Abstract
Rationale: β2-Adrenergic receptor agonists accelerate resolution of pulmonary edema in experimental and clinical studies.
Objectives: This clinical trial was designed to test the hypothesis that an aerosolized β2-agonist, albuterol, would improve clinical outcomes in patients with acute lung injury (ALI).
Methods: We conducted a multicenter, randomized, placebo-controlled clinical trial in which 282 patients with ALI receiving mechanical ventilation were randomized to receive aerosolized albuterol (5 mg) or saline placebo every 4 hours for up to 10 days. The primary outcome variable for the trial was ventilator-free days.
Measurements and Main Results: Ventilator-free days were not significantly different between the albuterol and placebo groups (means of 14.4 and 16.6 d, respectively; 95% confidence interval for the difference, −4.7 to 0.3 d; P = 0.087). Rates of death before hospital discharge were not significantly different between the albuterol and placebo groups (23.0 and 17.7%, respectively; 95% confidence interval for the difference, −4.0 to 14.7%; P = 0.30). In the subset of patients with shock before randomization, the number of ventilator-free days was lower with albuterol, although mortality was not different. Overall, heart rates were significantly higher in the albuterol group by approximately 4 beats/minute in the first 2 days after randomization, but rates of new atrial fibrillation (10% in both groups) and other cardiac dysrhythmias were not significantly different.
Conclusions: These results suggest that aerosolized albuterol does not improve clinical outcomes in patients with ALI. Routine use of β2-agonist therapy in mechanically ventilated patients with ALI cannot be recommended.
Clinical trial registered with www.clinicaltrials.gov (NCT 00434993).
Keywords: pulmonary edema, acute respiratory distress syndrome, alveolar epithelium
At a Glance Commentary
Scientific Knowledge on the Subject
Preclinical studies and one clinical trial suggested that β2-agonist therapy could reduce pulmonary edema in acute lung injury. However, the potential value of aerosolized β2-agonist therapy for treatment of acute lung injury has not been tested previously in a phase III, randomized clinical trial.
What This Study Adds to the Field
The results of this randomized double-blind clinical trial demonstrate that aerosolized β2-agonist therapy with albuterol did not improve clinical outcomes in patients with acute lung injury.
In patients with acute lung injury and the acute respiratory distress syndrome (ALI/ARDS), inflammation of the pulmonary circulation increases vascular permeability. Fluid leaks from blood vessels into the pulmonary interstitium and alveoli. Recovery from this form of acute respiratory failure requires that the pulmonary edema resolve. The resolution of alveolar edema is driven by active transport of sodium and chloride ions from the luminal space across both type I and type II alveolar epithelial cells, creating an osmotic gradient for the reabsorption of water (1–4). The rate of alveolar fluid transport is increased by endogenous or exogenous cyclic AMP stimulation, which increases ion transport through epithelial sodium channels and chloride channels such as the cystic fibrosis transmembrane regulator (5, 6). In the ex vivo human lung, the rate of alveolar fluid clearance can be doubled by treatment with a cyclic AMP β2-adrenergic receptor agonist (7, 8).
Alveolar epithelial fluid clearance is impaired during ALI/ARDS, and decreased resolution of alveolar edema is associated with increased mortality (9, 10). There are several potential mechanisms of decreased alveolar fluid clearance in ALI, including apoptosis and necrosis of alveolar epithelial cells (11, 12), decreased vectorial sodium and chloride transport secondary to inflammatory mediators (13–15), and reactive oxygen species (16).
On the basis of the preclinical observations that treatment with β2-agonists could reduce pulmonary edema and accelerate the rate of alveolar fluid clearance, our primary hypothesis was that treatment of patients with ALI/ARDS with β-agonist therapy would accelerate the resolution of alveolar edema and improve clinical outcomes (17, 18). In addition, there was preclinical evidence that β-agonists could reduce barrier injury in the lung (19), potentially reducing pulmonary edema by another mechanism. Both of these possibilities were in agreement with the results of a small randomized phase II trial in which intravenous albuterol reduced the quantity of pulmonary edema as measured by a thermal method in patients with ARDS (20).
We selected an aerosol route of delivery for the β-agonist in this trial because administration of β-agonists to the airspaces had reduced pulmonary edema in a preclinical model of acute lung injury (21), and we considered it likely to be a safe method of delivery of a β2-agonist. Moreover, in patients with ALI who received conventional doses of nebulized albuterol by inhalation, the levels of albuterol in undiluted pulmonary edema fluid (22) were sufficient to stimulate alveolar fluid clearance in the ex vivo human lung (22). Therefore, we conducted a multicenter, randomized, placebo-controlled clinical trial of aerosolized albuterol (also known as salbutamol) in patients with ALI to determine whether this therapy would increase ventilator-free days and decrease mortality. Preliminary data included in this article have been previously presented in abstract form (23).
Methods
Patients were enrolled from August 6, 2007 until July 7, 2008 at 33 hospitals of the National Heart, Lung, and Blood Institute ARDS Clinical Trials Network (Appendix for Network Participants). The trial was approved by the institutional review board at each hospital. Informed consent was obtained from patients or surrogates. A complete description of the methods used is available in the online supplement. Thirty-seven patients were coenrolled in a separate trial of a medical food and timing of enteral nutrition support (www.clinicaltrials.gov, NCT00609180).
Patients
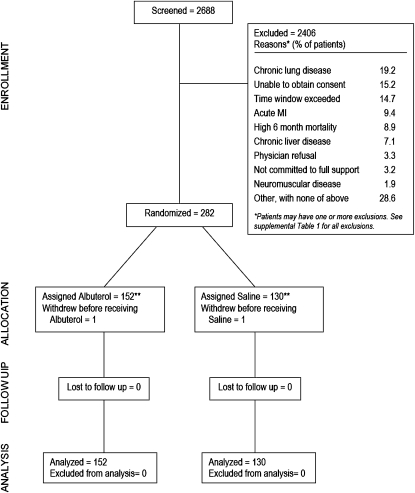
To be eligible, a patient had to be intubated and receiving mechanical ventilation, have bilateral pulmonary infiltrates consistent with edema on frontal chest radiograph, have a ratio of PaO2 to FiO2 (fraction of inspired oxygen) of 300 or less (adjusted for altitude as appropriate), and not have clinical evidence of left atrial hypertension. Exclusion criteria are shown in Figure 1.
