Abstract
Adenosine triphosphate (ATP) and its metabolite adenosine regulate airway mucociliary clearance via activation of purinoceptors. In this study, we investigated the contribution of goblet cells to airway epithelial ATP release. Primary human bronchial epithelial (HBE) cultures, typically dominated by ciliated cells, were induced to develop goblet cell metaplasia by infection with respiratory syncytial virus (RSV) or treatment with IL-13. Under resting conditions, goblet-cell metaplastic cultures displayed enhanced mucin secretion accompanied by increased rates of ATP release and mucosal surface adenosine accumulation as compared with nonmetaplastic control HBE cultures. Intracellular calcium chelation [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester] or disruption of the secretory pathways (nocodazole, brefeldin A, and N-ethylmaleimide) decreased mucin secretion and ATP release in goblet-cell metaplastic HBE cultures. Conversely, stimuli that triggered calcium-regulated mucin secretion (e.g., ionomycin or UTP) increased luminal ATP release and adenyl purine accumulation in control and goblet-cell metaplastic HBE cultures. Goblet cell–associated ATP release was not blocked by the connexin/pannexin hemichannel inhibitor carbenoxolone, suggesting direct nucleotide release from goblet cell vesicles rather than the hemichannel insertion. Collectively, our data demonstrate that nucleotide release is increased by goblet cell metaplasia, reflecting, at least in part, a mechanism tightly associated with goblet cell mucin secretion. Increased goblet cell nucleotide release and resultant adenosine accumulation provide compensatory mechanisms to hydrate mucins by paracrine stimulation of ciliated cell ion and water secretion and maintain mucociliary clearance, and to modulate inflammatory responses.
Keywords: goblet cell metaplasia, ATP release, mucin, airway epithelia, RSV
CLINICAL RELEVANCE.
This is the first study to demonstrate that goblet cells are important contributors of enhanced nucleotides and nucleosides in airway lumen during goblet cell metaplasia. Our data have relevance to a score of airway diseases characterized by goblet cell metaplasia, such as viral infection, asthma, and chronic obstructive pulmonary disease, by suggesting that increased goblet cell nucleotide release and the resultant adenosine accumulation provide compensatory mechanisms to hydrate mucins by paracrine stimulation of ciliated cell ion and water secretion and to modulate inflammatory responses.
Mucociliary clearance (MCC), a critical component of innate lung defense mediated by airway epithelia, requires coordination of airway surface liquid (ASL) hydration, ciliary beat, and mucin secretion. ATP and its metabolite adenosine are coordinators of these functions (1). ATP, released from airway epithelia to ASL, and adenosine activate epithelial cell surface P2Y2 and A2B purinoceptors, respectively, and regulate ion transport, maintain ASL hydration, and promote ciliary beat. The ATP-gated P2X4 receptor has also been proposed to regulate ion transport in airway epithelia (2, 3). In addition, ATP promotes mucin secretion via P2Y2 receptors expressed on goblet cells (1).
Airway epithelia are comprised of several different cell types, including basal, ciliated, and goblet cells. A crucial question in airway surface homeostasis is how goblet cells, which secrete mucins “dry” (i.e., without concurrent water secretion) (4), communicate with neighboring ciliated cells to secrete sufficient liquid for mucin hydration and maintenance of MCC. We hypothesize that goblet cells secrete ATP with mucins to signal to ciliated cells in a paracrine fashion, thus promoting ASL volume secretion. Indeed, previous studies illustrated that mucin granules of a goblet-like human airway epithelial Calu-3 cell line contain nucleotides (5) and that nucleotides are released from Calu-3 cells concomitantly with mucins (6). However, it is unknown whether nucleotide release is coordinated with mucin secretion in airway epithelia in vivo.
Well differentiated primary human bronchial epithelial (HBE) cell cultures simulate in vivo airway epithelia morphologically and physiologically. To test the hypothesis that airway epithelial goblet cells release ATP in association with mucins, well differentiated primary HBE cell cultures, typically dominated by ciliated cells, were studied under basal conditions and after maneuvers designed to produce goblet cell metaplasia. The first maneuver included infection of cultures with respiratory syncytial virus (RSV), a common respiratory pathogen among young children and elderly subjects with respiratory complications (e.g., chronic obstructive pulmonary disease). RSV induced massive goblet cell metaplasia in primary HBE cultures several weeks after infection. Second, goblet cell metaplasia was induced by IL-13, as previously described (7, 8). In each model, the links between mucin secretion and nucleotide release were tested under resting conditions and with pharmacological inhibition or stimuli, with a focus on granule-associated release pathways.
MATERIALS AND METHODS
Cell Culture
Primary HBE cultures were established from surgical specimens of main stem or lobar bronchi from healthy donors on Transwell supports (Corning, Lowell, MA) and maintained in an air–liquid interface (9). Use of the cells was approved by the University of North Carolina Institutional Review Boards.
RSV Infection of Cultures
Well differentiated HBE cultures were infected with a recombinant RSV expressing green fluorescent protein (GFP) (rgRSV) (10). RgRSV is similar to the parent wild-type RSV with respect to replication, tropism, and pathogenicity (10). RgRSV inactivated by UV light was used as a mock control. Viral infections were monitored by GFP expression in virus-infected cells. Cultures were studied at 3, 14, and 42 days after RSV infection.
IL-13 Treatment of Cultures
Well differentiated HBE cultures were treated with 10 ng/ml IL-13 (PeproTech, Rocky Hill, NJ) serosally for 5 days. ALI medium (made in house [9]) containing IL-13 was freshly prepared and replaced at each 48-hour time point. Cultures were studied at the end of the IL-13 treatment.
