Abstract
The airway epithelium represents the first point of contact for inhaled foreign organisms. The protective arsenal of the airway epithelium is provided in the form of physical barriers and a vast array of receptors and antimicrobial compounds that constitute the innate immune system. Many of the known innate immune receptors, including the Toll-like receptors and nucleotide oligomerization domain–like receptors, are expressed by the airway epithelium, which leads to the production of proinflammatory cytokines and chemokines that affect microorganisms directly and recruit immune cells, such as neutrophils and T cells, to the site of infection. The airway epithelium also produces a number of resident antimicrobial proteins, such as lysozyme, lactoferrin, and mucins, as well as a swathe of cationic proteins. Dysregulation of the airway epithelial innate immune system is associated with a number of medical conditions that can result in compromised immunity and chronic inflammation of the lung. This review focuses on the innate immune capabilities of the airway epithelium and its role in protecting the lung from infection as well as the outcomes when its function is compromised.
Keywords: innate immunity, respiratory, airway, signaling
CLINICAL RELEVANCE.
The airway epithelium represents the first point of contact for inhaled foreign organisms. The airway epithelium uses a number of physical barriers and a vast array of receptors and antimicrobial compounds that constitute the innate immune system. This review focuses on the innate immune capabilities of the airway epithelium and its role in protecting the lung from infection as well as the outcomes when its function is compromised.
The airway epithelium represents the first line of defense of the lung. Airway epithelial cells provide a mechanical barrier to prevent infection but also produce chemokines and cytokines, such as IL-6, CXCL8, IL-1β, GM-CSF, and G-CSF, that recruit and activate phagocytic cells to eradicate organisms and infected cells. Because the lung is normally sterile, interactions with microorganisms typically cause an inflammatory response. This response can be due to direct cytopathic effects caused by the organism or can occur as a result of the host response to these organisms. The airway fluid contains a number of resident antimicrobial compounds, such as cationic defensins, or larger proteins such as lysozyme. In additional to resident antimicrobial proteins, the airway epithelium expresses an array of sensors to detect pathogens. Immune signaling can be activated by intact bacteria, viruses, fungi, or, more commonly, by the components of these organisms that are shed and gain access to surface or intracellular receptors. Even in the absence of direct epithelial contact, these shed components, such as LPS and flagella, referred to as pathogen-associated molecular patterns (PAMPs), can permeate the respiratory mucus layer to gain access to epithelial receptors stimulating inflammation. It is the recognition of PAMPs that constitutes what the innate immune system largely senses. The mucosal response, in particular the innate response, maintains the sterility of the lower airways by efficiently clearing sensed pathogens and rapidly controlling secondary effects associated with neutrophils and their products.
It is critical to regulate the intensity and duration of the proinflammatory signaling initiated in the airway. Perhaps more than at any other site, excessive inflammation (i.e., acute pneumonia) is associated with respiratory compromise and must be tightly controlled. Thus, a major component of mucosal immunity is the activation of the regulatory components of the innate immune system, which includes expression of NF-κB, activator protein 1, IFN regulatory factors (IRFs), and mitogen-activated protein kinases (MAPKs) (1, 2).
TLR SIGNALING
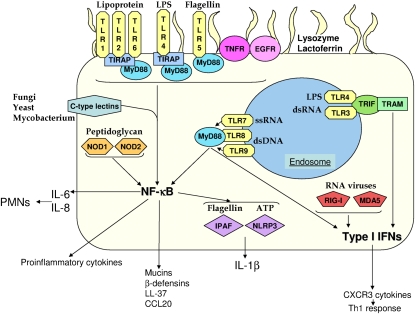
The Toll-like receptors (TLRs) are an important family of proteins involved in the recognition of microorganisms (Figure 1). The Toll protein was originally identified as being involved in dorsal-ventral patterning in Drosophila and later to be involved in fighting fungal infections (3, 4). Subsequent studies identified a number of homologs in humans that are involved in innate sensing of microbial products or PAMPs. The TLRs are integral membrane glycoproteins that, through homology, are part of a large family that includes IL-1 receptors (IL-1Rs). The cytoplasmic region contains a conserved TIR (Toll/IL-1R) domain (5), whereas the extracellular region differs between TLRs and IL-R by possessing leucine-rich repeats (LRRs), as opposed to an Ig-like domain. It is these LRRs that specify the target ligand for each TLR, also known as pattern recognition receptors.
Figure 1.
Innate immunity in the respiratory epithelium. Shown is an airway epithelial cell with the innate molecules discussed in this review and their ligands and surface receptors (Toll-like receptor (TLR)1, -2, -4, -5, -6; TNF receptor [TNFR]; and epidermal growth factor receptor (EGFR), endosomal receptors (TLR3, -4, -7, -8, and -9), cytosolic receptors (retinoic acid inducible gene [RIG]-I, melanoma differentiation–associated protein [MDA]5, nucleotide oligomerization domain [NOD]1, NOD2, IL1-β–converting enzyme protease activating factor [IPAF], and NOD-like receptor pyrin domain [NLRP3]) and antimicrobial proteins.
There have been 11 TLRs identified in humans. TLRs recognize a diverse array of microbial components, such as lipoproteins (TLR1, -2, and -6) (6–9), LPS (TLR4) (10), flagellin (TLR5) (11), DNA (TLR9) (12), and RNA (TLR3, -7, and -8) (13–15). The nature of the TLR10 ligand is unknown, whereas TLR11 has been shown to recognize uropathogenic E. coli (16) and a profilin-like molecule from Toxoplasma (17). TLRs1, -2, -4, -5, and -6 are located at the plasma membrane, with TLR3, -7, -8, and -9 in the endoplasmic reticulum, and are then chaperoned to endolysosomes (18).
Signal transduction from TLRs is typically referred to as MyD88-dependent or -independent. MyD88-dependent signaling (myeloid differentiation primary-response protein 88) (19) occurs through the adaptor protein MyD88 and its TLR binding partner toll–IL-1 receptor domain containing adaptor protein (TIRAP) (20). All TLRs, with the exception of TLR3, use MyD88-dependent signaling. TIRAP is not used by TLR5, -7, -8, or -9 (20). The importance of MyD88 is highlighted by the observation that protective immunity is lost to a small group of pyogenic organisms in humans with MyD88 mutations (21). The MyD88-independent arm (discussed below) is initiated by TLR3 and TLR4 through the TRIF-related adaptor molecule (22, 23) that couples endocytosis of TLR4 to the TIR-domain–containing adapter-inducing IFN-β (TRIF) adaptor (13, 24, 25). Activation of a TLR and subsequent signaling through MyD88 initiates an extensive signal transduction cascade that proceeds through a number of kinases and transcription factors, leading to phosphorylation of IκBa, an NF-κB inhibitory protein, and allowing NF-κB to activate expression of proinflammatory genes such as TNF, IL-1β, IL-6, and CXCL8 (26, 27).
TLR SIGNALING IN THE AIRWAY EPITHELIUM
The airway epithelium expresses the full complement of TLRs, but their distribution and the availability of adaptor proteins is important in determining their participation in signaling the presence of PAMPs. The expression of each TLR has been investigated in a variety of primary and immortalized cell lines from the upper and lower airways, with the strongest gene expression present for TLRs 2 through 6; the expression of TLRs 7 through 10 is variable depending on the cell type studied (2, 28–32). TLRs 1 through 6 and 9 are present on the cell surface, identified through flow cytometry (33). However, other studies point to a more even distribution of the receptors throughout airway epithelial cells (28). Adaptors such as MyD88 and CD14 are not seen on the cell surface (28, 33). Reduced surface expression of CD14 and low levels of MD2 production provides a potential mechanism for the low endogenous responsiveness of airway epithelial cells to LPS (34).
A number of TLRs are used by the airway to sense and initiate innate and adaptive immunity in response to pathogens. These organisms can induce the transcription of TLRs and their mobilization to the cell surface. Common airway pathogens, such as the viruses influenza, rhinovirus, and respiratory syncytial virus (RSV) and the bacteria Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pneumoniae, and Klebsiella pneumoniae, are detected through the presence of PAMPs on the epithelial cell surface. In some cases, expression of the TLRs is induced. TLR3 is important in the detection of a number of viruses, and its transcription is induced when a cell is infected (35–37). As a potential by-product of continual viral insult, TLR3 ligands (e.g., poly(I:C)) give the strongest proinflammatory response (2, 38). Bacterial infection with K. pneumoniae causes induction of genes encoding TLR2 and -4, sensors of liporotein and LPS, two important receptors for gram-negative bacterial pathogens (39). Expression of TLR4 on airway epithelial cells is crucial in the allergic response LPS, as demonstrated using bone marrow chimeric mice (40). Interaction of the epithelium with P. aeruginosa involves TLR2, -4, and -5 (41, 42). The flagella of P. aeruginosa, recognized by TLR5, induce mobilization of the receptor to the surface of the cell (43). P. aeruginosa flagella also interact with TLR2 and asialoGM1. This signaling through asialoGM1 is facilitated by a TLR2 lipid raft complex (caveolin-1) (43). The importance of MyD88 signaling in epithelial cells in response to P. aeruginosa was shown using bone marrow chimeras (44). During the early phase of clearance, MyD88 null mice that received normal bone marrow still faired worse, indicating that MyD88-dependent signaling of non–bone marrow derived cells was important in initial P. aeruginosa clearance.