Figure 1.
Enrollment and outcomes. *Patients may have had more than one reason for exclusion. The full exclusion criteria are listed in the online supplement. **Pharmacy errors resulted in the wrong treatment being given for one patient in each study group. These patients were assigned and analyzed on the basis of the treatment received. MI = mycocardial infarction.
A centralized web-based system was used to randomize patients to receive aerosolized albuterol or placebo. Patients were stratified according to hospital and presence of shock.
Procedures for Aerosolized Study Drug
Aerosolized albuterol sulfate (5.0 mg dissolved in saline) or a sterile, preservative-free 0.9% sterile sodium chloride placebo (Nephron Pharmaceuticals, Orlando, FL) was administered every 4 hours for 10 days after randomization or for 24 hours after extubation, whichever occurred first. If a patient's heart rate repeatedly exceeded a predefined age-based limit (see the online supplement) during administration of full-dose study medication (5.0 mg of active study medication or equal volume of placebo) the dose was reduced to 2.5 mg of active drug or equivalent volume of placebo for subsequent treatments or discontinued altogether.
Primary and Secondary End Points
The primary efficacy end point was the number of ventilator-free days (VFDs), defined as the number of days from randomization to Day 28 after achieving unassisted breathing for patients who maintained unassisted breathing for at least two consecutive calendar days. If a patient achieved unassisted breathing, subsequently required additional assisted breathing, and once again achieved unassisted breathing, we counted only the VFDs after beginning the final period of unassisted breathing. Patients who died before Day 28 were assigned zero VFDs. Mortality before hospital discharge with unassisted breathing on Day 60 and Day 90 were secondary efficacy end points. Patients alive and receiving unassisted breathing in the hospital on Day 60 or Day 90 were considered to have survived. The number of intensive care unit (ICU)–free days and the number of organ failure–free days (without Glasgow Coma Score) were measured as in previous trials (24–26).
Mechanical Ventilation and Hemodynamic Management
Simplified versions of the lower tidal volume and fluid-conservative hemodynamic management protocols used in previous ARDS Network trials were used (see the online supplement) (24–26).
Plasma Measurements
It was not possible to measure albuterol levels in the distal air spaces of the lung in this trial. To indirectly confirm that the delivery of aerosolized albuterol to the distal lung was sufficient, albuterol levels (NMS Labs, Willow Grove, PA) were measured in plasma obtained within 15 minutes of completion of administration of a dose of study drug on the day after enrollment in the first 100 patients. Endogenous catecholamines can also affect clearance of alveolar fluid through the same mechanism as albuterol (21). To compare baseline endogenous catecholamines between study groups, plasma epinephrine levels (Quest, Tucker, GA) were measured before administration of the first study drug dose and then on Day 1 in the first 100 patients. To assess effects of albuterol on inflammation, plasma was sampled for measurements of IL-6 and IL-8 at baseline and on Day 3. (R&D Systems [Minneapolis, MN] is the manufacturer of the IL-6 and IL-8 ELISA kits.)
Trial Design and Statistics
With a maximal enrollment of 1,000 patients and four planned interim analyses, the trial had a statistical power of 90.7% to detect a 2.25-day increase in VFDs, assuming a standard deviation of 10.5 days. The study was monitored using a group sequential design and could stop for either efficacy (if albuterol increased VFDs) or futility (if there was a low probability that the trial could demonstrate such an increase) (see the online supplement for more details). Asymmetric stopping boundaries were designed using α and β spending boundaries as described by DeMets and Ware (zl = 2.277, delta = 1.663, zu = 2.025, m = 4, mu = 3.3837) (27). An independent Data and Safety Monitoring Board (DSMB) conducted interim analyses after the enrollment of 100 patients and again after 276 patients. The primary safety end points were the number of adverse events and the proportions of patients in whom study drug was reduced in dosage or prematurely discontinued because of tachycardia or arrhythmia. The DSMB was advised to consider mortality differences in deciding whether to stop the trial and could have allowed the trial to continue past either an efficacy or futility boundary.
Means and standard deviations are reported for baseline continuous variables.
Percentages are reported for baseline categorical variables, with differences assessed by t tests and chi-square tests, respectively. The continuous outcome variables, VFDs, ICU-free days, and organ failure–free days, are reported as means and standard deviations, with differences assessed by analysis of variance controlling for baseline shock. Logistic regression controlling for baseline shock was used to analyze mortality. Adjusted mortality rates were calculated with seven baseline mortality-predicting covariates derived from a previous study of similar populations (22, 23). Kaplan-Meier curves were plotted for time to death and unassisted breathing. All analyses were performed with SAS 9.2 (SAS Institute, Cary, NC) on an intention-to-treat basis with two-sided P values equal to or less than 0.05 considered significant.
Results
There were 282 patients enrolled before the trial was stopped for futility by the DSMB. The DSMB stopped the trial because the observed ventilator-free day difference was unfavorable for albuterol treatment by –2.2 days, well past the futility boundary of –0.4 ventilator-free days.
The numbers of patients who were screened, enrolled, and excluded are shown in Figure 1. The study groups were well matched for most baseline demographic and physiological parameters (Table 1). A numerically larger proportion of patients in the albuterol group had received vasopressors at baseline within 24 hours of randomization (53 vs. 45%).
TABLE 1.