Cytokine Measurements
Twenty-four hours after fresh ALI medium (9) was added to the serosal side of cultures, serosal samples were collected for IL-8 measurements by ELISA (R&D Systems, Minneapolis, MN) (11).
ATP Measurements
ATP release was measured in real time using soluble luciferin (150 μM) (BD Biosciences, San Jose, CA) and luciferase (0.5 μg/culture) (Sigma, St. Louis, MO) in a Turner TD-20/20 luminometer (Turner Biosystems, Sunnyvale, CA) (12).
Measurements of Adenyl Purines
Cultures were rinsed bilaterally and preincubated for 2 hours with 300 μl mucosal Hanks' balanced salt solution. Mucosal samples were collected (100 μl), and ATP, adenosine diphosphate (ADP), adenosine monophosphate (AMP), and adenosine concentrations were measured by etheno-derivatization and HPLC analysis (13). In some experiments, UTP (100 μM) or ionomycin (5 μM) was added to the mucosal fluid 10 minutes before sample collection.
Measurement of Uridine Diphosphate Glucose
Cells were preincubated for 15 minutes mucosally with Hanks' balanced salt solution containing β,γ-methylene ATP (300 μM). Mucosal samples were collected (200 μl), and uridine diphosphate (UDP)-glucose was measured by HPLC analysis (17).
Mucin Secretion Measurements
Mucin secretion was measured by slot blot analysis or ELISA of the mucosal fluid (6, 14). The UNC-230 rabbit polyclonal anti-mucin common subunit antibody was used as a primary antibody in slot blots and ELISA to quantitate total polymeric mucins of all subtypes (14, 15).
Scoring of Goblet Cell Numbers
Immunohistochemistry was performed on the whole-mounted Transwells to differentially stain mucin-containing cells (by periodic acid-Schiff ) and ciliated cells (by monoclonal antitubulin antibody [Sigma]) (16). Horizontal (X-Y) dual images with differential interference contrast (to visualize cellular outlines) and laser (to visualize immunohistochemical signals) were obtained by laser confocal microscopy (LSM 510; Carl Zeiss, Oberkochen, Germany) for quantitation of goblet and nongoblet cell numbers.
Statistical Analysis
Data were expressed as mean values ± SE. Where appropriate, data were analyzed by Student's t test or ANOVA with GraphPad InStat software (GraphPad, La Jolla, CA). Statistical significance was defined as P < 0.05.
RESULTS
Enhanced Mucin Secretion, ATP Release, and Adenosine Accumulation in RSV-Induced Goblet-Cell Metaplastic Cultures
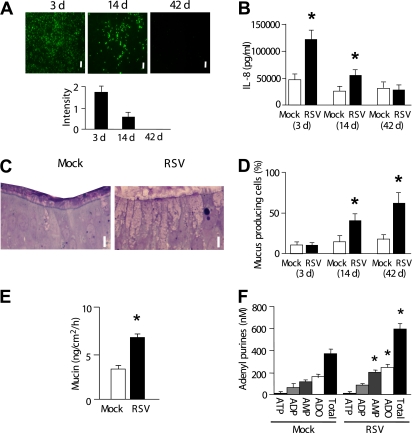
Well differentiated cultures of normal HBE cells are dominated by ciliated cells (typically ∼90%), which are specifically susceptible to RSV infection (10). After HBE cultures were exposed to a recombinant, GFP-bearing RSV (rgRSV), the number of infected cells, as indicated by GFP-associated fluorescence, was maximal at 3 days after infection and waned over 42 days (Figure 1A) (10). Airway epithelial inflammation, as measured by IL-8, was also maximal at 3 days after RSV infection and waned in parallel with the decrease in the number of virus-positive cells (Figure 1B). An increase in histologically defined goblet cell numbers was observed at 14 days after RSV infection (Figure 1C). Goblet cell numbers continued to increase at 42 days after infection, approaching approximately 50% of the cells, with a concomitant decrease in ciliated cell numbers, as quantitated by tubulin–periodic acid-Schiff staining (Figure 1D).
Figure 1.
Time course of respiratory syncytial virus (RSV) infection, RSV-induced goblet cell metaplasia, and extracellular adenyl purine profiles. The data were obtained at 3, 14, and 42 days after RSV (or mock) infection. (A) Top: Live intracellular RSV intensity visualized by green fluorescent protein fluorescence of the recombinant virus (rgRSV). Bar, 30 μm. Bottom: Quantitation of green fluorescent protein fluorescence intensity (by Image J). (B) IL-8 levels in serosal medium. (C) Representative x-z sectioned histological pictures (Richardson staining) of primary human bronchial epithelial cultures at 14 days after mock (left) or RSV (right) infection. Bar, 10 μm. (D) Percentages of mucus-producing cells (goblet cells) quantitated in laser confocal microscopy images of differential immunostaining for ciliated cells (red; tubulin) and goblet cells (green; periodic acid-Schiff) overlayed with differential interference contrast images. (E) Mucin secretion rates in control and RSV-induced goblet-cell metaplastic cultures as measured by ELISA over 12 hours. Mucosal surfaces were washed, and cultures were rested for 24 hours. At t = 0, 3, 6, 9, and 12 hours, 75 μl Dulbecco's modified Eagle's medium was added on cultures mucosally. Cultures were incubated for 10 minutes at 37°C and sampled for ELISA. Mucin secretion rates over 12 hours were calculated from the amount of mucins in samples at each time point. (F) Concentrations of adenyl purines in airway surface liquid sampled from resting cultures at 42 days after mock (left blocks) or RSV (right blocks) infection, as analyzed by etheno-derivatization. *Significant difference (P < 0.05) over mock-infected control cultures. Values are mean ± SE of four Transwells/subject established from three different subjects.