TLR REGULATION OF MUCIN PRODUCTION
Mucin gene expression is also regulated by proinflammatory/TLR signaling. Mucins are glycoproteins that constitute mucus, an important barrier component of the respiratory epithelium. Mucus not only partakes in the normal mucocilliary clearance of the lung but also keeps the airway hydrated and traps particulate matter and potential pathogens. There are a large number of mucin genes, of which at least 12 are expressed in the airway (45). The most abundant mucins expressed are MUC1, MUC2, and MUC5AC; each is induced by a variety of gram-positive and gram-negative pathogens as well as viruses (46–51). Induction of mucin gene expression has been observed with TNF (52) and CXCL8 (53). Direct stimulation of TLR2 (54) and TLR3 (55) induces mucin expression as well as activating MAPK (56) and inducing epidermal growth factor receptor (EGFR) signaling (55, 57). It is also likely that mucins feedback into the TLR signaling pathways. Ueno and colleagues (58) showed that MUC1 plays an antiinflammatory role by negatively regulating signaling as a result of TLR2, -3, -4, -5, -7, and -9 signaling. A comprehensive review of mucins in the airway has recently been published (59). There is a need to maintain balance of production and clearance of mucins and mucus in the airway, as can be seen in chronic diseases such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and asthma, which typically result in increased levels of mucus that reduce airway function (60–62). As an indication that excessive mucus is deleterious, mice lacking MUC1 were able to better clear P. aeruginosa with enhanced neutrophil recruitment and higher proinflammatory cytokine production (63).
MODIFICATION OF TLR SIGNALING IN DISEASE STATES
An altered ability to sense pathogens by TLRs can have a significant impact on health. Although viruses are not sensed by TLR4, infection with RSV induces expression of TLR4, resulting in a sensitized state to LPS and enhancing inflammatory signaling (64). Secondary bacterial pneumonia after influenza infection is associated with significant morbidity and mortality. Desensitization of TLRs by viral PAMPs may contribute to enhanced susceptibility to bacterial infection. The desensitization leads to reduced chemokine production and NF-κB activation (65). A tolerance state after repeated exposure is also the basis of the hygiene hypothesis in asthma, whereby exposure early in life to PAMPs reduces the likelihood of hyperinflammation later in life (66–68).
Increased TLR signaling is associated with several pulmonary diseases. Exposure to cigarette smoke has been shown to increase TLR4 expression, leading to heightened CXCL8 production and additional recruitment of polymorphonuclear cells to the airways (69). In patients with CF, there is typically a hyperinflammatory state in the lungs, which is also seen in CF cell lines with increased CXCL8 and NF-κB signaling (28, 70). This increased signaling is not a consequence of TLR4, which is reported to be reduced in CF (71). Although CF inflammation is enhanced by the sensing of flagellin by TLR5, P. aeruginosa typically becomes nonmotile in chronic infections of patients with CF over time (72), and loss of motility is not necessarily coupled with a loss of inflammatory activity (73). Changes in P. aeruginosa LPS also occur in the CF lung (74). Modification of the lipid A portion of P. aeruginosa in vivo was associated with resistance to antimicrobial peptides and increased proinflammatory signaling (75). A reduced ability to activate TLR signaling is also problematic. Mutations in TLR4 are associated with increased risk of infection after surgery and display reduced cytokine production in the context of ventilator-associated pneumonia (76).
TYPE I IFNS
Type I IFN signaling often involves the activation of an endosomally located sensor and, via the TRIF adaptor (TLR3 and -4), initiates the production of IFN-β via TANK binding kinase (TBK)1 and phosphorylated IRF3, -5, and -7 (Figure 1) (77–80). Interaction of IFN-β with its heterodimeric receptor (IFN-α/β receptor [IFNAR]) results in dimerization and phosphorylation of STAT1/2 via Jak1 and Tyk2, leading to the downstream transcription of many genes, including CXCL10 (81–85). It has been shown in the airway epithelium that IFNAR is located basolaterally in differentiated cells (86). Signaling through IFNAR also results in the activation of the MAPK and PI3K pathways (87, 88) and leads to NF-κB activation that can in turn activate type I IFN signaling (89).
Many bacterial pathogens, both intracellular and extracellular, induce the type I IFN response via recognition of PAMPs such as proteins, LPS, and DNA (90–92). TLRs3, -4, -7, -8, and -9 (93–96), nucleotide oligomerization domain (NOD) (97, 98), and RNA polymerase III, which was identified as a sensor for cytosolic DNA (99, 100), as well as DAI/Zbp1 (DNA-dependent activator of IFN genes) (101), can activate type I IFN signaling.
Viruses are potent activators of type I IFN signaling through endosomal TLRs as well as the retinoic acid inducible gene [RIG]-like receptors. The proteins that are able to recognize RNA viruses are RIG-I (102) and melanoma differentiation–associated protein 5 (MDA5) (103, 104), which converge to the mitochondrial-bound IPS-1 (also called mitochondrial antiviral signaling protein) (105, 106) before the signal goes to TBK1 and IRF3 and IRF7. RIG-I and MDA5 are produced in the airway epithelium and respond to a number of pathogens such as influenza, rhinovirus, and RSV (35–37, 107).
How nonphagocytic cells such as airway mucosal cells produce type I IFNs in response to extracellular pathogens is ill defined. Most of the pathogens studied to date that activate type I IFN signaling are intracellular in nature, and their signaling pathways have been studied in the context of DCs or macrophages. Recently, the importance of epithelial type I IFN signaling was shown (108) using a mouse lacking STAT1 in epithelial cells. In that study, STAT1 null mice were irradiated and reconstituted with healthy bone marrow. These epithelial-specific STAT1 null mice were still highly susceptible to viral infection, indicating that epithelial STAT1 signaling was important in mediating viral clearance. S. aureus induces type I IFN in the airway epithelium, a process dependent on the virulence factor, protein A (91).
The outcome of this type I IFN response is variable and dependent upon the organism and the nature of the infection. The ability to induce production of type I IFNs is a critical component of the host response to influenza infection (109) but has much more variable consequences in response to bacterial infection. Infection of Ifnar−/− mice by the intracellular organisms Listeria and Legionella have opposite consequences, with the Ifnar−/− mice being significantly protected from Listeriosis (110) but with enhanced susceptibility to Legionella (111). Many extracellular bacteria shed PAMPs in the airway that can be internalized by airway cells and gain access to receptors linked to type I IFN signaling, thereby functioning more like viruses in stimulating innate immune responses. The clinical outcome of these type I IFN signaling responses differs according to the specific organism. For example, type I IFN contributes to S. aureus virulence in the setting of pneumonia (91), possibly due to TNF-induced death (112, 113), but contributes to the clearance of S. pneumoniae (114). Consistent with type I IFN activation via LPS (115), mice lacking TRIF (116) or IRF3 (117) have reduced capacity to clear P. aeruginosa infection, indicating a role for type I IFNs in protection. A similar observation was observed with E. coli in a pneumonia model with TRIF-null mice (118). Type I IFN signaling also contributes to the development of secondary bacterial pneumonia after influenza infection (119). In inflammatory diseases such as COPD, higher levels of type I IFN production are observed (120), whereas nasal epithelial cells from smokers have reduced expression of type I IFN receptors, kinases, and reduced type I IFN–dependent cytokines after influenza infection (121).
CXCR3
One group of cytokines that is regulated by type I IFNs provides a link between innate and adaptive immunity. The CXCR3 ligands CXCL9 (MIG), CXCL10 (IP-10), and CXCL11 (I-TAC) provide a mechanism for epithelial and other resident cells to recruit T cells (122–128). The production of CXCR3 chemokines such as CXCL10 preferentially attracts Th1 T cells (Figure 1) (127, 129) while antagonizing the recruitment of Th2 T cells (126).
The CXCR3 receptor and the CXCR3 cytokines are expressed in airway epithelial cells and are induced upon bacterial and viral stimulation in the airway (130–134). This results in CD4+ T-cell chemotaxis (135) and contributes to inflammation (136, 137). The CXCR3 cytokines can exert direct antibacterial effects against gram-positive and gram-negative organisms (136, 138). CXCL9 has a bactericidal effect on S. pneumonia; however, CXCL9 knockout mice were not attenuated in pneumococcal clearance from the lung (130).
A correlation exists between respiratory infections and levels of CXCR3 cytokines, particularly CXCL10. Levels of CXCL10 correlated to disease severity, viral titer, and number of lymphocytes in patients infected with rhinovirus (134). Elevated levels of CXCR3-positive cells and cytokines have also been observed in smokers and patients with COPD and bronchitis (139–141).
NOD-LIKE RECEPTORS
The NOD-like receptor (NLR) family encompasses a family of proteins that sense PAMPs in the cytosol. The best characterized members of this family are the NOD proteins NOD1 and NOD2. The NODs contain a caspase recruitment domain (CARD), NOD, and LRR domains. The NOD proteins were initially observed for their role in NF-κB induction and Crohn's disease (NOD2) (142–146). NOD1 primarily senses gram-negative peptidoglycan, which contains γ-d-glutamyl-meso-diaminopimelic acid (147, 148), whereas NOD2 is considered a general sensor of peptidolgycan through recognition of muramyl dipeptide (Figure 1) (149). Signal transduction from either NOD converges on the RIP2 kinase that leads to NF-κB activation (150).