BASELINE CHARACTERISTICS OF PATIENTS
| Characteristic | Albuterol Group (n = 152) | Placebo Group (n = 130) | P Value |
| Age | 52 ± 16 | 51 ± 16 | 0.481 |
| Female sex, % | 44 | 45 | 0.826 |
| Race or ethnic group, % | 0.932 | ||
| White, not Hispanic | 68 | 70 | |
| Black, not Hispanic | 17 | 15 | |
| Hispanic | 10 | 11 | |
| Other, N/A | 5 | 5 | |
| Diabetes, % | 22 | 21 | 0.745 |
| Medical ICU, % | 77 | 72 | 0.368 |
| APACHE III score* | 94.1 ± 28.7 | 91.5 ± 29.6 | 0.467 |
| Vasopressor use, % | 53 | 45 | 0.146 |
| No. of nonpulmonary organ or system failures (maximum of 5)† | 1.5 ± 1.0 | 1.4 ± 1.0 | 0.703 |
| Mean arterial pressure, cm of water | 76.0 ± 12.4 | 76.0 ± 15.8 | 0.968 |
| Central venous pressure, cm H2O | 11.5 ± 5.7 | 11.7 ± 4.5 | 0.820 |
| Prestudy fluid intake, ml/24 h | 4,914 ± 3,144 | 5,249 ± 4,169 | 0.444 |
| Albumin, g/dl | 2.3 ± 0.6 | 2.3 ± 0.7 | 0.903 |
| Tidal volume, ml/kg | 7.1 ± 1.7 | 7.0 ± 1.5 | 0.618 |
| Plateau airway pressure, cm H2O | 23.8 ± 5.2 | 23.7 ± 6.2 | 0.857 |
| PEEP, cm H2O | 9.2 ± 3.5 | 9.2 ± 3.7 | 0.936 |
| Minute ventilation, L/min | 11.8 ± 4.5 | 11.3 ± 3.4 | 0.298 |
| Respiratory rate, breaths/min | 25.4 ± 7.4 | 25.0 ± 7.2 | 0.591 |
| PaO2/FiO2 | 170 ± 84 | 171 ± 75 | 0.870 |
| Oxygenation index | 11.2 ± 7.1 | 10.8 ± 7.3 | 0.713 |
| Primary cause of lung injury, % | 0.343 | ||
| Aspiration | 23 | 14 | |
| Multiple transfusion | 2 | 2 | |
| Other | 7 | 5 | |
| Pneumonia | 38 | 41 | |
| Sepsis | 24 | 29 | |
| Trauma | 7 | 10 |
Definition of abbreviations: APACHE = Acute Physiology, Age, and Chronic Health Evaluation; FiO2 = fraction of inspired oxygen, corrected for altitude; ICU = intensive care unit; PEEP = positive end-expiratory pressure.
Plus–minus values represent means ± SD.
Scores for the APACHE III can range from 0 to 299, with higher scores indicating a higher probability of death.
Patients were monitored for cardiovascular, central nervous system, coagulation, renal, and hepatic failure.
The on-study clinical variables for Days 2 and 4 are summarized in Table 2. Heart rate was modestly higher in the albuterol group than in the placebo group on Day 2 (P < 0.05). Vasopressor use continued to be greater after randomization in the albuterol group than in the placebo group on Day 2 (P < 0.01).
TABLE 2.
ON-STUDY PARAMETERS AND VARIABLES
| Day 2 | Day 4 | |||
| Variable | Albuterol | Placebo | Albuterol | Placebo |
| Heart rate, beats/min | 99 ± 20 | 94 ± 17* | 96 ± 18 | 93 ± 17 |
| No. of patients | 148 | 127 | 130 | 114 |
| Mean arterial pressure, mm Hg | 82 ± 16 | 84 ± 14 | 83 ± 16 | 87 ± 15 |
| No. of patients | 148 | 127 | 130 | 114 |
| CVP, mm Hg | 11.1 ± 5.3 | 10.1 ± 4.9 | 9.7 ± 4.9 | 9.0 ± 5.9 |
| No. of patients | 133 | 111 | 98 | 88 |
| Minute ventilation, L/min | 11.1 ± 3.9 | 11.1 ± 3.4 | 11.7 ± 3.8 | 11.1 ± 3.1 |
| No. of patients | 133 | 113 | 98 | 88 |
| Vasopressors, % | 32 ± 4 | 18 ± 3† | 18 ± 3 | 17 ± 3 |
| No. of patients | 148 | 127 | 130 | 114 |
| Cumulative fluid balance, ml | 1,969 ± 3,052 | 1,542 ± 3,390 | 2,988 ± 6,614 | 1,905 ± 6,388 |
| No. of patients | 148 | 128 | 133 | 118 |
| Creatinine, mg/dl | 1.8 ± 1.5 | 1.6 ± 1.5 | 1.6 ± 1.4 | 1.6 ± 1.4 |
| No. of patients | 145 | 126 | 126 | 116 |
| Tidal volume, ml/kg | 6.6 ± 1.5 | 6.6 ± 1.2 | 7.0 ± 3.9 | 6.6 ± 1.0 |
| No. of patients | 119 | 101 | 85 | 75 |
| Plateau airway pressure, cm H2O | 21.7 ± 5.4 | 21.9 ± 6.2 | 20.5 ± 5.0 | 22.3 ± 5.8 |
| No. of patients | 101 | 87 | 67 | 61 |
| PEEP, cm H2O | 8.0 ± 3.1 | 7.9 ± 3.1 | 7.4 ± 2.8 | 7.4 ± 3.3 |
| No. of patients | 133 | 114 | 98 | 90 |
| PaO2/FiO2 | 203 ± 76 | 206 ± 86 | 226 ± 107 | 209 ± 83 |
| No. of patients | 120 | 100 | 79 | 77 |
| Oxygenation index | 8.3 ± 5.5 | 8.3 ± 5.5 | 7.1 ± 3.8 | 8.4 ± 7.8 |
| No. of patients | 114 | 91 | 72 | 71 |
Definition of abbreviations: CVP = central venous pressure; FiO2 = fraction of inspired oxygen, corrected for altitude; PEEP = positive end-expository pressure.
