janelle MF, Tremblay GM. Anti-inflammatory effect of pre-elafin in lipopolysaccharide-induced acute lung inflammation. Biol Chem 2002;383:1249–1256 [DOI] [PubMed] [Google Scholar]
- 45.Janelle MF, Doucet A, Bouchard D, Bourbonnais Y, Tremblay GM. Increased local levels of granulocyte colony-stimulating factor are associated with the beneficial effect of pre-elafin (SKALP/trappin-2/WAP3) in experimental emphysema. Biol Chem 2006;387:903–909 [DOI] [PubMed] [Google Scholar]
- 46.Sakashita A, Nishimura Y, Nishiuma T, Takenaka K, Kobayashi K, Kotani Y, Yokoyama M. Neutrophil elastase inhibitor (sivelestat) attenuates subsequent ventilator-induced lung injury in mice. Eur J Pharmacol 2007;571:62–71 [DOI] [PubMed] [Google Scholar]
- 47.Zeiher BG, Artigas A, Vincent JL, Dmitrienko A, Jackson K, Thompson BT, Bernard G. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med 2004;32:1695–1702 [DOI] [PubMed] [Google Scholar]
- 48.Kaynar AM, Houghton AM, Lum EH, Pitt BR, Shapiro SD. Neutrophil elastase is needed for neutrophil emigration into lungs in ventilator-induced lung injury. Am J Respir Cell Mol Biol 2008;39:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson K, Rabinovitch M. Exogenous leukocyte and endogenous elastases can mediate mitogenic activity in pulmonary artery smooth muscle cells by release of extracellular-matrix bound basic fibroblast growth factor. J Cell Physiol 1996;166:495–505 [DOI] [PubMed] [Google Scholar]
- 50.Wigle DA, Thompson KE, Yablonsky S, Zaidi SH, Coulber C, Jones PL, Rabinovitch M. AML1-like transcription factor induces serine elastase activity in ovine pulmonary artery smooth muscle cells. Circ Res 1998;83:252–263 [DOI] [PubMed] [Google Scholar]
- 51.Numanami H, Koyama S, Sato E, Haniuda M, Nelson DK, Hoyt JC, Freels JL, Habib MP, Robbins RA. Serine protease inhibitors modulate chemotactic cytokine production by human lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol 2003;284:L882–L890 [DOI] [PubMed] [Google Scholar]
- 52.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol 2003;28:555–562 [DOI] [PubMed] [Google Scholar]
- 53.Butler MW, Robertson I, Greene CM, O'Neill SJ, Taggart CC. Elafin prevents lipopolysaccharide-induced AP-1 and NF-KB activation via an effect on the ubiquitin-proteasome pathway. J Biol Chem 2006;281:34730–34735 [DOI] [PubMed] [Google Scholar]
- 54.Liu YY, Liao SK, Huang CC, Tsai YH, Quinn DA, Li LF. Role for nuclear factor-kappaB in augmented lung injury because of interaction between hyperoxia and high stretch ventilation. Transl Res 2009;154:228–240 [DOI] [PubMed] [Google Scholar]
- 55.Buczek-Thomas JA, Lucey EC, Stone PJ, Chu CL, Rich CB, Carreras I, Goldstein RH, Foster JA, Nugent MA. Elastase mediates the release of growth factors from lung in vivo. Am J Respir Cell Mol Biol 2004;31:344–350 [DOI] [PubMed] [Google Scholar]
- 56.McGowan SE. Influences of endogenous and exogenous TGF-beta on elastin in rat lung fibroblasts and aortic smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 1992;263:L257–L263 [DOI] [PubMed] [Google Scholar]
- 57.Ferrari G, Pintucci G, Seghezzi G, Hyman K, Galloway AC, Mignatti P. VEGF, a prosurvival factor, acts in concert with TGF-beta1 to induce endothelial cell apoptosis. Proc Natl Acad Sci USA 2006;103:17260–17265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandriota SJ, Menoud PA, Pepper MS. Transforming growth factor beta 1 down-regulates vascular endothelial growth factor receptor 2/flk-1 expression in vascular endothelial cells. J Biol Chem 1996;271:11500–11505 [DOI] [PubMed] [Google Scholar]
- 59.Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 2003;163:2575–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 2004;31:650–656 [DOI] [PubMed] [Google Scholar]
- 61.Lee CT, Fein AM, Lippmann M, Holtzman H, Kimbel P, Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med 1981;304:192–196 [DOI] [PubMed] [Google Scholar]
- 62.McGuire WW, Spragg RG, Cohen AB, Cochrane CG. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest 1982;69:543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McClintock DE, Starcher B, Eisner MD, Thompson BT, Hayden DL, Church GD, Matthay MA. Higher urine desmosine levels are associated with mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L566–L571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Z, Beach D, Su L, Zhai R, Christiani DC. A genome-wide expression analysis in blood identifies pre-elafin as a biomarker in ARDS. Am J Respir Cell Mol Biol 2008;38:724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tejera P, Wang Z, Zhai R, Su L, Sheu CC, Taylor DM, Chen F, Gong MN, Thompson BT, Christiani DC. Genetic polymorphisms of peptidase inhibitor 3 (elafin) are associated with acute respiratory distress syndrome. Am J Respir Cell Mol Biol 2009;41:696–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stiskal JA, Dunn MS, Shennan AT, O'Brien KK, Kelly EN, Koppel RI, Cox DW, Ito S, Chappel SL, Rabinovitch M. Alpha1-proteinase inhibitor therapy for the prevention of chronic lung disease of prematurity: randomized, controlled trial. Pediatrics 1998;101:89–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.