Because NLRs are relatively new, the knowledge of NLRs in the airway is still developing. Both NOD proteins are expressed in the airway epithelium and are induced with bacterial stimuli (30, 151–154). In the context of polymicrobial colonization in the airway, the pore-forming toxin pneumolysin from S. pneumoniae facilitates entry of peptidoglycan from Haemophilus influenzae to activate NOD1 (155). In vivo studies have shown that the NODs are involved in pulmonary clearance of a number of bacterial pathogens (156–159); in some cases they appear to have redundant roles, with attenuated clearance only observed in RIP2 knockout mice (157). Genetic polymorphisms in nod1 have been linked to asthma (160).
BACTERIAL ACTIVATION OF THE INFLAMMASOME
The inflammasome is the term applied to the assembly of a number of proteins, including an NLR, pro–caspase-1, and the adapter apoptosis-associated speck-like protein (ASC) (161). An outcome of inflammasome activation is the production of caspase-1, which cleaves pro-proteins of IL-1β and IL-18 to their biologically active forms (162). Pro–IL-1β production is mediated by induction of the IL-1β gene through TLR and NOD stimulation, which is then processed by caspase-1 produced by recognition by the NLRs (163). The consequence of inflammasome activation is a form of cell death termed “pyroptosis.” Pyroptosis results in membrane disruption and the release of IL-1β and other inflammatory cytokines (164). Two other NLR proteins are involved in inflammasome activation, an area that has not been studied in detail in the airway but is important in pulmonary defenses (165, 166).
IL1-β–converting enzyme protease activating factor (IPAF), also known as NLRC4 (NLR CARD domain), recognizes cytosolic flagellin (Figure 1) (167, 168), including that of P. aeruginosa (169, 170). Extracellular flagellin is not recognized by IPAF. Activation of IPAF via flagellin is complex because it involves the delivery of the ligand via a functional type III secretion system. In the case of P. aeruginosa, two different type III secreted toxins have been shown to inhibit caspase-1–dependent cytokine production (169–172). In human epithelial cells, it has been shown that IPAF controls replication of Legionella pneumophila (173).
A second inflammasome NLR is NLR pyrin domain (NLRP3). NLRP3 senses multiple PAMPS, such as peptidoglycan (174) and RNA (175), and results in an inflammasome if ATP is sensed or bacterial toxins facilitate entry of stimulating ligands (Figure 1) (176–179). NLRP3 has been shown to sense asbestos and uric acid as a result of lung injury (180–182). NLRP3 is present in the nasal epithelium, and in vivo NLRP3 null mice show reduced inflammation to bacterial and viral challenge but poor survival, showcasing the requirement for inflammation in clearing infections (151, 165, 166, 183).
NON-TLR SIGNALING
There are a number of receptors present on the cell surface that signal through a number of pathways that are not related to the TLRs or NLRs. These receptors, three of which are TNF receptor (TNFR)1, EGFR, and C-type lectins, respond to host components but are also used by pathogens and can be important in defense.
TNFR1
TNF is a major proinflammatory cytokine whose expression is briskly activated in response to many types of infection; thus, it is not surprising that many different cell types in the lung express receptors to TNF (TNFRs) (184). In the airway epithelium, TNFR1 is abundant on the cell surface (Figure 1) (185) and is linked to many signaling cascades involved in host defense. One of the most striking examples for the involvement of TNFR1 in host defense is its interaction with protein A from S. aureus. The IgG binding domain of protein A, which recognizes the Fc region of IgG and Fab of VH3 (185–187), activates the TNF cascade, inducing CXCL8 expression via TRAF2/p38 MAPK and NF-κB. This interaction is critical in the pathogenesis of S. aureus pneumonia because spa null mutants do not cause infection and TNFR1 null mice are highly resistant to infection (185, 187).TNFR1 signaling appears to be the primary sensing mechanism for S. aureus in the airway because MyD88 is not important in S. aureus pneumonia models in vivo (188). A similar requirement for TNFR1 in causing pneumonia was observed with Stenotrophomonas maltophilia, an opportunistic pathogen for patients with CF. TNFR1 mice faired significantly better for pneumonia and bacteremia when intranasally infected with S. maltophilia (189).
Elevated levels of TNFR1 expression have been observed in CF epithelial cells, and Burkholderia cenocepacia, also a CF pathogen, activates TNFR1 as well (190). TNFR1 is also important for the clearance of P. aeruginosa (191). TNFR1 also regulates expression of MUC1, which is an important anti-inflammatory component and binding site for P. aeruginosa on airway epithelial cells (47, 52, 63, 192).
EGFR
EGFR plays a number of roles in epithelial signaling in response to airway infection. EGFR is located apically on airway epithelial cells (Figure 1) and induces production of CXCL8 in response to a variety of stimuli (193, 194). S. aureus interacts with EGFR through the IgG binding domain of protein A to activate TNF converting enzyme (TACE) (also called ADAM17). TACE participates in the regulation of inflammatory signaling by cleaving TNFR1 from the epithelial surface and inducing IL-6R shedding (195) and trans-signaling. The protein A-EGFR interaction induces TACE through a c-Src-erk1/2–mediated cascade (194). This signaling is not due to TGF-α because inhibition of protein A-EGFR binding prevented EGFR phosphorylation and TNFR1 cleavage.
EGFR signaling is central to the induction of mucin production in the airway. Activation of EGFR results in increased production of MUC5AC in the airway epithelium (196), and P. aeruginosa induces MUC5AC via activation of MAPK and EGFR (57, 197, 198). Increased mucin is a response to tobacco smoke (197), and EGFR serves as a gateway for cigarette smoke to mediate its damaging effects on adherens junctions and Wnt/β-catenin signaling (199). TACE is an integral component of this response because inhibiting TACE prevents the increased mucin expression as a result of reduce TGF-α shedding (198)
An interplay exists between EGFR signaling and the TLRs. TLR2, -3, -5, and -6 have been shown to activate EGFR. The mechanism leading to induction of CXCL8 occurs via a Duox1–TACE–TGF-α–EGFR pathway, with TGF-α acting as the ligand for EGFR signaling induced by the TLRs (200–202).
C-TYPE LECTINS
The C-type lectin family of proteins has an important physical role in mediating cell–cell adhesion but also recognizes carbohydrates, an important mechanism to sense fungal, yeast, and mycobacterial infections (203). C-type lectins possess a distinct protein fold, termed the carbohydrate recognition domain, which is generated by two conserved disulfide bonds between cysteine residues at the base of a double loop structure (204). Members of this family include dectin-1, dectin-2, and mincle. The C-type lectins can recognize the β-glycans present on fungi, yeast, and mycobacterial cell walls (205, 206)
Dectin-1 has been shown to be important in Pneumocystis carinii respiratory infection (207), whereas its role in Candida albicans depends on the infection model (206, 207). Dectin-1 also plays a significant role in inflammatory signaling in response to Aspergillus fumigatus (208). Dectin-2 is another C-type lectin involved in sensing yeast that is expressed in the lung (209). Dectin-2 shows a preference for hyphae of C. albicans (210) and is important in host defense (211). A third C-type lectin is Mincle, which is been shown to be required for proinflammatory signaling in response to C. albicans (212). The CARD9 adaptor mediates dectin-1 and dectin-2 signaling in CARD9 (213, 214), and mice lacking CARD9 were unable to control respiratory infection of Mycobacterium tuberculosis (215).
The biology of the C-type lectins has been mainly characterized in myeloid cells. Their role, if any, in airway epithelial cells is not fully understood. One study has identified production of dectin-1 in airway epithelial cells (216), contrasting earlier work (217). Production of dectin-1 in A549 cells was induced upon stimulation with M. tuberculosis, and internalization of the organism was partially blocked by silencing dectin-1 (216).
ANTIMICROBIAL PRODUCTS
In response to the recognition of PAMPs via TLRs and NLRs, the airway itself participates in microbial killing. The airway secretes a number of antimicrobial products that act directly on invading pathogens (Figure 1). These products are resident in the airway fluid and inducible upon recognition of pathogen. The antimicrobial molecules produced by the airway can be small cationic molecules, such as the β-defensins, LL-37, and CCL20, or larger proteins, such lysozyme, lactoferrin, and mucin.
β-DEFENSINS
β-Defensins are small cationic peptides that play an important role in host defense against microbial pathogens in the airway epithelium. There are six β-defensins identified in humans (hBD1–6). Although hBD5 and hBD6 have shown antimicrobial activity, they are not expressed in the respiratory epithelium (218–220). hBD1 is constitutively expressed in the epithelium, whereas hBD2, -3, and -4 can be induced by a variety of bacterial, fungal, and viral pathogens (221–227).
Significant evidence exists for the regulation of β-defensin expression by TLRs. Initial evidence observed that proinflammatory cytokines such as TNF and IL-1β could induce expression of hBD2 (228, 229). Subsequently it was found that hBD2 could be induced through TLR2 signaling (230, 231). Interfering with NF-κB signaling abolishes this response (232), as does blocking MyD88 or Mal/TIRAP (233). The TLR4 signaling complex and its MyD88 portion of signaling are involved in β-defensin expression (229, 233, 234). Microbial DNA through TLR9 (31), bacterial flagellin through TLR5 (235), and viral dsRNA through TLR3 (235) induce β-defensin expression in the respiratory epithelium. β-Defensins can also induce signaling of T cells and dendritic cells by binding to the chemokine receptor CCR6 (236).
Levels of β-defensin expression correlate to lung disease. Elevated levels of hBD2 are associated with inflammation in patients with CF, inflammatory lung disease, and deterioration of lung function. β-Defensins are not usually detected in healthy bronchoalveolar lavage samples (221, 237, 238). Mucoid strains of P. aeruginosa, as selected in chronic infections in the CF lung, were capable of inducing hBD2, whereas nonmucoid strains were not (239). The ability to express β-defensins has also been shown to be reduced with long-term smoking (240) and may contribute to lung disease.