Plus–minus values represent means ± SD.
0.01 < P ≤ 0.05.
P ≤ 0.01.
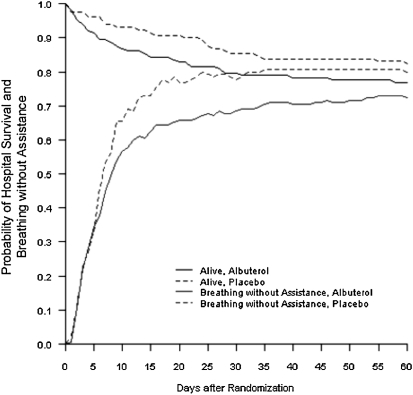
The primary outcome, the number of VFDs to Day 28, was not significantly different between study groups (Table 3). Mortality before hospital discharge to Days 60 and 90 and the number of organ failure–free days were also not significantly different between the two groups (Table 3). The number of intensive care unit–free days was lower in the albuterol group. The probabilities of survival and discharge while breathing without assistance in the albuterol and placebo groups are shown in Figure 2.
TABLE 3.
MAIN OUTCOME VARIABLES
| Outcome | Albuterol Group (n = 152) | Placebo Group (n = 130) | Difference (95% CI) | P Value |
| Ventilator-free days from Day 1 to Day 28 | 14.4 ± 0.9 | 16.6 ± 0.9 | −2.2 (−4.7, 0.3) | 0.087 |
| Death before discharge home to Day 60, % | ||||
| Unadjusted | 23.0 ± 3.4 | 17.7 ± 3.3 | 5.3 (−4.0, 14.7) | 0.302 |
| Adjusted for differences in baseline covariates | 22.2 ± 7.6 | 18.7 ± 7.3 | 3.5 (−6.4, 13.4) | 0.400 |
| Death before discharge home to Day 90, % | 24.3 ± 3.5 | 18.5 ± 3.4 | 5.9 (−3.7, 15.4) | 0.261 |
| No. of days not spent in an intensive care unit from Day 1 to Day 28 | 13.5 ± 0.8 | 16.2 ± 0.8 | −2.7 (−4.9, −0.4) | 0.023 |
| No. of days without failure of circulatory, coagulation, hepatic, or renal organs from Day 1 to Day 28 | 14.2 ± 0.9 | 15.9 ± 1.0 | −1.7 (−4.3, 0.9) | 0.226 |
Definition of abbreviation: CI = confidence interval.
Plus–minus values represent means ± SD. The median (interquartile range) for ventilator-free days in the albuterol group was 20 (0, 24.5) versus 21 (7–24) in the placebo group.
Figure 2.
Probabilities of survival and of discharge home and breathing without assistance, from the day of randomization (Day 0) to Day 60, according to whether patients received albuterol or placebo.
Because there were trends at baseline toward greater vasopressor use (shock) in the albuterol group, we conducted an adjusted analysis to test for differences in mortality. The variables for adjustment were age, APACHE (Acute Physiology, Age, and Chronic Health Evaluation) III, plateau airway pressure, missing recorded plateau airway pressure, the number of organ failures, the number of hospital days before enrollment in the trial, and the alveolar–arterial oxygen difference, as previously described (25, 28). With this adjustment, there was no significant difference in mortality before hospital discharge (Table 3).
According to the predefined analysis plan, differences in the main outcomes between the albuterol and placebo groups were calculated both for the subset of patients whose baseline PaO2/FiO2 ratios were less than 200 (ARDS; 68% of the 282 enrolled patients) and for the subset of patients who were in shock at the time of randomization, defined as need for vasopressors except for dopamine (<6 μg/kg/min). There were no significant differences between albuterol and placebo patients for the main outcome variables in the ARDS subgroup (PaO2/FiO2 < 200) (Table 4), but ICU-free days were significantly lower in the albuterol group in the shock subset (Table 5). Mortality was not significantly different in this subgroup.
TABLE 4.
MAIN OUTCOME VARIABLES: PATIENTS WITH BASELINE PaO2/FiO2 LESS THAN 200
| Outcome | Albuterol Group (n = 109) | Placebo Group (n = 92) | Difference (95% CI) | P Value |
| Ventilator-free days from Day 1 to Day 28* | 20 (14, 22) | 21 (19, 23) | 0 (0, 3) | 0.227 |
| Death before discharge home, % | ||||
| Unadjusted | 22.0 ± 4.0 | 17.4 ± 4.0 | 4.6 (−6.4, 15.6) | 0.484 |
| Adjusted for differences in baseline covariates | 21.0 ± 8.8 | 18.5 ± 8.6 | 2.5 (−9.2, 14.2) | 0.467 |
| No. of days not spent in an intensive care unit from Day 1 to Day 28* | 16 (11, 20) | 20 (17, 21) | 1 (0, 4) | 0.100 |
| No. of days without failure of circulatory, coagulation, hepatic, or renal organs from Day 1 to Day 28* | 21 (20, 21) | 21 (19, 23) | 0 (0, 2) | 0.320 |
Definition of abbreviation: CI = confidence interval.
Plus–minus values represent means ± SD.
Results are presented as median with interquartile ranges in parentheses. P values were calculated by Kruskal-Wallis test.
TABLE 5.