Consistent with the increase in goblet cell numbers, an increase in mucin release from resting cultures was detected at 42 days after RSV infection (Figure 1E). Mucosal ATP concentrations were not different between RSV- and mock-infected HBE cultures at any time point as measured by real-time luminometry or etheno-derivatization (∼1–5 nM) (Figure 1F). However, enhanced concentrations of AMP, adenosine, and total adenyl purine species were observed in RSV, as compared with mock-infected cultures, at 42 days after infection (Figure 1F). These results are consistent with the notion that goblet-cell metaplastic cultures exhibit increased release of ATP, ADP, and AMP (5) and that these nucleotides are rapidly metabolized to AMP or adenosine by ecto-ATPases (5, 12, 18, 19).
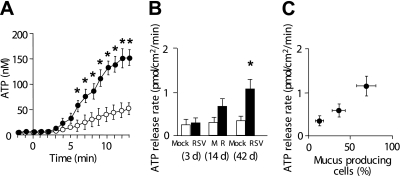
We examined the extent to which increased AMP and adenosine concentrations associated with goblet cell metaplasia reflected an increase in ATP release and ecto-hydrolysis at the cell surface. When cell surface ecto-ATPases were inhibited by the addition of ecto-ATPase inhibitors (β,γ-methylele-ATP, ebselen, and levamisole) to the luminal cell surface (Figure 2A) (12), ATP accumulated over time in ASL at similar rates in RSV- and mock-infected cultures at 3 and 14 days after infection (Figure 2B). However, the ATP accumulation rates from RSV-infected cultures increased up to approximately 3-fold as compared with mock-infected cultures at 42 days after infection (Figures 2A and 2B). ATP release rates were proportional to the increase in goblet cell numbers over the time course of RSV infection (Figure 2C).
Figure 2.
ATP release rates from cultures after RSV infection. (A) ATP accumulation in airway surface liquid when epithelial cell surface-mediated ATP hydrolysis was inhibited by the addition of ecto-ATPase inhibitors (β, γ-methylene-ATP [300 μM], ebselen [30 μM], and levamisole [10 mM]) to airway surface liquid at t = 0. Open circles and solid circles indicate mock- and RSV-infected cultures, respectively, at 42 days after infection. (B) ATP release rates in resting cultures at 3, 14, and 42 days after RSV (or mock) infection, as measured from ATP accumulation rates by using the method depicted in Fig. 2A and Ref. 12. (C) Correlation between goblet cell percentages (data imported from Fig. 1D) and ATP release rates (data imported from Fig. 2B). *Significant difference (P < 0.05) over mock-infected control cultures. Values are mean ± SE of four Transwells/subject established from three different subjects.
Enhanced ATP Release from IL-13–Induced Goblet-Cell Metaplastic Cultures
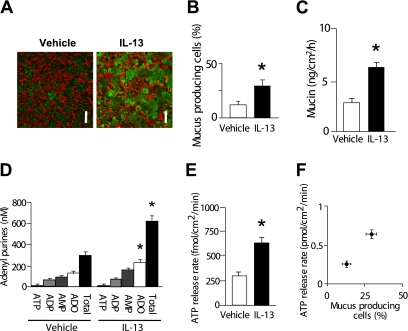
Viral infection can trigger cellular responses additional to goblet cell metaplasia, which may also contribute to the observed increment in nucleotide release. Thus, a “sterile” model (i.e., IL-13–induced goblet cell metaplasia) was used to independently validate the results illustrated above. After IL-13 treatment of HBE cultures for 5 days, an increase in goblet cell numbers (Figures 3A and 3B) was accompanied by increased mucin secretion (Figure 3C), increased adenosine and total adenyl purine accumulation in ASL (Figure 3D), and increased ATP release rates (Figure 3E). ATP release rates were proportional to goblet cell numbers (Figure 3F).
Figure 3.
Goblet cell metaplasia after IL-13 treatment. (A) Representative images of differential immunohistochemistry for ciliated cells (red; tubulin) and goblet cells (green; periodic acid-Schiff) after 5 days of treatment with vehicle (left) or IL-13 (right). Bar, 30 μm. (B) Goblet cell percentages in vehicle- or IL-13–treated cultures quantitated from the confocal images as represented in A. (C) Mucin secretion rates in control and IL-13–treated goblet-cell metaplastic cultures as measured by ELISA over 12 hours. (D) Concentrations of adenyl purines in airway surface liquid sampled from vehicle- or IL-13–treated cultures under resting condition, as measured by etheno-derivatization. (E) ATP release rates in vehicle- or IL-13–treated cultures. (F) Correlation between goblet cell percentages (data imported from B) and ATP release rates (data imported from E). *Significant difference (P < 0.05) over vehicle-treated control cultures. Values are mean ± SE of three Transwells/subject established from three different subjects.