LL37
Another cationic host peptide peptide is LL-37, the only human member of the cathelicidin family of antimicrobial peptides (241). LL-37 is generated by the respiratory epithelium and possesses broad spectrum antimicrobial activity (242) that, when overexpressed in murine models, enhances bacterial clearance (243). Elevated levels of LL-37 have been observed in CF samples, correlating with severity of disease (237). This inflammatory correlate may be related to cell death because apoptosis of respiratory epithelial cells has been observed with physiologically relevant levels of LL-37 (244).
LL-37 is induced by bacterial and mycobacterial infections, and this is dependent on MAPK (245–247). LL-37 is also capable of activating MAPK to induce CXCL8 secretion via activation of EGFR and IL-6 via NF-κB (248, 249). Although not investigated in epithelial cells, LL-37 can be induced by a variety of TLRs in macrophages (250).
CCL20
CCL20 (also known as LARC and MIP-3α) is another protein similar to the defensins. CCL20 is expressed in the respiratory epithelium and is stimulated by a variety of microorganisms, including bacteria and the dust mite (251, 252). CCL20 has also been shown to be regulated by TLR2, -3, and -5 as well as TNF (253–256). By interacting with CCR6, CCL20 is able to attract immature DC and T cells. Clinically, elevated levels are observed in patients with CF (253), and cigarette smoke retards its induction (254).
LACTOFERRIN AND LYSOZYME
The large and abundant antimicrobial proteins in the airway are lysozyme and lactoferrin. Both proteins have proven antibacterial properties but act with differing mechanisms (257). Lysozyme targets the β, 1→4 glycosidic bond between N-acetylglucosamine and N-acetylmuraminic acid in peptidoglycan (258) and subsequently is effective against gram–positive pathogens (257). Levels of lysozyme produced by epithelial cells correlate well to clearance of invading pathogens, and transgenic mice expressing elevated levels of lysozyme have significantly improved clearance of bacteria (259–261). Lactoferrin chelates iron away from bacteria but also has direct antimicrobial properties (257, 262, 263). Lactoferrin works with lysozyme to kill gram–negative pathogens by disrupting their membrane to expose susceptible peptidoglycan (264). A number of studies have investigated the correlation between elevated levels of lysozyme and lactoferrin in patients with CF (265) as well as individuals with chronic bronchitis and asymptomatic smokers, indicating a potential contribution to inflammation (266).
CONCLUSION
The airway epithelium is an important part of the innate immune system. Its collection of surface, endosomal, and cytosolic sensors that activate numerous proinflammatory signaling pathways and resident antimicrobial peptides offers significant mechanisms to deal with invading pathogens. It is a tremendously complex system, with many coregulated components. There is likely even greater complexity than we now appreciate; additional receptors are being identified continuously as is an appreciation for their role in epithelial cells. Despite the large amount of experimental data accrued, many questions remain. It remains unclear how the airway epithelium discriminates between commensal flora and pathogens that often colonize (S. pneumoniae or S. aureus) from the bacteria which initiate invasive infection. Not only does the host actively respond to the perceived threat of infection, but the organisms readily adapt to immune pressure, activating and repressing specific genes to facilitate proliferation despite the many effectors of immune clearance. A great deal has been learned by exploiting murine models of infection, which, despite their limitations, have facilitated a basic understanding of the major components of the innate immune system and their role in host defense of the respiratory tract. The importance of the innate immune system is highlighted by susceptibility to pathogens in specific transgenic mice studies and the correlations that exist with diseased states such as COPD, CF, and cigarette smoking. More complex models, such as the newly developed CF pig (267–269), as well as detailed genetic studies of polymorphisms in TLRs, NODs, and other receptors, should provide even more insights into the mechanisms through which the respiratory mucosa initiates host defenses against such a variety of pathogens.
FUTURE DIRECTION: THE ROLE OF EPITHELIAL SIGNALING IN MUCOSAL IMMUNITY
The participation of the airway epithelium in mucosal defenses has been well established; there is no question that airway epithelial cells provide much more than just a mechanical barrier to infection. However, many unanswered questions remain. The presence of the full complement of innate immune receptors, TLRs, NLRs, and the diverse intracellular receptors linked to the type I IFN cascade indicate that many airway epithelial cells have the potential to respond to a wide range of pathogens. What may be limiting is whether specific PAMPs can gain access to the corresponding receptors and whether they are superficially exposed or intracellular. Thus, the ability of the mucosal epithelium to distinguish commensal flora, which does not activate immune responses, from pathogens that do may lie in the ability of the pathogen to stimulate intracellular signaling. For many bacteria and viruses, this may include activating receptors linked to the type I IFN cascade, which are intracellular. The relative amounts and distribution of these receptors could account for major differences in the activation of epithelial cells at specific sites to respond to specific pathogens (e.g., the lack of TLR4 on the surface of polarized epithelial cells). A better understanding of how the mucosal epithelium responds to airway PAMPs and how signals from commensals are processed to prevent excessive damaging inflammatory responses and how the presence of a real pathogen is rapidly amplified to protect the lung are questions that are being actively investigated.
Supplementary Material
This work was supported by National Institutes of Health grants 5R21AI083491, 2R01HL079395, and 5R01HL073989 (A.P.).
Originally Published in Press as DOI: 10.1165/rcmb.2011-0011RT on February 17, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Yoshikawa T, Hill TE, Yoshikawa N, Popov VL, Galindo CL, Garner HR, Peters CJ, Tseng CT. Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome-associated coronavirus infection. PLoS ONE 2010;5:e8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol 2004;31:358–364. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 1985;42:779–789. [DOI] [PubMed] [Google Scholar]
- 4.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996;86:973–983. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–820. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 2001;13:933–940. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 2002;169:10–14. [DOI] [PubMed] [Google Scholar]
- 8.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 1999;285:736–739. [DOI] [PubMed] [Google Scholar]
- 9.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 1999;274:17406–17409. [DOI] [PubMed] [Google Scholar]
- 10.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001;410:1099–1103. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000;408:740–745. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001;413:732–738. [DOI] [PubMed] [Google Scholar]
- 14.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004;303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 15.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004;303:1526–1529. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 2004;303:1522–1526. [DOI] [PubMed] [Google Scholar]
- 17.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 2005;308:1626–1629. [DOI] [PubMed] [Google Scholar]
- 18.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 2008;452:234–238. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 1998;2:253–258. [DOI] [PubMed] [Google Scholar]
- 20.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 2002;420:329–333. [DOI] [PubMed] [Google Scholar]
- 21.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 2008;321:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med 2003;198:1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 2003;4:1144–1150. [DOI] [PubMed] [Google Scholar]
- 24.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 2003;424:743–748. [DOI] [PubMed] [Google Scholar]
- 25.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 2008;9:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 1998;9:143–150. [DOI] [PubMed] [Google Scholar]
- 27.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem 1990;265:21128–21133. [PubMed] [Google Scholar]
- 28.Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 2004;30:777–783. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong L, Medford AR, Uppington KM, Robertson J, Witherden IR, Tetley TD, Millar AB. Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. Am J Respir Cell Mol Biol 2004;31:241–245. [DOI] [PubMed] [Google Scholar]
- 30.Mayer AK, Muehmer M, Mages J, Gueinzius K, Hess C, Heeg K, Bals R, Lang R, Dalpke AH. Differential recognition of TLR-dependent microbial ligands in human bronchial epithelial cells. J Immunol 2007;178:3134–3142. [DOI] [PubMed] [Google Scholar]
- 31.Platz J, Beisswenger C, Dalpke A, Koczulla R, Pinkenburg O, Vogelmeier C, Bals R. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol 2004;173:1219–1223. [DOI] [PubMed] [Google Scholar]
- 32.Homma T, Kato A, Hashimoto N, Batchelor J, Yoshikawa M, Imai S, Wakiguchi H, Saito H, Matsumoto K. Corticosteroid and cytokines synergistically enhance toll-like receptor 2 expression in respiratory epithelial cells. Am J Respir Cell Mol Biol 2004;31:463–469. [DOI] [PubMed] [Google Scholar]
- 33.Greene CM, Carroll TP, Smith SG, Taggart CC, Devaney J, Griffin S, O'Neill SJ, McElvaney NG. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J Immunol 2005;174:1638–1646. [DOI] [PubMed] [Google Scholar]