MAIN OUTCOME VARIABLES: PATIENTS WITH BASELINE SHOCK
| Outcome | Albuterol Group (n = 68) | Placebo Group (n = 55) | Difference (95% CI) | P Value |
| Ventilator-free days from Day 1 to Day 28* | 5 (0, 18) | 18 (10, 22) | 0 (0, 6) | 0.095 |
| Death before discharge home, % | ||||
| Unadjusted | 36.8 ± 5.8 | 27.3 ± 6.0 | 9.5 (−6.9, 25.9) | 0.265 |
| Adjusted for differences in baseline covariates | 35.3 ± 12.1 | 28.9 ± 11.8 | 6.4 (−10.3, 23.0) | 0.385 |
| No. of days not spent in an intensive care unit from Day 1 to Day 28* | 5 (0, 15) | 17 (12, 20) | 3 (0, 9) | 0.018 |
| No. of days without failure of circulatory, coagulation, hepatic, and renal organs from Day 1 to Day 28* | 15 (3, 21) | 19 (15, 21) | 1 (0, 7) | 0.120 |
Definition of abbreviation: CI = confidence interval.
Plus–minus values represent means ± SD.
Results are presented as median with interquartile ranges in parentheses. P values were calculated by Kruskal-Wallis test.
Because β2-agonists can reduce inflammation, we measured concentrations of two proinflammatory cytokines that were reduced by a lower tidal volume strategy in a previous study (28). However, we found that the concentrations of IL-6 and IL-8 in the plasma were not significantly different between the placebo and albuterol groups at either baseline or on Day 3 (Table E1 in the online supplement).
Plasma epinephrine concentrations were 10−9 M or less in most patients, even those in shock (Table E2 and Figure E1). On the basis of previous studies, this level of epinephrine should not have stimulated the resolution of alveolar edema (29, 30). Baseline epinephrine levels were significantly higher in the albuterol-treated patients, although this difference was modest. There were no significant differences in the levels of epinephrine on Day 1 between the groups (Table E2).
There was no detectable plasma albuterol in any of the 50 placebo patients. In contrast, plasma albuterol levels were within the anticipated concentration range of approximately 10−8 M in 46 of the 51 albuterol study group patients. On the basis of studies in which plasma and pulmonary edema fluid levels of albuterol were compared after aerosolized albuterol delivery (22), this concentration should have been sufficient to accelerate alveolar fluid clearance.
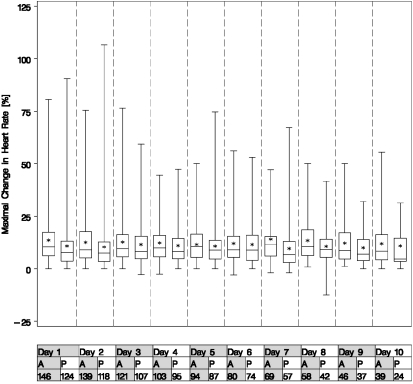
The mean maximal change in heart rate in the 15 minutes after nebulization was 12.5 beats/minute in the albuterol group versus 10.4 beats/minute in the placebo group (P = 0.01) (Figure 3). Nebulization of study drug was interrupted because of tachycardia in 8 and 3% of patients in the albuterol and placebo groups, respectively (P = 0.08). New onset of atrial fibrillation occurred in 10% of patients in both the albuterol and placebo groups (P = 0.90). The number of adverse events (58 and 41 in albuterol and placebo groups, respectively; P = 0.13) and events resulting in discontinuation of study drug (6 vs. 7 albuterol vs. placebo) were similar.
Figure 3.
Boxplot of maximal changes in heart rate from immediately before each nebulization treatment to the 15-minute interval after treatment. Upper and lower margins of the boxes represent the 75th and 25th quartiles, respectively. The bars in the boxes represent medians. The asterisks indicate mean values. The mean maximal change in heart rate over 10 days was 12.6 beats/minute after albuterol administration and 10.5 beats/minute after placebo administration (P = 0.02 by analysis of variance).
Discussion
In this randomized clinical trial, there were no significant differences in the number of ventilator-free days, mortality or organ failure–free days between patients with ALI/ARDS who received aerosolized albuterol or placebo. Patients randomized to albuterol had numerically higher APACHE III scores and were numerically more likely to be in shock at baseline, but mortality was not significantly different after adjustment for these and other prognostic variables (Table 3). There were also no significant differences in any of the main outcomes in the predefined subgroup with baseline PaO2/FiO2 less than 200. ICU-free days were fewer with albuterol in the predefined shock subset, although VFDs and mortality were not significantly different.
This trial was stopped by the DSMB because the primary end point, VFDs, had crossed predefined futility boundaries, making the probability of a positive trial low. Our asymmetric stopping boundaries were designed to stop early if the trial was unlikely to show benefit if continued to 1,000 subjects. We did not design the study with symmetric stopping boundaries because we did not think it was scientifically or ethically justified to prove that albuterol was harmful. The stopping boundary for the VFD difference (albuterol – placebo) at the time of the DSMB meeting that stopped the trial (266 patients at that time) was –0.4 ventilator-free days. The observed ventilator-free day difference was –2.2 days, well past the futility stopping boundary. We had designed the trial to detect a benefit of 2.25 ventilator-free days. The likelihood of detecting this difference if the study continued to 1,000 patients was small. In addition, the mortality trend was also unfavorable for the albuterol group at the time of the DSMB recommendation to stop the trial. Thus, there was even some concern that albuterol could be harmful. Thus, all of these issues combined with the low likelihood for success with albuterol treatment resulted in the DSMB terminating the trial, which was in agreement with the a priori–determined futility stopping rules.
Because the confidence intervals for mortality were wide at this early stopping point (–6.4 to 13.4% for adjusted analyses of mortality), it is not possible to completely exclude either clinical benefit (i.e., type II error) or harm, an issue that was well discussed in a prior editorial in this journal regarding another trial (31). Early stopping, wide confidence intervals, and multiple comparisons also preclude a definitive conclusion of harm, although we recognize that there were significantly fewer ICU-free days in the albuterol-treated patients (Table 3).