Pharmacologic Inhibition of Vesicular Release under Basal Condition
Because goblet cell mucin granule secretion involves Ca2+–regulated exocytosis (20), we tested the effect of the Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester (BAPTA-AM) on mucin secretion and ATP release rates. BAPTA-AM inhibited mucin secretion in control (non–goblet-cell metaplastic) cultures and, to a greater extent, in RSV- or IL-13–treated goblet-cell metaplastic cultures (Figure 4A). ATP release rates were also inhibited by BAPTA-AM in control cultures and, to a greater extent, in goblet-cell metaplastic cultures (Figure 4B). Agents that disrupt the secretory pathway (e.g., nocodazole, which disrupts microtubules; brefeldin A, which dissembles the Golgi complex; and N-ethylmaleimide, which inhibits docking of secretory granules to plasma membrane) also inhibited mucin secretion in goblet-cell metaplastic cultures (Figure 4C). The effects of these inhibitors were not significant over sample variability in nonmetaplastic control cultures (Figure 4C). ATP release rates in response to each reagent mirrored the pattern of mucin secretion, that is secretory pathway inhibitors were effective in inhibiting ATP release in goblet-cell metaplastic, but not in control, cultures (Figure 4D).
Figure 4.
ATP and mucin secretion from goblet-cell metaplastic cultures. (A and B) Amounts of secreted mucins as measured by slot blot (A) and ATP release rates (B) in control, RSV-infected (42 d), or IL-13–treated cultures. These cultures were treated with BAPTA-AM (100 μM, 30 min) (black bars) or vehicle (white bars) immediately before the mucin or ATP measurement assays. (C and D) Amounts of secreted mucins as measured by slot blot (C) and ATP release rates (D) in control, RSV-infected, or IL-13–treated cultures. These cultures were treated with secretory pathway inhibitors: nocodazole (Noco; 20 μM, 4 h), brefeldin A (BFA) (40 μM, 2.5 h), and N-ethylmaleimide (NEM) (1 mM, 15 min) or vehicle immediately before the mucin or ATP measurement assays. For slot blots (A and C), 300 μl Hanks' balanced salt solution buffered with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid was added to mucosal culture surface, and 100 μl was sampled 15 minutes later. Signal intensity in slot blots was expressed as relative to that of mock-infected cultures without inhibitor treatment. *Significant difference (P < 0.05) between vehicle and the reagent treatment. †Significant difference (P < 0.05) over nonmetaplastic control subjects under the same reagent treatment. Values are mean ± SE of four Transwells/subject established from three different subjects.
Increased Nucleotide Release Coupled to Stimulated Mucin Secretion
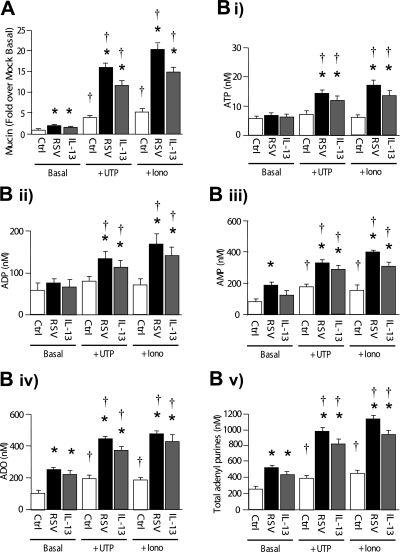
To further investigate the relationship between rates of mucin secretion and nucleotide release, we measured nucleotide release under conditions of agonist-promoted, Ca2+–regulated mucin granule secretion (21). As predicted on the basis of previous studies, apical treatment of control HBE cultures with UTP or ionomycin for 10 minutes resulted in increased secretion of mucins (Figure 5A) (6, 22–25). Mucin secretion in response to these agonists was markedly enhanced in RSV and IL-13–induced goblet-cell metaplastic cultures (Figure 5A).
Figure 5.
Enhanced nucleotide release associated with robust mucin secretion. (A) Mucin secretion after UTP (100 μM, 10 min) or ionomycin (5 μM, 10 min) treatment in control, RSV-infected (42 d), or IL-13–treated cultures. (B) Adenyl purine profiles at basal (left), UTP-treated (middle), or ionomycin-treated (right) conditions in control, RSV-infected (42 d), or IL-13–treated cultures. *Significant difference (P < 0.05) over nonmetaplastic control subjects. †Significant difference (P < 0.05) over basal values (i.e., without UTP or ionomycin treatment). Values are mean ± SE of three Transwells/subject established from three different subjects.
As observed with mucin secretion, UTP and ionomycin treatment increased AMP, adenosine, and total adenyl purine concentrations in ASL in control (non–goblet-cell metaplastic) cultures (Figure 5B), consistent with a robust increase in ATP/ADP/AMP release rates with rapid hydrolysis to metabolic products. In goblet-cell metaplastic (RSV or IL-13–induced) cultures, UTP and ionomycin treatment produced large increases in adenyl purine concentrations, including ATP and ADP, consistent with greatly increased rates of nucleotide release (Figure 5B). The patterns of increased release rates triggered by ionomycin or UTP were similar between mucin (Figure 5A) nucleotides/nucleosides (Figure 5B) in all cultures. The increase in nucleotide release in response to stimuli was proportional to goblet cell number and the increase in mucin secretion.
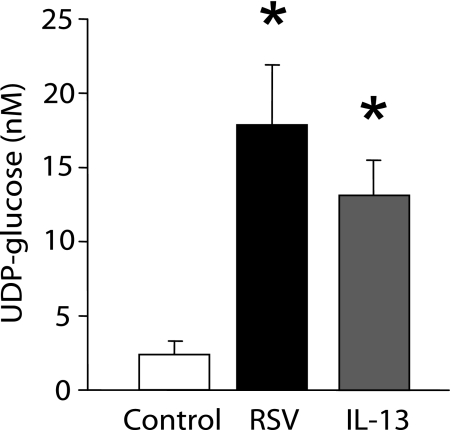
UDP-Glucose Release Rates Correlate Mucin Granule Release Rates
UDP-glucose is a sugar nucleotide that is a natural agonist for the P2Y14 receptor. Recent reports indicated that UDP-glucose is contained in Golgi-derived vesicles and released via an exocytotic mechanism (26, 27). Indeed, UDP-glucose has recently been shown to be released concomitantly with mucins from goblet-like Calu-3 cells, suggesting that the nucleotide–sugar release may be a marker for vesicular exocytosis of nucleotides (6). Thus, we investigated whether release of this nucleotide–sugar is increased in goblet-cell metaplastic cultures, consistent with the postulated granule-associated release of mucins and purine nucleotides. UDP-glucose concentrations in ASL were, in fact, significantly greater in goblet-cell metaplastic (RSV or IL-13–induced) cultures than in nonmetaplastic control HBE cultures (Figure 6).