- 34.Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, McCray PB Jr. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol 2004;287:L428–L437. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, Zhao Y, McHenry CL, Burgens RV, Miller DJ, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol 2009;183:6989–6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing Z, Harper R, Anunciacion J, Yang Z, Gao W, Qu B, Guan Y, Cardona CJ. Host immune and apoptotic responses to avian influenza virus H9N2 in human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 2011;44:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol 2007;81:1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Matsukura S, Watanabe S, Adachi M, Suzaki H. Involvement of Toll-like receptors in the immune response of nasal polyp epithelial cells. Clin Immunol 2007;124:345–352. [DOI] [PubMed] [Google Scholar]
- 39.Regueiro V, Moranta D, Campos MA, Margareto J, Garmendia J, Bengoechea JA. Klebsiella pneumoniae increases the levels of Toll-like receptors 2 and 4 in human airway epithelial cells. Infect Immun 2009;77:714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 2009;15:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raoust E, Balloy V, Garcia-Verdugo I, Touqui L, Ramphal R, Chignard M. Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS ONE 2009;4:e7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2007;292:L312–L322. [DOI] [PubMed] [Google Scholar]
- 43.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol 2004;30:627–634. [DOI] [PubMed] [Google Scholar]
- 44.Hajjar AM, Harowicz H, Liggitt HD, Fink PJ, Wilson CB, Skerrett SJ. An essential role for non-bone marrow-derived cells in control of Pseudomonas aeruginosa pneumonia. Am J Respir Cell Mol Biol 2005;33:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. [DOI] [PubMed] [Google Scholar]
- 46.Voynow JA, Selby DM, Rose MC. Mucin gene expression (MUC1, MUC2, and MUC5/5AC) in nasal epithelial cells of cystic fibrosis, allergic rhinitis, and normal individuals. Lung 1998;176:345–354. [DOI] [PubMed] [Google Scholar]
- 47.Choi S, Park YS, Koga T, Treloar A, Kim KC. TNF-{alpha} is a key regulator of MUC1, an anti-inflammatory molecule during airway Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol 2011;44:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat Med 2002;8:41–46. [DOI] [PubMed] [Google Scholar]
- 49.Dohrman A, Miyata S, Gallup M, Li JD, Chapelin C, Coste A, Escudier E, Nadel J, Basbaum C. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim Biophys Acta 1998;1406:251–259. [DOI] [PubMed] [Google Scholar]
- 50.Hewson CA, Haas JJ, Bartlett NW, Message SD, Laza-Stanca V, Kebadze T, Caramori G, Zhu J, Edbrooke MR, Stanciu LA, et al. Rhinovirus induces MUC5AC in a human infection model, & in vitro via NF-{kappa}B & EGFR pathways. Eur Respir J 2010;36:1425–1435. [DOI] [PubMed] [Google Scholar]
- 51.Kraft M, Adler KB, Ingram JL, Crews AL, Atkinson TP, Cairns CB, Krause DC, Chu HW. Mycoplasma pneumoniae induces airway epithelial cell expression of MUC5AC in asthma. Eur Respir J 2008;31:43–46. [DOI] [PubMed] [Google Scholar]
- 52.Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y, Kim KC. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol 2007;293:L693–L701. [DOI] [PubMed] [Google Scholar]
- 53.Bautista MV, Chen Y, Ivanova VS, Rahimi MK, Watson AM, Rose MC. IL-8 regulates mucin gene expression at the posttranscriptional level in lung epithelial cells. J Immunol 2009;183:2159–2166. [DOI] [PubMed] [Google Scholar]
- 54.Chen R, Lim JH, Jono H, Gu XX, Kim YS, Basbaum CB, Murphy TF, Li JD. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2–TAK1-dependent p38 MAPK-AP1 and IKKbeta-IkappaBalpha-NF-kappaB signaling pathways. Biochem Biophys Res Commun 2004;324:1087–1094. [DOI] [PubMed] [Google Scholar]
- 55.Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, Chen Y. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol 2009;40:610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, Nadel JA, Prince A, Basbaum CB. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA 1997;94:967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohri K, Ueki IF, Shim JJ, Burgel PR, Oh YM, Tam DC, Dao-Pick T, Nadel JA. Pseudomonas aeruginosa induces MUC5AC production via epidermal growth factor receptor. Eur Respir J 2002;20:1263–1270. [DOI] [PubMed] [Google Scholar]
- 58.Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, Kim KC. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol 2008;38:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest 2009;135:505–512. [DOI] [PubMed] [Google Scholar]
- 60.Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, Barnes PJ, Ciaccia A, Cavallesco G, Chung KF, et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology 2004;45:477–484. [DOI] [PubMed] [Google Scholar]
- 61.Zuelzer WW, Newton WA Jr. The pathogenesis of fibrocystic disease of the pancreas; a study of 36 cases with special reference to the pulmonary lesions. Pediatrics 1949;4:53–69. [PubMed] [Google Scholar]
- 62.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992;101:916–921. [DOI] [PubMed] [Google Scholar]
- 63.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, Chen W, Cross AS, Gendler SJ, Gewirtz AT, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 2006;176:3890–3894. [DOI] [PubMed] [Google Scholar]
- 64.Monick MM, Yarovinsky TO, Powers LS, Butler NS, Carter AB, Gudmundsson G, Hunninghake GW. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J Biol Chem 2003;278:53035–53044. [DOI] [PubMed] [Google Scholar]
- 65.Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med 2008;205:323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 2001;358:1129–1133. [DOI] [PubMed] [Google Scholar]
- 67.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 2002;347:869–877. [DOI] [PubMed] [Google Scholar]
- 68.Prefontaine D, Banville-Langelier AA, Fiset PO, Guay J, An J, Mazer M, Hamid Q, Mazer BD. Children with atopic histories exhibit impaired lipopolysaccharide-induced Toll-like receptor-4 signalling in peripheral monocytes. Clin Exp Allergy 2010;40:1648–1657. [DOI] [PubMed] [Google Scholar]
- 69.Pace E, Ferraro M, Siena L, Melis M, Montalbano AM, Johnson M, Bonsignore MR, Bonsignore G, Gjomarkaj M. Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 2008;124:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl- channel function. Am J Physiol Lung Cell Mol Physiol 2001;281:L71–L78. [DOI] [PubMed] [Google Scholar]
- 71.John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir Cell Mol Biol 2010;42:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahenthiralingam E, Campbell ME, Speert DP. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun 1994;62:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blohmke CJ, Victor RE, Hirschfeld AF, Elias IM, Hancock DG, Lane CR, Davidson AG, Wilcox PG, Smith KD, Overhage J, et al. Innate immunity mediated by TLR5 as a novel antiinflammatory target for cystic fibrosis lung disease. J Immunol 2008;180:7764–7773. [DOI] [PubMed] [Google Scholar]
- 74.Ernst RK, Moskowitz SM, Emerson JC, Kraig GM, Adams KN, Harvey MD, Ramsey B, Speert DP, Burns JL, Miller SI. Unique lipid a modifications in Pseudomonas aeruginosa isolated from the airways of patients with cystic fibrosis. J Infect Dis 2007;196:1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ernst RK, Yi EC, Guo L, Lim KB, Burns JL, Hackett M, Miller SI. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 1999;286:1561–1565. [DOI] [PubMed] [Google Scholar]
- 76.Kumpf O, Giamarellos-Bourboulis EJ, Koch A, Hamann L, Mouktaroudi M, Oh DY, Latz E, Lorenz E, Schwartz DA, Ferwerda B, et al. Influence of genetic variations in TLR4 and TIRAP/Mal on the course of sepsis and pneumonia and cytokine release: an observational study in three cohorts. Crit Care 2010;14:R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 2003;4:491–496. [DOI] [PubMed] [Google Scholar]
- 78.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 2000;13:539–548. [DOI] [PubMed] [Google Scholar]
- 79.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005;434:772–777. [DOI] [PubMed] [Google Scholar]
- 80.Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgerald KA, Golenbock DT. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem 2005;280:17005–17012. [DOI] [PubMed] [Google Scholar]
- 81.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol 2001;167:5887–5894. [DOI] [PubMed] [Google Scholar]
- 82.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci USA 1998;95:15623–15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta S, Yan H, Wong LH, Ralph S, Krolewski J, Schindler C. The SH2 domains of Stat1 and Stat2 mediate multiple interactions in the transduction of IFN-alpha signals. EMBO J 1996;15:1075–1084. [PMC free article] [PubMed] [Google Scholar]
- 84.Muller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur AG, Barbieri G, Witthuhn BA, Schindler C, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature 1993;366:129–135. [DOI] [PubMed] [Google Scholar]
- 85.Watling D, Guschin D, Muller M, Silvennoinen O, Witthuhn BA, Quelle FW, Rogers NC, Schindler C, Stark GR, Ihle JN, et al. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature 1993;366:166–170. [DOI] [PubMed] [Google Scholar]
- 86.Ciencewicki JM, Brighton LE, Jaspers I. Localization of type I interferon receptor limits interferon-induced TLR3 in epithelial cells. J Interferon Cytokine Res 2009;29:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.David M, Petricoin E III, Benjamin C, Pine R, Weber MJ, Larner AC. Requirement for MAP kinase (ERK2) activity in interferon alpha- and interferon beta-stimulated gene expression through STAT proteins. Science 1995;269:1721–1723. [DOI] [PubMed] [Google Scholar]
- 88.Rani MR, Hibbert L, Sizemore N, Stark GR, Ransohoff RM. Requirement of phosphoinositide 3-kinase and Akt for interferon-beta-mediated induction of the beta-R1 (SCYB11) gene. J Biol Chem 2002;277:38456–38461. [DOI] [PubMed] [Google Scholar]
- 89.Lenardo MJ, Fan CM, Maniatis T, Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell 1989;57:287–294. [DOI] [PubMed] [Google Scholar]