Patients were monitored for potential adverse effects from albuterol in this trial, including tachycardia, cardiac arrhythmias, and hypokalemia. Heart rates were modestly elevated in the albuterol group, but there was no evidence of serious adverse effects, including ventricular dysrhythmias or atrial fibrillation. However, it is possible that β-agonist therapy had an unrecognized adverse effect in the patients with ALI included in this trial.
Cumulative fluid balance in this trial does not strictly reflect conservative fluid management as reported in a previous trial (26). For this trial we used a simplified fluid-conservative protocol (included in the online supplement). This may explain why net fluid balance was greater in the current trial. In addition, the patient populations were somewhat different. The proportion of patients with shock was higher in the current trial than in the previous trial (approximately one-half vs. approximately one-third). This may account for some of the differences in cumulative fluid balance between studies, as the fluid management protocol does not apply when patients are in shock.
There are several possible explanations for why β2-adrenergic therapy did not improve outcomes in this clinical trial. First, aerosol delivery to edematous alveoli might have been inadequate. In an observational study, 2.5 mg of albuterol administered by aerosol inhalation to patients with ALI resulted in albuterol concentrations in undiluted pulmonary edema fluid of 10−6 M (22), which is on the plateau of the dose–response curve for increasing the rate of alveolar fluid clearance in the human lung (29, 30). In the current trial a higher dose of albuterol, 5.0 mg, was administered. Several additional measures were taken to maximize drug delivery (see the online supplement). Even with these measures, it is possible that the aerosolized drug was delivered predominantly to aerated, nonedematous alveoli. The aerosolized drug could then have been absorbed from the aerated alveoli, yielding the observed concentration of approximately 10−8 M albuterol in plasma but without adequate drug delivered to injured, edematous alveoli.
A second possible explanation for the lack of benefit of albuterol is that the injured alveolar epithelium may have been unable to respond to a β2-adrenergic agonist. A substantially intact alveolar epithelial barrier is required for net alveolar fluid clearance to occur (2). Impaired alveolar fluid clearance is characteristic of patients with ALI (9, 10) and in experimental models of lung injury (4). Aerosolized study drug was administered for 10 days or for 24 hours after extubation, anticipating that in some patients, repair of the alveolar epithelium could require several days before β-agonists could up-regulate alveolar fluid clearance. Short-term experimental studies suggest that β2-agonists can decrease pulmonary edema in the early phase of lung injury (21), but in many patients with ALI/ARDS the epithelium is denuded, with apoptosis and necrosis, and therefore may not be able to respond to β-agonists (11, 12).
A third possibility is that down-regulation of β2-adrenergic receptors occurred during the course of albuterol therapy. However, most experimental studies suggest that down-regulation of β2-receptors is a minor factor (32, 33). A fourth possibility is that there were differences between study groups in the proportions of patients with genetic variants that determine individual responses to β2-adrenergic stimulation in the distal lung epithelium, as reported in patients with asthma (34).
Last, it is possible that with lung-protective ventilation and a fluid-conservative hemodynamic strategy, there was little opportunity to further enhance alveolar fluid clearance with albuterol. In an experimental model of ALI, lung-protective ventilation with lower tidal volumes reduced pulmonary edema in part by up-regulating alveolar fluid clearance (35). In addition, arterial oxygenation improved and plateau pressures were lower in a prior trial in patients with ALI/ARDS who received a fluid-conservative strategy (24), suggesting that this approach reduced pulmonary edema.
Nebulized bronchodilators are used frequently in patients with acute respiratory failure without known airway disease (36). Potential beneficial effects of this therapy include enhanced mucociliary clearance (37), improved lung mechanics (38), and decreased work of breathing (39). The results of the present trial suggest that despite these potential beneficial effects, β-agonist therapy does not improve clinical outcomes. On the other hand, the cost of nebulized bronchodilator treatments can add several hundred dollars to the cost of medical care for acute respiratory failure (36). Therefore, clinicians should carefully consider the circumstances under which they prescribe β-agonists in patients with ALI/ARDS.
Last, the overall mortality in this 282-patient trial was 20.5% compared with 25.5% in the fluid-conservative arm of a previous ARDS Network clinical trial of hemodynamic strategies (40). This lower mortality is particularly noteworthy because the proportion of patients who were in shock at the time of randomization was higher in the current trial than in the hemodynamic management trial (44 vs. 37%). These data suggest that outcomes from ALI and ARDS are better now because of improvements in supportive clinical care.
In summary, the results of this clinical trial suggest that aerosolized albuterol does not improve clinical outcomes in patients with ALI. Routine use of β2-agonist therapy in mechanically ventilated patients with ALI cannot be recommended.
Supplementary Material
Acknowledgments
The authors thank Nephron Pharmaceuticals (Orlando, FL) for providing albuterol and placebo solutions. The authors are indebted to the intensive care unit personnel, especially respiratory therapists and nurses, and to our patients and their families, who supported this trial. The authors appreciate the assistance of Andrew Manies in the preparation of this manuscript.
Footnotes
Supported by National Heart, Lung, and Blood Institute contracts NO1-HR-56165–56713.
Author contributions: Conception, design, and execution of the trial—all of the authors; analysis and interpretation—M.A.M., R.G.B., K.D.L., D.S., B.T.T.; drafting of the manuscript for content—all of the authors.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201012-2090OC on May 11, 2011
Author Disclosure: M.A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.G.B. has received fees for board activities from AstraZeneca (AZ). S.C. has received consultancy fees from Asthmatx. I.S.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.E. is an employee of Genentech. R.D.H. holds stock in Discovery Laboratories, Inc. S.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.H.K. has received fees for board activities from CareFusion, and consultancy fees from Phillips Healthcare and Covidien Healthcare. K.D.L. has not received funding from Abbott and CMIC; however, she has received donated reagents from them; she holds stock in Amgen; she has received lecture fees from the American Society of Nephrologists, and has been employed by the American Society of Nephrologists as an associate editor for NephSAP. NM has received consultancy fees from CareFusion. M.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.S. has received consultancy fees from Averion International, Mylan, and Posen; he holds stock in Averion International. J.S. does not have a financial relationship with a commercial entity that has an interest in subject of this manuscript. B.T.T. has received consultancy fees from Lilly and AZ.