Figure 6.
UDP-glucose release from goblet-cell metaplastic cultures. UDP-glucose concentrations in the sampled airway surface liquid from control, RSV-infected (42 d), or IL-13–treated cultures. After 2 hours of incubation with mucosal assay buffer, 300 μM β, γ-methylene ATP was added to airway surface liquid to inhibit UDP-glucose hydrolysis and was incubated for additional 15 minutes. *Significant difference over mock-infected cultures (P < 0.05). Values are mean ± SE of four Transwells/subject established from two different subjects.
Inhibition of Hemichannels Does Not Affect ATP Release from Goblet Cells
Cellular ATP release can occur via vesicular exocytosis or via conductive (i.e., channel- or transporter-mediated) pathways (28). Our observation of coordinated release of mucins and nucleotides suggests the involvement of vesicular secretory pathways in nucleotide release from goblet cells. However, our data do not distinguish between nucleotide release via vesicular pathways and vesicle-mediated insertion of a conductive pathway into the plasma membrane.
Connexin and pannexin hemichannels have been proposed to conduct ATP in well differentiated primary HBE cultures and in A549 lung epithelial cells based on the effect of pharmacological inhibitors (29). In addition, a recent study has identified pannexin 1 as a putative ATP release channel in primary human airway epithelia based on the effect of pharmacological inhibitors and knock-down experiments (30). In that study, Ransford and colleagues illustrated that primary HBE cultures displayed strong immunoreactivity against a 40-kD protein by Western blot using a pannexin 1 antibody and that ciliated cells in primary HBE cultures and human tracheal sections exhibited strong immunofluorescence by confocal microscopy using the same antibody. The immunofluorescence was also apparent, albeit diffuse, in cells that resembled (in morphology) native goblet cells in human tracheal sections, raising the possibility that pannexin 1 may contribute to ATP release from goblet cells.
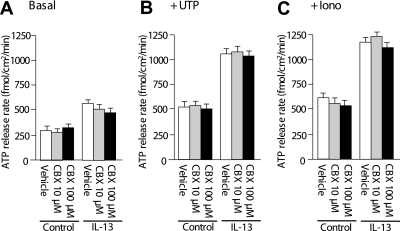
Therefore, we examined whether connexin and pannexin hemichannels contribute to ATP release in our model of goblet cell metaplasia. HBE cultures were treated with carbenoxolone, a nonselective blocker of connexin and pannexin hemichannels. Preincubation of HBE cultures with carbenoxolone for 15 minutes at 10 and 100 μM, to inhibit pannexins and connexins, respectively, did not affect ATP release from control or IL-13–induced goblet-cell metaplastic HBE cultures under resting conditions (Figure 7A). ATP release under stimulation with UTP (Figure 7B) or ionomycin (Figure 7C) was also not affected by carbenoxolone in control or IL-13–induced goblet-cell metaplastic cultures. These observations suggest that connexin and pannexin hemichannels are not involved in ATP release that is coupled with mucin secretion.
Figure 7.
ATP release from human bronchial epithelial cultures treated with connexin or pannexin hemichannels. (A) Control and IL-13–treated goblet-cell metaplastic cultures at resting conditions were preincubated with carbenoxolone (CBX) of indicated concentrations or vehicle for 15 minutes, and ATP release rates were measured. (B and C) After the preincubation with CBX or vehicle, cultures were stimulated with UTP (100 μM) (B) or ionomycin (5 μM) (C), and ATP release rates were measured. Values are mean ± SE of three Transwells/subject established from three different subjects.
DISCUSSION
Our study demonstrated an association between goblet cell metaplasia and increased rates of nucleotide release in a model relevant to in vivo airway epithelia, primary HBE cultures. Our data further demonstrated that nucleotide release occurs coordinately with mucin secretion and that goblet cells can be important contributors to nucleotide and nucleoside concentrations in ASL. Nucleotide release coordinated with mucin secretion enables goblet cells to signal neighboring ciliated cells via purinoceptors to increase ion and liquid secretion and, hence, properly hydrate newly released mucins.
Goblet cell metaplasia was induced in primary HBE cultures via two independent approaches, RSV infection and IL-13 treatment. In both models, ATP release and mucin secretion rates directly correlated with goblet cell number (Figures 2C and 3). We also observed that nonstimulated (i.e., “basal”) mucin secretion and ATP release rates were similarly reduced by an intracellular Ca2+ chelator, BAPTA-AM (Figures 4A and 4B). These data suggest that mucin secretion and ATP release from resting goblet cells are not completely “constitutive” (i.e., independent of regulation by second messengers) but likely consisted of “constitutive” and “basally Ca2+–regulated” components. We speculate that both processes may reflect basally regulated rates of granule release as proposed by Davis and Dickey (20).