- 90.Charrel-Dennis M, Latz E, Halmen KA, Trieu-Cuot P, Fitzgerald KA, Kasper DL, Golenbock DT. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe 2008;4:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martin FJ, Gomez MI, Wetzel DM, Memmi G, O'Seaghdha M, Soong G, Schindler C, Prince A. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J Clin Invest 2009;119:1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol 2002;3:392–398. [DOI] [PubMed] [Google Scholar]
- 93.Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol 2009;10:587–594. [DOI] [PubMed] [Google Scholar]
- 94.Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol 2005;5:675–687. [DOI] [PubMed] [Google Scholar]
- 95.Bogdan C, Mattner J, Schleicher U. The role of type I interferons in non-viral infections. Immunol Rev 2004;202:33–48. [DOI] [PubMed] [Google Scholar]
- 96.Mancuso G, Midiri A, Biondo C, Beninati C, Zummo S, Galbo R, Tomasello F, Gambuzza M, Macri G, Ruggeri A, et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J Immunol 2007;178:3126–3133. [DOI] [PubMed] [Google Scholar]
- 97.Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, Behr MA, Fitzgerald KA, Sassetti CM, Kelliher MA. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog 2009;5:e1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leber JH, Crimmins GT, Raghavan S, Meyer-Morse NP, Cox JS, Portnoy DA. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog 2008;4:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol 2009;10:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 2009;138:576–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007;448:501–505. [DOI] [PubMed] [Google Scholar]
- 102.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 2004;5:730–737. [DOI] [PubMed] [Google Scholar]
- 103.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA 2006;103:8459–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006;441:101–105. [DOI] [PubMed] [Google Scholar]
- 105.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 2005;6:981–988. [DOI] [PubMed] [Google Scholar]
- 106.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005;122:669–682. [DOI] [PubMed] [Google Scholar]
- 107.Opitz B, Rejaibi A, Dauber B, Eckhard J, Vinzing M, Schmeck B, Hippenstiel S, Suttorp N, Wolff T. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol 2007;9:930–938. [DOI] [PubMed] [Google Scholar]
- 108.Shornick LP, Wells AG, Zhang Y, Patel AC, Huang G, Takami K, Sosa M, Shukla NA, Agapov E, Holtzman MJ. Airway epithelial versus immune cell Stat1 function for innate defense against respiratory viral infection. J Immunol 2008;180:3319–3328. [DOI] [PubMed] [Google Scholar]
- 109.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science 1994;264:1918–1921. [DOI] [PubMed] [Google Scholar]
- 110.O'Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med 2004;200:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, Ishii KJ, Akira S, Colonna M, Chen ZJ, et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med 2009;206:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huys L, Van Hauwermeiren F, Dejager L, Dejonckheere E, Lienenklaus S, Weiss S, Leclercq G, Libert C. Type I interferon drives tumor necrosis factor-induced lethal shock. J Exp Med 2009;206:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yarilina A, Park-Min KH, Antoniv T, Hu X, Ivashkiv LB. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol 2008;9:378–387. [DOI] [PubMed] [Google Scholar]
- 114.Weigent DA, Huff TL, Peterson JW, Stanton GJ, Baron S. Role of interferon in streptococcal infection in the mouse. Microb Pathog 1986;1:399–407. [DOI] [PubMed] [Google Scholar]
- 115.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009;458:1191–1195. [DOI] [PubMed] [Google Scholar]
- 116.Power MR, Li B, Yamamoto M, Akira S, Lin TJ. A role of Toll-IL-1 receptor domain-containing adaptor-inducing IFN-beta in the host response to Pseudomonas aeruginosa lung infection in mice. J Immunol 2007;178:3170–3176. [DOI] [PubMed] [Google Scholar]
- 117.Carrigan SO, Junkins R, Yang YJ, Macneil A, Richardson C, Johnston B, Lin TJ. IFN regulatory factor 3 contributes to the host response during Pseudomonas aeruginosa lung infection in mice. J Immunol 2010;185:3602–3609. [DOI] [PubMed] [Google Scholar]
- 118.Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol 2007;178:3153–3160. [DOI] [PubMed] [Google Scholar]
- 119.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest 2009;119:1910–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schneider D, Ganesan S, Comstock AT, Meldrum CA, Mahidhara R, Goldsmith AM, Curtis JL, Martinez FJ, Hershenson MB, Sajjan U. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;182:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Horvath KM, Brighton LE, Zhang W, Carson JL, Jaspers I. Epithelial cells from smokers modify dendritic cell responses in the context of influenza infection. Am J Respir Cell Mol Biol (In press) [DOI] [PMC free article] [PubMed]
- 122.Satoh J, Nanri Y, Tabunoki H, Yamamura T. Microarray analysis identifies a set of CXCR3 and CCR2 ligand chemokines as early IFNbeta-responsive genes in peripheral blood lymphocytes in vitro: an implication for IFNbeta-related adverse effects in multiple sclerosis. BMC Neurol 2006;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wenzel J, Schmidt R, Proelss J, Zahn S, Bieber T, Tuting T. Type I interferon-associated skin recruitment of CXCR3+ lymphocytes in dermatomyositis. Clin Exp Dermatol 2006;31:576–582. [DOI] [PubMed] [Google Scholar]
- 124.Kelly-Scumpia KM, Scumpia PO, Delano MJ, Weinstein JS, Cuenca AG, Wynn JL, Moldawer LL. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med 2010;207:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watanabe T, Asano N, Fichtner-Feigl S, Gorelick PL, Tsuji Y, Matsumoto Y, Chiba T, Fuss IJ, Kitani A, Strober W. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest 2010;120:1645–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G, Baggiolini M, Clark-Lewis I. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J Biol Chem 2001;276:2986–2991. [DOI] [PubMed] [Google Scholar]
- 127.Qian C, An H, Yu Y, Liu S, Cao X. TLR agonists induce regulatory dendritic cells to recruit Th1 cells via preferential IP-10 secretion and inhibit Th1 proliferation. Blood 2007;109:3308–3315. [DOI] [PubMed] [Google Scholar]
- 128.Manicone AM, Burkhart KM, Lu B, Clark JG. CXCR3 ligands contribute to Th1-induced inflammation but not to homing of Th1 cells into the lung. Exp Lung Res 2008;34:391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Debes GF, Dahl ME, Mahiny AJ, Bonhagen K, Campbell DJ, Siegmund K, Erb KJ, Lewis DB, Kamradt T, Hamann A. Chemotactic responses of IL-4-, IL-10-, and IFN-gamma-producing CD4+ T cells depend on tissue origin and microbial stimulus. J Immunol 2006;176:557–566. [DOI] [PubMed] [Google Scholar]
- 130.Eliasson M, Morgelin M, Farber JM, Egesten A, Albiger B. Streptococcus pneumoniae induces expression of the antibacterial CXC chemokine MIG/CXCL9 via MyD88-dependent signaling in a murine model of airway infection. Microbes Infect 2010;12:565–573. [DOI] [PubMed] [Google Scholar]
- 131.Kelsen SG, Aksoy MO, Yang Y, Shahabuddin S, Litvin J, Safadi F, Rogers TJ. The chemokine receptor CXCR3 and its splice variant are expressed in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L584–L591. [DOI] [PubMed] [Google Scholar]
- 132.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol 1999;162:3549–3558. [PubMed] [Google Scholar]
- 133.Pechkovsky DV, Goldmann T, Ludwig C, Prasse A, Vollmer E, Muller-Quernheim J, Zissel G. CCR2 and CXCR3 agonistic chemokines are differently expressed and regulated in human alveolar epithelial cells type II. Respir Res 2005;6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol 2005;289:L85–L95. [DOI] [PubMed] [Google Scholar]
- 135.Escotte S, Al Alam D, Le Naour R, Puchelle E, Guenounou M, Gangloff SC. T cell chemotaxis and chemokine release after Staphylococcus aureus interaction with polarized airway epithelium. Am J Respir Cell Mol Biol 2006;34:348–354. [DOI] [PubMed] [Google Scholar]
- 136.Egesten A, Eliasson M, Johansson HM, Olin AI, Morgelin M, Mueller A, Pease JE, Frick IM, Bjorck L. The CXC chemokine MIG/CXCL9 is important in innate immunity against Streptococcus pyogenes. J Infect Dis 2007;195:684–693. [DOI] [PubMed] [Google Scholar]
- 137.Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, Rosa R, Di Salvo J, Mudgett J, Peterson LB, et al. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol 2003;73:771–780. [DOI] [PubMed] [Google Scholar]
- 138.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol 2001;167:623–627. [DOI] [PubMed] [Google Scholar]
- 139.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, Bellettato CM, Papi A, Corbetta L, Zuin R, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;165:1404–1409. [DOI] [PubMed] [Google Scholar]
- 140.Costa C, Rufino R, Traves SL, Lapa ESJR, Barnes PJ, Donnelly LE. CXCR3 and CCR5 chemokines in induced sputum from patients with COPD. Chest 2008;133:26–33. [DOI] [PubMed] [Google Scholar]
- 141.Woodman L, Sutcliffe A, Kaur D, Berry M, Bradding P, Pavord ID, Brightling CE. Chemokine concentrations and mast cell chemotactic activity in BAL fluid in patients with eosinophilic bronchitis and asthma, and in normal control subjects. Chest 2006;130:371–378. [DOI] [PubMed] [Google Scholar]
- 142.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001;411:603–606. [DOI] [PubMed] [Google Scholar]
- 143.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 144.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 2001;276:4812–4818. [DOI] [PubMed] [Google Scholar]
- 145.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep 2001;2:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bertin J, Nir WJ, Fischer CM, Tayber OV, Errada PR, Grant JR, Keilty JJ, Gosselin ML, Robison KE, Wong GH, et al. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kappaB. J Biol Chem 1999;274:12955–12958. [DOI] [PubMed] [Google Scholar]
- 147.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol 2003;4:702–707. [DOI] [PubMed] [Google Scholar]
- 148.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 2003;300:1584–1587. [DOI] [PubMed] [Google Scholar]