Appendix for Network Participants: University of Washington, Harborview—L. Hudson*, C. Hough, M. Neff, K. Sims, T. Watkins; Baystate Medical Center—J. Steingrub*, M. Tidswell, L. DeSouza, C. Kardos, L. Kozikowski, K. Kozikowski,; Baylor College of Medicine—K. Guntupalli, V. Bandi, C. Pope; Johns Hopkins Hospital—R. Brower*, H. Fessler, D. Hager, P. Mendez-Tellez, K. Oakjones, D. Needham; Johns Hopkins Bayview Medical Center—J. Sevransky, A. Workneh, S. Han, S. Murray; University of Maryland—C. Shanholtz, G. Netzer, P. Rock, A. Sampaio, J. Titus, T. Harrington; Washington Hospital Center—D. Herr, B. Lee, N. Bolouri; Cleveland Clinic Foundation—H. P. Wiedemann*, R. W. Ashton, D. A. Culver, T. Frederick, J. J. Komara, J. A. Guzman, A. J. Reddy; University Hospitals of Cleveland—R. Hejal, M. Andrews, D. Haney; MetroHealth Medical Center—A. F. Connors, S. Lasalvia, J. D. Thornton, E. L. Warren; University of Colorado Health Science Centers—M. Moss*, A. Benson, E. Burnham, B. Clark, L. Gray, C. Higgins, B. J. Maloney, M. Mealer; National Jewish Health—S. Frankel; St. Anthony's Hospital—T. Bost, P. Dennen, K. Hodgin; Denver Health Medical Center—I. Douglas, K. Overdier, K. Thompson, R. Wolken; Duke University—N. MacIntyre*, L. Brown, C. Cox, M. Gentile, J. Govert, N. Knudsen; University of North Carolina—S. Carson, L. Chang, J. Lanier; Vanderbilt University—A. P. Wheeler*, G. R. Bernard, M. Hays, S. Mogan, T. Rice; Wake Forest University—R. D. Hite*, P. E. Morris, A. Howard, A. Harvey, K. Bender; Moses Cone Memorial Hospital—P. Wright, S. Gross, J. McLean, A. Overton; University of Virginia—J. Truwit, K. Enfield, M. Marshall; LDS Hospital—T. Clemmer, L. Weaver; Cottonwood Hospital—M. Zenger, J. Krueger; Intermountain Medical Center—A. Morris*, A. Ahmed, A. Austin, N. Dean, C. Grissom, E. Hirshberg, N. Kumar, R. Miller, L. Napoli, J. Orme, S. Pandita, G. Schreiber, L. Struck, F. Thomas, G. Thomsen; McKay-Dee Hospital—C. Lawton, F. Leung, P. Kim, T. Fujii, J. Baughman, B. Kerwin, D. Hanselman; Utah Valley Regional Medical Center—K. Sundar, W. Alward, T. Hill, E. Campbell, K. Ludwig, D. Nielsen, M. Pearce; University of California, San Francisco—M. A. Matthay*, C. Calfee, B. Daniel, M. Eisner, O. Garcia, E. Johnson, R. Kallet, K. Kordesch, K. Liu, H. Zhou; University of California, San Francisco, Fresno—M. W. Peterson, J. Blaauw; University of California, Davis—T. Albertson, E. Vlastelin; Mayo Foundation—R. Hubmayr*, D. Brown, O. Gajic, R. Hinds, S. Holets, D. J. Kor, M. Passe; Louisiana State University—B. deBoisblanc*, P. Lauto, C. Romaine, G. Meyaski, J. Hunt, A. Marr; Louisiana State University—Earl K. Long; Baton Rouge General Medical Center Mid-City and Baton Rouge General Medical Center Bluebonnet—S. Brierre, C. LeBlanc; Alton-Ochsner Clinic Foundation—D. Taylor, S. Jain, L.Seoane; Tulane University—F. Simeone, J. Fearon, J. Duchesne; Clinical Coordinating Center (Massachusetts General Hospital and Harvard Medical School): D. Schoenfeld*, M. Aquino, D. Dorer, M. Guha, E. Hammond, N. Lavery, P. Lazar, I. Molina, R. Morse, C. Oldmixon, B. Rawal, N. Ringwood, A. Shui, E. Smoot, B. T. Thompson; National Heart, Lung, and Blood Institute: A. Harabin, S. Bredow, M. Waclawiw, G. Weinmann; Data and Safety Monitoring Board: R. G. Spragg (chair), A. Slutsky, M. Levy, B. Markovitz, E. Petkova, C. Weijer; Protocol Review Committee: J. Sznajder (chair), M. Begg, E. Israel, J. Lewis, P. Parsons.
* Denotes Principal Investigator.