ATP release and mucin secretion were similarly inhibited by reagents that inhibit secretory pathways (e.g., nocodazole, brefeldin A, and N-ethylmaleimide) in goblet-cell metaplastic cultures (Figures 4C and 4D). Conversely, agonists that increased mucin exocytosis and secretion (i.e., UTP and ionomycin) (Figure 5A) also increased nucleotide release in control (non–goblet-cell metaplastic) cultures and to a greater extent in goblet-cell metaplastic cultures (Figure 5B). The increase in nucleotide release in response to stimuli was in proportion to the goblet cell numbers and the increase in mucin secretion. Because the use of a pharmacological agent could elicit off-target effects, it is important to confirm the results with several different agents or methods.
Our recent studies indicate that a nucleotide–sugar UDP-glucose is secreted concomitantly with mucins from goblet-like cell lines (6). Thus, although the focus of this study was on the release of adenine nucleotides that enhance MCC functions, we investigated a correlation between goblet cell metaplasia and release of a nucleotide sugar that is known to be secreted via a vesicular-mediated mechanism (27). Goblet-cell metaplastic cultures released UDP-glucose at greater rates than nonmetaplastic control cultures, strengthening the notion that increased vesicular-mediated release produced paralleled increases in mucin and nucleotide sugar concentrations in ASL (Figure 6). UDP-glucose may modulate airway luminal innate immune responses, given that its receptor, P2Y14, is expressed in neutrophils, lymphocytes, and macrophages (31–33) and that UDP-glucose has been reported to promote secretion of inflammatory mediators from lung epithelial cell lines (34).
The simplest hypothesis to account for the association between nucleotide release and mucin secretion is that nucleotides and mucins are contained in common granules and are released when vesicles fuse with the apical membrane. Recent studies have supported this hypothesis by demonstrating that subapical granules of goblet-like cell lines contain mucins and a spectrum of adenyl nucleotides (5) and are apically secreted via Ca2+–regulated exocytosis, producing simultaneous mucin secretion and nucleotide release (6). The increased concentrations of a range of adenyl purine species on airway surfaces with goblet cell metaplasia (Figure 5B) could reflect selective release of ATP from mucin granules with extensive ecto-ATP metabolism. Conversely, predictions from mathematical models (18) and data from isolated mucin granules from the Calu-3 lung epithelial cell line (5) suggest that granules exocytotically release a spectrum of adenyl nucleotides (ATP, ADP, and AMP).
An alternative hypothesis is that ATP release channels could be expressed in the mucin granule membrane and inserted into the plasma membrane during granule exocytosis. However, carbenoxolone, an inhibitor of the primary candidate for ATP release channels in airway epithelia (i.e., connexin and pannexin hemichannels) (29, 30), failed to affect basal or stimulated ATP release from control or goblet-cell metaplastic cultures (Figure 7). Thus, it is unlikely that ATP release from airway epithelial goblet cells is predominantly mediated by vesicular insertion of connexin and pannexin ATP-releasing hemichannels.
Primary HBE cultures, reflecting in vivo airway morphology, are dominated by ciliated cells (∼90%) under normal conditions. Data that ATP release rates (in the absence of shear stress) correlated with the number of mucus-producing cells (Figures 2C and 3F) illustrate that mucus cells are a major contributor to ATP release under such conditions. Data that HBE cultures with 10 and 70% goblet cells released 0.3 and 1.2 pmol/cm2/min of ATP, respectively (Figure 2C), suggest that, if the correlation between the percentage of goblet cells and ATP release rates is linear, cultures with 0 and 100% goblet cells would release 0.15 and 1.65 pmol/cm2/min of ATP, respectively. Thus, it is estimated that goblet cells contributed to ATP release approximately 11 times more than ciliated cells under resting conditions.
On the other hand, airway cells in vivo are constantly exposed to shear stress (e.g., from breathing and coughing). Mechanisms of ATP release and contributions of ciliated versus goblet cells to ATP release under shear stress are likely to be different from those in the absence of shear stress. A previous study demonstrated that shear stress caused an approximately 30-fold increase in ATP release as compared with resting conditions (i.e., to a rate of 9 pmol/cm2/min) in ciliated cell–dominant (∼90%) primary human airway cultures (19). These data suggest that ciliated cells are a significant contributor to ATP release under shear stress.
Our data may have relevance to chronic airway diseases. For example, increased ATP release and adenosine accumulation on the surfaces of goblet-cell metaplastic airway epithelia may be particularly pertinent to asthma pathogenesis. Increased airway adenosine concentrations are a hallmark of asthma (35, 36), and increased airway ATP concentrations have been speculated to play a key role in asthma pathogenesis by activating dendritic cell functions (37). However, the source for extracellular airway ATP and adenosine in asthma has been unclear. Our studies, using IL-13–treated cultures as a model of asthma-induced goblet cell metaplasia, suggest that metaplastic goblet cells could represent an important source of ASL nucleotides under stimulated conditions (Figure 5B).
Our data may also be relevant to viral airways infection. Common acute viral infections (e.g., RSV) produce respiratory symptoms consistent with mucus hyperproduction and inflammation that may persist for many weeks after initial infection (38, 39). Data from models that span this time frame (e.g., virus-infected mice and primary HBE cultures) suggest that this late postviral phase is associated with goblet cell metaplasia (40, 41). However, the cellular origins of increased mucus production and inflammatory signals that produce the postviral syndrome have not been unambiguously identified. The current study demonstrated that RSV induces goblet cell metaplasia in HBE cultures, which not only provides a source of increased mucin secretion but also increases ASL nucleotides and nucleosides that may participate in inflammatory signaling (Figures 1 and 2) (42–50).