- 149.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 2003;278:8869–8872. [DOI] [PubMed] [Google Scholar]
- 150.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J 2008;27:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bogefors J, Rydberg C, Uddman R, Fransson M, Mansson A, Benson M, Adner M, Cardell LO. Nod1, Nod2 and Nalp3 receptors, new potential targets in treatment of allergic rhinitis? Allergy 2010;65:1222–1226. [DOI] [PubMed] [Google Scholar]
- 152.Opitz B, Puschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, Schumann RR, Suttorp N, Hippenstiel S. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem 2004;279:36426–36432. [DOI] [PubMed] [Google Scholar]
- 153.Travassos LH, Carneiro LA, Girardin SE, Boneca IG, Lemos R, Bozza MT, Domingues RC, Coyle AJ, Bertin J, Philpott DJ, et al. Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J Biol Chem 2005;280:36714–36718. [DOI] [PubMed] [Google Scholar]
- 154.Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM, van Crevel R, Adema GJ, Ottenhoff TH, Van der Meer JW, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 2005;1:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ratner AJ, Aguilar JL, Shchepetov M, Lysenko ES, Weiser JN. Nod1 mediates cytoplasmic sensing of combinations of extracellular bacteria. Cell Microbiol 2007;9:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 2010;16:228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Frutuoso MS, Hori JI, Pereira MS, Junior DS, Sonego F, Kobayashi KS, Flavell RA, Cunha FQ, Zamboni DS. The pattern recognition receptors Nod1 and Nod2 account for neutrophil recruitment to the lungs of mice infected with Legionella pneumophila. Microbes Infect 2010;12:819–827. [DOI] [PubMed] [Google Scholar]
- 158.Shimada K, Chen S, Dempsey PW, Sorrentino R, Alsabeh R, Slepenkin AV, Peterson E, Doherty TM, Underhill D, Crother TR, et al. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog 2009;5:e1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zola TA, Lysenko ES, Weiser JN. Mucosal clearance of capsule-expressing bacteria requires both TLR and nucleotide-binding oligomerization domain 1 signaling. J Immunol 2008;181:7909–7916. [DOI] [PubMed] [Google Scholar]
- 160.Hysi P, Kabesch M, Moffatt MF, Schedel M, Carr D, Zhang Y, Boardman B, von Mutius E, Weiland SK, Leupold W, et al. NOD1 variation, immunoglobulin E and asthma. Hum Mol Genet 2005;14:935–941. [DOI] [PubMed] [Google Scholar]
- 161.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417–426. [DOI] [PubMed] [Google Scholar]
- 162.Yamin TT, Ayala JM, Miller DK. Activation of the native 45-kDa precursor form of interleukin-1-converting enzyme. J Biol Chem 1996;271:13273–13282. [DOI] [PubMed] [Google Scholar]
- 163.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev 2009;227:106–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009;7:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog 2010;6:e1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Thomas PG, Dash P, Aldridge JR Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 2009;30:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 2006;7:569–575. [DOI] [PubMed] [Google Scholar]
- 168.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol 2006;7:576–582. [DOI] [PubMed] [Google Scholar]
- 169.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci USA 2008;105:2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol 2007;37:3030–3039. [DOI] [PubMed] [Google Scholar]
- 171.Galle M, Schotte P, Haegman M, Wullaert A, Yang HJ, Jin S, Beyaert R. The Pseudomonas aeruginosa Type III secretion system plays a dual role in the regulation of caspase-1 mediated IL-1beta maturation. J Cell Mol Med 2008;12:1767–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med 2007;204:3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vinzing M, Eitel J, Lippmann J, Hocke AC, Zahlten J, Slevogt H, N'Guessan PD, Gunther S, Schmeck B, Hippenstiel S, et al. NAIP and Ipaf control Legionella pneumophila replication in human cells. J Immunol 2008;180:6808–6815. [DOI] [PubMed] [Google Scholar]
- 174.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol 2004;14:1929–1934. [DOI] [PubMed] [Google Scholar]
- 175.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 2006;440:233–236. [DOI] [PubMed] [Google Scholar]
- 176.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006;440:228–232. [DOI] [PubMed] [Google Scholar]
- 177.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol 2005;175:7611–7622. [DOI] [PubMed] [Google Scholar]
- 178.Harder J, Franchi L, Munoz-Planillo R, Park JH, Reimer T, Nunez G. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J Immunol 2009;183:5823–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 2007;26:433–443. [DOI] [PubMed] [Google Scholar]
- 180.Gasse P, Riteau N, Charron S, Girre S, Fick L, Petrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med 2009;179:903–913. [DOI] [PubMed] [Google Scholar]
- 181.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–241. [DOI] [PubMed] [Google Scholar]
- 182.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol 2009;183:2008–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nakane A, Minagawa T, Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun 1988;56:2563–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med 2004;10:842–848. [DOI] [PubMed] [Google Scholar]
- 186.Moks T, Abrahmsen L, Nilsson B, Hellman U, Sjoquist J, Uhlen M. Staphylococcal protein A consists of five IgG-binding domains. Eur J Biochem 1986;156:637–643. [DOI] [PubMed] [Google Scholar]
- 187.Gomez MI, O'Seaghdha M, Magargee M, Foster TJ, Prince AS. Staphylococcus aureus protein A activates TNFR1 signaling through conserved IgG binding domains. J Biol Chem 2006;281:20190–20196. [DOI] [PubMed] [Google Scholar]
- 188.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol 2004;172:3377–3381. [DOI] [PubMed] [Google Scholar]
- 189.Waters VJ, Gomez MI, Soong G, Amin S, Ernst RK, Prince A. Immunostimulatory properties of the emerging pathogen Stenotrophomonas maltophilia. Infect Immun 2007;75:1698–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sajjan US, Hershenson MB, Forstner JF, LiPuma JJ. Burkholderia cenocepacia ET12 strain activates TNFR1 signalling in cystic fibrosis airway epithelial cells. Cell Microbiol 2008;10:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol 1999;276:L715–L727. [DOI] [PubMed] [Google Scholar]
- 192.Lillehoj EP, Hyun SW, Kim BT, Zhang XG, Lee DI, Rowland S, Kim KC. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2001;280:L181–L187. [DOI] [PubMed] [Google Scholar]
- 193.Subauste MC, Proud D. Effects of tumor necrosis factor-alpha, epidermal growth factor and transforming growth factor-alpha on interleukin-8 production by, and human rhinovirus replication in, bronchial epithelial cells. Int Immunopharmacol 2001;1:1229–1234. [DOI] [PubMed] [Google Scholar]
- 194.Gomez MI, Seaghdha MO, Prince AS. Staphylococcus aureus protein A activates TACE through EGFR-dependent signaling. EMBO J 2007;26:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gomez MI, Sokol SH, Muir AB, Soong G, Bastien J, Prince AS. Bacterial induction of TNF-alpha converting enzyme expression and IL-6 receptor alpha shedding regulates airway inflammatory signaling. J Immunol 2005;175:1930–1936. [DOI] [PubMed] [Google Scholar]
- 196.Burgel PR, Lazarus SC, Tam DC, Ueki IF, Atabai K, Birch M, Nadel JA. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol 2001;167:5948–5954. [DOI] [PubMed] [Google Scholar]
- 197.Basbaum C, Li D, Gensch E, Gallup M, Lemjabbar H. Mechanisms by which gram-positive bacteria and tobacco smoke stimulate mucin induction through the epidermal growth factor receptor (EGFR). Novartis Found Symp 2002;248:171–176; discussion 176–180, 277–182. [PubMed] [Google Scholar]
- 198.Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 2003;100:11618–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen YT, Gallup M, Nikulina K, Lazarev S, Zlock L, Finkbeiner W, McNamara N. Cigarette smoke induces epidermal growth factor receptor-dependent redistribution of apical MUC1 and junctional beta-catenin in polarized human airway epithelial cells. Am J Pathol 2010;177:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Koff JL, Shao MX, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol 2008;294:L1068–L1075. [DOI] [PubMed] [Google Scholar]
- 201.Shaykhiev R, Behr J, Bals R. Microbial patterns signaling via Toll-like receptors 2 and 5 contribute to epithelial repair, growth and survival. PLoS ONE 2008;3:e1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nakanaga T, Nadel JA, Ueki IF, Koff JL, Shao MX. Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Physiol Lung Cell Mol Physiol 2007;292:L1289–L1296. [DOI] [PubMed] [Google Scholar]
- 203.Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem 1988;263:9557–9560. [PubMed] [Google Scholar]
- 204.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J 2005;272:6179–6217. [DOI] [PubMed] [Google Scholar]
- 205.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med 2002;196:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007;8:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 2007;8:39–46. [DOI] [PubMed] [Google Scholar]
- 208.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 2005;1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gavino AC, Chung JS, Sato K, Ariizumi K, Cruz PD Jr. Identification and expression profiling of a human C-type lectin, structurally homologous to mouse dectin-2. Exp Dermatol 2005;14:281–288. [DOI] [PubMed] [Google Scholar]
- 210.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD Jr, Ariizumi K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem 2006;281:38854–38866. [DOI] [PubMed] [Google Scholar]
- 211.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010;32:681–691. [DOI] [PubMed] [Google Scholar]
- 212.Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 2008;180:7404–7413. [DOI] [PubMed] [Google Scholar]
- 213.Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006;442:651–656. [DOI] [PubMed] [Google Scholar]