References
- 1.Crandall ED, Matthay MA. Alveolar epithelial transport: basic science to clinical medicine. Am J Respir Crit Care Med 2001;163:1021–1029 [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600 [DOI] [PubMed] [Google Scholar]
- 3.Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol 2005;289:L685–L695 [DOI] [PubMed] [Google Scholar]
- 4.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol 2006;35:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 2002;119:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 2006;103:4964–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakuma T, Okaniwa G, Nakada T, Nishimura T, Fujimura S, Matthay MA. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med 1994;150:305–310 [DOI] [PubMed] [Google Scholar]
- 8.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol 2007;293:L52–L59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 1990;142:1250–1257 [DOI] [PubMed] [Google Scholar]
- 10.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376–1383 [DOI] [PubMed] [Google Scholar]
- 11.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis 1977;116:589–615 [DOI] [PubMed] [Google Scholar]
- 12.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and Fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol 2002;161:1783–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, et al. Transforming growth factor-β1 decreases expression of the epithelial sodium channel αENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 2003;278:43939–43950 [DOI] [PubMed] [Google Scholar]
- 14.Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF. Interleukin-1 βdecreases expression of the epithelial sodium channel α-subunit in alveolar epithelial cells via a p38 MAPK–dependent signaling pathway. J Biol Chem 2005;280:18579–18589 [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Fang X, Dolganov G, Fremont RD, Bastarache JA, Ware LB, Matthay MA. Acute lung injury edema fluid decreases net fluid transport across human alveolar epithelial type II cells. J Biol Chem 2007;282:24109–24119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song W, Matalon S. Modulation of alveolar fluid clearance by reactive oxygen–nitrogen intermediates. Am J Physiol Lung Cell Mol Physiol 2007;293:L855–L858 [DOI] [PubMed] [Google Scholar]
- 17.Mutlu GM, Sznajder JI. β2-Agonists for treatment of pulmonary edema: ready for clinical studies? Crit Care Med 2004;32:1607–1608 [DOI] [PubMed] [Google Scholar]
- 18.Matthay MA, Abraham E. β-Adrenergic agonist therapy as a potential treatment for acute lung injury. Am J Respir Crit Care Med 2006;173:254–255 [DOI] [PubMed] [Google Scholar]
- 19.Khimenko PL, Barnard JW, Moore TM, Wilson PS, Ballard ST, Taylor AE. Vascular permeability and epithelial transport effects on lung edema formation in ischemia and reperfusion. J Appl Physiol 1994;77:1116–1121 [DOI] [PubMed] [Google Scholar]
- 20.Perkins GD, McAuley DF, Thickett DR, Gao F. The β-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 2006;173:281–287 [DOI] [PubMed] [Google Scholar]
- 21.McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of β2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate–dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med 2004;32:1470–1476 [DOI] [PubMed] [Google Scholar]
- 22.Atabai K, Ware LB, Snider ME, Koch P, Daniel B, Nuckton TJ, Matthay MA. Aerosolized β2-adrenergic agonists achieve therapeutic levels in the pulmonary edema fluid of ventilated patients with acute respiratory failure. Intensive Care Med 2002;28:705–711 [DOI] [PubMed] [Google Scholar]
- 23.Matthay MA, Brower R, Thompson BT, Schoenfeld D, Eisner M, Carson S, Moss M, Douglas I, Hite RD, MacIntyre N. Randomized, placebo controlled trial of an aerosolized β2-adrenergic agonist (albuterol) for the treatment of acute lung injury [abstract]. Am J Respir Crit Care Med 2009;179:A2166 [Google Scholar]
- 24.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349 [DOI] [PubMed] [Google Scholar]
- 25.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327–336 [DOI] [PubMed] [Google Scholar]
- 26.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr., Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 27.DeMets DL, Ware JH. Asymmetric group sequential boundaries for monitoring clinical trials. Biometrica 1982;69:661–663 [Google Scholar]
- 28.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 29.Sakuma T, Folkesson HG, Suzuki S, Okaniwa G, Fujimura S, Matthay MA. β-Adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med 1997;155:506–512 [DOI] [PubMed] [Google Scholar]
- 30.Sakuma T, Gu X, Wang Z, Maeda S, Sugita M, Sagawa M, Osanai K, Toga H, Ware LB, Folkesson G, et al. Stimulation of alveolar epithelial fluid clearance in human lungs by exogenous epinephrine. Crit Care Med 2006;34:676–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenfeld GD, Abraham E. When is a negative phase II trial truly negative? Am J Respir Crit Care Med 2008;178:554–555 [DOI] [PubMed] [Google Scholar]
- 32.Sartori C, Fang X, McGraw DW, Koch P, Snider ME, Folkesson HG, Matthay MA. Selected contribution: long-term effects of β2-adrenergic receptor stimulation on alveolar fluid clearance in mice. J Appl Physiol 2002;93:1875–1880 [DOI] [PubMed] [Google Scholar]
- 33.Maron MB, Folkesson HG, Stader SM, Hodnichak CM. Impaired alveolar liquid clearance after 48-h isoproterenol infusion spontaneously recovers by 96 h of continuous infusion. Am J Physiol Lung Cell Mol Physiol 2006;291:L252–L256 [DOI] [PubMed] [Google Scholar]
- 34.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, Cooper DM, Fahy JV, Fish JE, Ford JG, et al. The effect of polymorphisms of the β2-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med 2000;162:75–80 [DOI] [PubMed] [Google Scholar]
- 35.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med 2002;165:242–249 [DOI] [PubMed] [Google Scholar]
- 36.Chang LH, Honiden S, Haithcock JA, Das AM, Short KA, Nierman DM, Carson SS. Utilization of bronchodilators in ventilated patients without obstructive airways disease. Respir Care 2007;52:154–158 [PubMed] [Google Scholar]
- 37.Bennett WD. Effect of β-adrenergic agonists on mucociliary clearance. J Allergy Clin Immunol 2002;110:S291–S297 [DOI] [PubMed] [Google Scholar]
- 38.Wright PE, Carmichael LC, Bernard GR. Effect of bronchodilators on lung mechanics in the acute respiratory distress syndrome (ARDS). Chest 1994;106:1517–1523 [DOI] [PubMed] [Google Scholar]
- 39.Mancebo J, Amaro P, Lorino H, Lemaire F, Harf A, Brochard L. Effects of albuterol inhalation on the work of breathing during weaning from mechanical ventilation. Am Rev Respir Dis 1991;144:95–100 [DOI] [PubMed] [Google Scholar]
- 40.Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, Hall J, Israel E, Jain M, Needham DM, et al. Beyond mortality: future clinical research in acute lung injury [NHLBI workshop report]. Am J Respir Crit Care Med 2010;181:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.