In summary, nucleotide release rates increase in parallel to mucin secretion rates in RSV- and IL-13–induced goblet-cell metaplastic HBE. The increase in goblet-cell nucleotide release proportional to increased mucin secretion may provide a compensatory mechanism to hydrate newly secreted mucus and promote airway surface clearance. In parallel, increased release into the extracellular environment of adenine nucleotides and nucleosides may promote airway luminal inflammatory responses via purinoceptors by stimulating secretion of inflammatory mediators from airway epithelia (42–44), secretion of airway and alveolar fluids (19, 45), mucin gene transcription (43, 46), neutrophil migration (47, 48), and regulating endothelial barrier function (49, 50). Accordingly, goblet cell–mediated increases in ATP, adenosine, and UDP-glucose concentrations in airway lumens may coordinately activate innate host defense mucociliary clearance and cellular inflammatory pathways. These compensatory mechanisms are parts of the complex pathophysiologic scheme pertinent to many chronically diseased airways characterized by goblet cell metaplasia.
Acknowledgments
The authors thank Ms. Catharina van Heusden for the etheno-derivatization and HPLC analysis of nucleotides and nucleosides, the UNC Immunotechnology Core for cytokine measurement, Dr. Scott Randell and the Cystic Fibrosis Center Tissue Core for supplying primary HBE cell cultures, and the UNC Michael Hooker Microscope Facility for the microscopy studies.
This work was supported by Cystic Fibrosis Foundation grants OKADA06I0 (S.F.O) and R026-CR07 (R.C.B) and by National Institutes of Health grants P01 HL034322 (R.C.B. and E.R.L), P30 DK065988, R01 HL092964, and PS0 HL084934 (R.C.B.).
Originally Published in Press as DOI: 10.1165/rcmb.2010-0253OC on October 8, 2010
Author Disclosure: L.H.A. received a sponsored grant from CFF ($10,001–$50,000). R.C.B. received consultancy fees from Gilead Sciences ($1001–$5000), Pulmatrix ($5001–$10,000), and Parion Sciences and Inspire Pharmaceuticals ($10,001–$50,000 each). R.C.B. serves on the advisory board of Parion Sciences for $10,001 to $50,000 and owns stock in Parion Sciences and Inspire Pharmaceuticals for more than $100,001 each. S.M.K. received sponsored grants from Cystic Fibrosis Foundation ($50,001–$100,000) and NIH and AstraZeneca (more than $100,001 each). E.R.L. received a sponsored grant from NIH (more than $100,001). S.F.O. received sponsored grants from Inspire Pharmaceuticals ($10,001–$50,000) and Cystic Fibrosis Foundation ($10,001–$50,000). R.P. received sponsored grants from Cystic Fibrosis Foundation ($50,001–$100,000) and from NIH (more than $100,001). C.W.D. received sponsored grants from Syntaxin Ltd. and AstraZeneca ($10,001–$50,000 each).
References
- 1.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol 2009;9:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zsembery A, Fortenberry JA, Liang L, Bebok Z, Tucker TA, Boyce AT, Braunstein GM, Welty E, Bell PD, Sorscher EJ, et al. Extracellular zinc and ATP restore chloride secretion across cystic fibrosis airway epithelia by triggering calcium entry. J Biol Chem 2004;279:10720–10729. [DOI] [PubMed] [Google Scholar]
- 3.Zsembery A, Boyce AT, Liang L, Peti-Peterdi J, Bell PD, Schwiebert EM. Sustained calcium entry through p2x nucleotide receptor channels in human airway epithelial cells. J Biol Chem 2003;278:13398–13408. [DOI] [PubMed] [Google Scholar]
- 4.Verdugo P, Deyrup-Olsen I, Aitken M, Villalon M, Johnson D. Molecular mechanism of mucin secretion: I. The role of intragranular charge shielding. J Dent Res 1987;66:506–508. [DOI] [PubMed] [Google Scholar]
- 5.Kreda SM, Seminario-Vidal L, van Heusden CA, O'Neal W, Jones L, Boucher RC, Lazarowski ER. Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol 2010. [DOI] [PMC free article] [PubMed]
- 6.Kreda SM, Okada SF, van Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 2007;584:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atherton HC, Jones G, Danahay H. Il-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol 2003;285:L730–L739. [DOI] [PubMed] [Google Scholar]
- 8.Laoukili J, Perret E, Willems T, Minty A, Parthoens E, Houcine O, Coste A, Jorissen M, Marano F, Caput D, et al. Il-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest 2001;108:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 2005;107:183–206. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 2002;76:5654–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O'Neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem 2005;280:17798–17806. [DOI] [PubMed] [Google Scholar]
- 12.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 2006;281:22992–23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (vdac-1) contributes to atp release and cell volume regulation in murine cells. J Gen Physiol 2004;124:513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol 2008;586:1977–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheehan JK, Boot-Handford RP, Chantler E, Carlstedt I, Thornton DJ. Evidence for shared epitopes within the ‘naked’ protein domains of human mucus glycoproteins: a study performed by using polyclonal antibodies and electron microscopy. Biochem J 1991;274:293–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi AH, Salmon WC, Chua M, Davis CW. Calcium signaling in human airway goblet cells following purinergic activation. Am J Physiol Lung Cell Mol Physiol 2007;292:L92–L98. [DOI] [PubMed] [Google Scholar]
- 17.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol 2003;63:1190–1197. [DOI] [PubMed] [Google Scholar]