- 214.Bi L, Gojestani S, Wu W, Hsu YM, Zhu J, Ariizumi K, Lin X. CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans. J Biol Chem 2010;285:25969–25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dorhoi A, Desel C, Yeremeev V, Pradl L, Brinkmann V, Mollenkopf HJ, Hanke K, Gross O, Ruland J, Kaufmann SH. The adaptor molecule CARD9 is essential for tuberculosis control. J Exp Med 2010;207:777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lee HM, Yuk JM, Shin DM, Jo EK. Dectin-1 is inducible and plays an essential role for mycobacteria-induced innate immune responses in airway epithelial cells. J Clin Immunol 2009;29:795–805. [DOI] [PubMed] [Google Scholar]
- 217.Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am J Respir Cell Mol Biol 2005;32:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jones DE, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett 1993;315:187–192. [DOI] [PubMed] [Google Scholar]
- 219.Huang L, Ching CB, Jiang R, Leong SS. Production of bioactive human beta-defensin 5 and 6 in Escherichia coli by soluble fusion expression. Protein Expr Purif 2008;61:168–174. [DOI] [PubMed] [Google Scholar]
- 220.Yamaguchi Y, Nagase T, Makita R, Fukuhara S, Tomita T, Tominaga T, Kurihara H, Ouchi Y. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol 2002;169:2516–2523. [DOI] [PubMed] [Google Scholar]
- 221.Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, Conway BA, Greenberg EP, Valore EV, Welsh MJ, Ganz T, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA 1998;95:14961–14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.McCray PB Jr, Bentley L. Human airway epithelia express a beta-defensin. Am J Respir Cell Mol Biol 1997;16:343–349. [DOI] [PubMed] [Google Scholar]
- 223.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson JM. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest 1998;102:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Alekseeva L, Huet D, Femenia F, Mouyna I, Abdelouahab M, Cagna A, Guerrier D, Tichanne-Seltzer V, Baeza-Squiban A, Chermette R, et al. Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol 2009;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 2001;276:5707–5713. [DOI] [PubMed] [Google Scholar]
- 226.Xu Z, Zhong Z, Huang L, Peng L, Wang F, Cen P. High-level production of bioactive human beta-defensin-4 in Escherichia coli by soluble fusion expression. Appl Microbiol Biotechnol 2006;72:471–479. [DOI] [PubMed] [Google Scholar]
- 227.Duits LA, Nibbering PH, van Strijen E, Vos JB, Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS. Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol 2003;38:59–64. [DOI] [PubMed] [Google Scholar]
- 228.Jang BC, Lim KJ, Paik JH, Kwon YK, Shin SW, Kim SC, Jung TY, Kwon TK, Cho JW, Baek WK, et al. Up-regulation of human beta-defensin 2 by interleukin-1beta in A549 cells: involvement of PI3K, PKC, p38 MAPK, JNK, and NF-kappaB. Biochem Biophys Res Commun 2004;320:1026–1033. [DOI] [PubMed] [Google Scholar]
- 229.Tsutsumi-Ishii Y, Nagaoka I. Modulation of human beta-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J Immunol 2003;170:4226–4236. [DOI] [PubMed] [Google Scholar]
- 230.Wang X, Zhang Z, Louboutin JP, Moser C, Weiner DJ, Wilson JM. Airway epithelia regulate expression of human beta-defensin 2 through Toll-like receptor 2. FASEB J 2003;17:1727–1729. [DOI] [PubMed] [Google Scholar]
- 231.Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismuller KH, Godowski PJ, Ganz T, Randell SH, Modlin RL. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol 2003;171:6820–6826. [DOI] [PubMed] [Google Scholar]
- 232.Birchler T, Seibl R, Buchner K, Loeliger S, Seger R, Hossle JP, Aguzzi A, Lauener RP. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur J Immunol 2001;31:3131–3137. [DOI] [PubMed] [Google Scholar]
- 233.MacRedmond R, Greene C, Taggart CC, McElvaney N, O'Neill S. Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide. Respir Res 2005;6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Diamond G, Russell JP, Bevins CL. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA 1996;93:5156–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Scharf S, Vardarova K, Lang F, Schmeck B, Opitz B, Flieger A, Heuner K, Hippenstiel S, Suttorp N, N'Guessan PD. Legionella pneumophila induces human beta defensin-3 in pulmonary cells. Respir Res 2010;11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999;286:525–528. [DOI] [PubMed] [Google Scholar]
- 237.Chen CI, Schaller-Bals S, Paul KP, Wahn U, Bals R. Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J Cyst Fibros 2004;3:45–50. [DOI] [PubMed] [Google Scholar]
- 238.Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol 1999;31:645–651. [DOI] [PubMed] [Google Scholar]
- 239.Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, Schroder JM. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol 2000;22:714–721. [DOI] [PubMed] [Google Scholar]
- 240.Herr C, Beisswenger C, Hess C, Kandler K, Suttorp N, Welte T, Schroeder JM, Vogelmeier C. Suppression of pulmonary innate host defence in smokers. Thorax 2009;64:144–149. [DOI] [PubMed] [Google Scholar]
- 241.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta 2006;1758:1408–1425. [DOI] [PubMed] [Google Scholar]
- 242.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA 1998;95:9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bals R, Weiner DJ, Meegalla RL, Wilson JM. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest 1999;103:1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Barlow PG, Li Y, Wilkinson TS, Bowdish DM, Lau YE, Cosseau C, Haslett C, Simpson AJ, Hancock RE, Davidson DJ. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol 2006;80:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mendez-Samperio P, Miranda E, Trejo A. Expression and secretion of cathelicidin LL-37 in human epithelial cells after infection by Mycobacterium bovis Bacillus Calmette-Guerin. Clin Vaccine Immunol 2008;15:1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Braff MH, Jones AL, Skerrett SJ, Rubens CE. Staphylococcus aureus exploits cathelicidin antimicrobial peptides produced during early pneumonia to promote staphylokinase-dependent fibrinolysis. J Infect Dis 2007;195:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med 2002;165:992–995. [DOI] [PubMed] [Google Scholar]
- 248.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol 2003;171:6690–6696. [DOI] [PubMed] [Google Scholar]
- 249.Pistolic J, Cosseau C, Li Y, Yu JJ, Filewod NC, Gellatly S, Rehaume LM, Bowdish DM, Hancock RE. Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-kappaB signaling pathway. J Innate Immun 2009;1:254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rivas-Santiago B, Hernandez-Pando R, Carranza C, Juarez E, Contreras JL, Aguilar-Leon D, Torres M, Sada E. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun 2008;76:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sim SH, Liu Y, Wang D, Novem V, Sivalingam SP, Thong TW, Ooi EE, Tan G. Innate immune responses of pulmonary epithelial cells to Burkholderia pseudomallei infection. PLoS ONE 2009;4:e7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol 2009;123:612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Starner TD, Barker CK, Jia HP, Kang Y, McCray PB Jr. CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol 2003;29:627–633. [DOI] [PubMed] [Google Scholar]
- 254.Crane-Godreau MA, Maccani MA, Eszterhas SK, Warner SL, Jukosky JA, Fiering S. Exposure to cigarette smoke disrupts CCL20-mediated antimicrobial activity in respiratory epithelial cells. Open Immunol J 2009;2:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sirard JC, Didierlaurent A, Cayet D, Sierro F, Rumbo M. Toll-like receptor 5- and lymphotoxin beta receptor-dependent epithelial Ccl20 expression involves the same NF-kappaB binding site but distinct NF-kappaB pathways and dynamics. Biochim Biophys Acta 2009;1789:386–394. [DOI] [PubMed] [Google Scholar]
- 256.Torres D, Dieudonne A, Ryffel B, Vilain E, Si-Tahar M, Pichavant M, Lassalle P, Trottein F, Gosset P. Double-stranded RNA exacerbates pulmonary allergic reaction through TLR3: implication of airway epithelium and dendritic cells. J Immunol 2010;185:451–459. [DOI] [PubMed] [Google Scholar]
- 257.Travis SM, Conway BA, Zabner J, Smith JJ, Anderson NN, Singh PK, Greenberg EP, Welsh MJ. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol 1999;20:872–879. [DOI] [PubMed] [Google Scholar]
- 258.Wadstrom T, Hisatsune K. Bacteriolytic enzymes from Staphylococcus aureus: specificity of action of endo-beta-N-acetylglucosaminidase. Biochem J 1970;120:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Akinbi HT, Epaud R, Bhatt H, Weaver TE. Bacterial killing is enhanced by expression of lysozyme in the lungs of transgenic mice. J Immunol 2000;165:5760–5766. [DOI] [PubMed] [Google Scholar]
- 260.Dajani R, Zhang Y, Taft PJ, Travis SM, Starner TD, Olsen A, Zabner J, Welsh MJ, Engelhardt JF. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am J Respir Cell Mol Biol 2005;32:548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 1996;14:104–112. [DOI] [PubMed] [Google Scholar]
- 262.Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature 2002;417:552–555. [DOI] [PubMed] [Google Scholar]
- 263.Arnold RR, Cole MF, McGhee JR. A bactericidal effect for human lactoferrin. Science 1977;197:263–265. [DOI] [PubMed] [Google Scholar]
- 264.Ellison RT III, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest 1991;88:1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sagel SD, Sontag MK, Accurso FJ. Relationship between antimicrobial proteins and airway inflammation and infection in cystic fibrosis. Pediatr Pulmonol 2009;44:402–409. [DOI] [PubMed] [Google Scholar]
- 266.Thompson AB, Bohling T, Payvandi F, Rennard SI. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med 1990;115:148–158. [PubMed] [Google Scholar]
- 267.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 2008;321:1837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2010;2:29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 2010;143:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.