- 18.Zuo P, Picher M, Okada SF, Lazarowski ER, Button B, Boucher RC, Elston TC. Mathematical model of nucleotide regulation on airway epithelia. Implications for airway homeostasis. J Biol Chem 2008;283:26805–26819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, et al. Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem 2005;280:35751–35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol 2008;70:487–512. [DOI] [PubMed] [Google Scholar]
- 21.Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol 2008;163:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KC, Park HR, Shin CY, Akiyama T, Ko KH. Nucleotide-induced mucin release from primary hamster tracheal surface epithelial cells involves the P2u purinoceptor. Eur Respir J 1996;9:1579. [PubMed] [Google Scholar]
- 23.Ko KH, Lee CJ, Shin CY, Jo M, Kim KC. Inhibition of mucin release from airway goblet cells by polycationic peptides. Am J Physiol 1999;277:L811–L815. [DOI] [PubMed] [Google Scholar]
- 24.Abdullah LH, Davis CW. Regulation of airway goblet cell mucin secretion by tyrosine phosphorylation signaling pathways. Am J Physiol Lung Cell Mol Physiol 2007;293:L591–L599. [DOI] [PubMed] [Google Scholar]
- 25.Abdullah LH, Davis SW, Burch L, Yamauchi M, Randell SH, Nettesheim P, Davis CW. P2u purinoceptor regulation of mucin secretion in spoc1 cells, a goblet cell line from the airways. Biochem J 1996;316:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esther CR Jr, Sesma JI, Dohlman HG, Ault AD, Clas ML, Lazarowski ER, Boucher RC. Similarities between UDP-glucose and adenine nucleotide release in yeast: involvement of the secretory pathway. Biochemistry 2008;47:9269–9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sesma JI, Esther CR Jr, Kreda SM, Jones L, O'Neal W, Nishihara S, Nicholas RA, Lazarowski ER. Endoplasmic reticulum/golgi nucleotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem 2009;284:12572–12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnstock G. Unresolved issues and controversies in purinergic signalling. J Physiol 2008;586:3307–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seminario-Vidal L, Kreda S, Jones L, O'Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J Biol Chem 2009;284:20638–20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 2009;41:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in human neutrophils. Eur J Pharmacol 2006;543:166–173. [DOI] [PubMed] [Google Scholar]
- 32.Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in murine T-lymphocytes. Br J Pharmacol 2005;146:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myrtek D, Muller T, Geyer V, Derr N, Ferrari D, Zissel G, Durk T, Sorichter S, Luttmann W, Kuepper M, et al. Activation of human alveolar macrophages via p2 receptors: coupling to intracellular Ca2+ increases and cytokine secretion. J Immunol 2008;181:2181–2188. [DOI] [PubMed] [Google Scholar]
- 34.Muller T, Bayer H, Myrtek D, Ferrari D, Sorichter S, Ziegenhagen MW, Zissel G, Virchow JC Jr, Luttmann W, Norgauer J, et al. The P2Y14 receptor of airway epithelial cells: coupling to intracellular Ca2+ and IL-8 secretion. Am J Respir Cell Mol Biol 2005;33:601–609. [DOI] [PubMed] [Google Scholar]
- 35.Csoma Z, Huszar E, Vizi E, Vass G, Szabo Z, Herjavecz I, Kollai M, Horvath I. Adenosine level in exhaled breath increases during exercise-induced bronchoconstriction. Eur Respir J 2005;25:873–878. [DOI] [PubMed] [Google Scholar]
- 36.Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar Vilagos G, Herjavecz I, Horvath I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J 2002;20:1393–1398. [DOI] [PubMed] [Google Scholar]
- 37.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 2007;13:913–919. [DOI] [PubMed] [Google Scholar]
- 38.Kesson AM. Respiratory virus infections. Paediatr Respir Rev 2007;8:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lugo RA, Nahata MC. Pathogenesis and treatment of bronchiolitis. Clin Pharm 1993;12:95–116. [PubMed] [Google Scholar]
- 40.Miller AL, Gerard C, Schaller M, Gruber AD, Humbles AA, Lukacs NW. Deletion of CCR1 attenuates pathophysiologic responses during respiratory syncytial virus infection. J Immunol 2006;176:2562–2567. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, Pickles RJ. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol 2005;79:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Z, Bettega K, Carroll S, Buchholz KR, Machen TE. Role of Ca2+ in responses of airway epithelia to Pseudomonas aeruginosa, flagellin, ATP, and thapsigargin. Am J Physiol Lung Cell Mol Physiol 2007;292:L353–L364. [DOI] [PubMed] [Google Scholar]
- 43.McNamara N, Gallup M, Sucher A, Maltseva I, McKemy D, Basbaum C. AsialoGM1 and TLR5 cooperate in flagellin-induced nucleotide signaling to activate Erk1/2. Am J Respir Cell Mol Biol 2006;34:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribeiro CM, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic fibrosis airway epithelial Ca2+ i signaling: the mechanism for the larger agonist-mediated Ca2+ i signals in human cystic fibrosis airway epithelia. J Biol Chem 2005;280:10202–10209. [DOI] [PubMed] [Google Scholar]
- 45.Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 2004;286:L112–L120. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto K, Graham BS, Ho SB, Adler KB, Collins RD, Olson SJ, Zhou W, Suzutani T, Jones PW, Goleniewska K, et al. Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am J Respir Crit Care Med 2004;170:306–312. [DOI] [PubMed] [Google Scholar]
- 47.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem 2008;283:28480–28486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 2006;314:1792–1795. [DOI] [PubMed] [Google Scholar]
- 49.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res 2006;99:1100–1108. [DOI] [PubMed] [Google Scholar]
- 50.Weissmuller T, Eltzschig HK, Colgan SP. Dynamic purine signaling and metabolism during neutrophil-endothelial interactions. Purinergic Signal 2005;1:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]