Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a potent environmental toxicant. Epidemiological studies have associated TCDD exposure with the development of chronic obstructive pulmonary disease, which is manifested by mucous/goblet cell hyperplasia. The purpose of this research was to elucidate the pathway/mechanisms that lead to TCDD-induced gene expression in both primary normal human bronchial epithelial cells and an immortalized cell line, HBE1, under air–liquid interface conditions. TCDD exposure induced a time-dependent elevation of MUC5AC mRNA and protein synthesis, and cytochrome p450 1A1 (CYP1A1) expression in these cells. Treatment with an aryl hydrocarbon receptor antagonist had no effect on TCDD-induced MUC5AC expression, but significantly suppressed CYP1A1 induction. However, treatments with inhibitors of signaling pathways and the expression of dominant negative mutants of epidermal growth factor receptor (EGFR), extracellular signal–regulated kinase (ERK) and p38, but not the inhibition of c-Jun N-terminal kinase pathway, abrogated MUC5AC induction, but not that of CYP1A1. These effects also occurred at the MUC5AC promoter–reporter level using the chimeric construct for a transient transfection study. Western blot analysis confirmed the phosphorylation of activated EGFR, ERK, and p38 signaling molecules, but not the c-Jun N-terminal kinase, in cells after TCDD exposure. Specificity protein 1 (Sp1) phosphorylation also occurred in cells after TCDD exposure. Both MUC5AC expression and the promoter activity were inhibited by mithramycin A, an inhibitor specific to Sp1-based transcription. These results lead to the conclusion that TCDD induced MUC5AC expression through a noncanonical aryl hydrocarbon receptor–independent, EGFR/ERK/p38–mediated signaling pathway–mediated/Sp1-based transcriptional mechanism.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin; aryl hydrocarbon receptor; airways; mucin; signaling
CLINICAL RELEVANCE.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is one of the most toxic man-made chemical that the exposure has caused the development of lung disease of chronic obstructive pulmonary diseases (COPD). This study demonstrates that TCDD can also stimulate airway mucin gene expression. This effect is consistent with the pathogenesis of COPD development. A better understanding of the signaling pathways and molecular mechanism in the regulation may provide the therapeutic targets for the clinical intervention of the lung pathogenesis due to TCDD exposure.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is one of the most toxic man-made chemicals, and is ubiquitously present in the environment. It is a member of the halogenated aromatic hydrocarbon family that also includes dibenzo-p-dioxins, dibenzofurans, biphenyls, and related compounds (1). TCDD has been studied extensively, and is known to have many toxicological effects, including tumorigenesis, immunotoxicity, reproductive and developmental toxicity, and wasting syndrome (2). Epidemiological reports have also associated TCDD exposure with an increased incidence of chronic obstructive pulmonary disease (COPD) (3–6). The most comprehensive epidemiological study follows the mortality of a large Italian population after an industrial accident released large amounts of TCDD into the environment in 1976. This investigation demonstrated a higher incidence of lymphatic and hematopoietic neoplasms, in addition to an increase in COPD. One of the major clinical hallmarks of COPD is mucous/goblet cell hyperplasia in the airways (6).
Classically, TCDD functions through the aryl hydrocarbon receptor (AhR) (7–11). According to this model, TCDD binds to cytoplasmic AhR, which associates with heat shock protein 90 and X-associated protein 2, resulting in nuclear translocation of the complex. Once in the nucleus, dissociation of heat shock protein 90 and X-associated protein 2 leads to dimerization of the AhR nuclear translocator. This heterodimer then recognizes a specific DNA sequence, the dioxin response element of 5′-TNGCGTG-3′ in the 5′ region of the target gene, which in turn regulates downstream gene expression (12, 13). An example of classical AhR-dependent gene regulation is TCDD-induced cytochrome p450 1A1 (CYP1A1) expression. Although it is less well characterized, TCDD can also activate epidermal growth factor receptor (EGFR) and its downstream mitogen-activated protein kinase (MAPK) pathways (14, 15).
In this study, we demonstrate that TCDD could stimulate MUC5AC mRNA and protein expression and CYP1A1 expression, but not MUC5B, in human bronchial epithelial (HBE) cells and cell line HBE1. Both MUC5AC and MUC5B are two major gel-forming mucins, which are frequently elevated in various airway diseases (16–18). The regulation of these mucin genes in airway epithelial cells has been extensively studied. These studies have led to the identification of the involvement of EGFR/MAPK signaling pathways and various putative transcriptional factor–binding sites responsible for cytokine/mediator-enhanced gene expression. Our recent work has demonstrated the importance of proximal specificity protein 1 (Sp1) sites at both MUC5AC and MUC5B promoter regions, and that they are involved in both the basal and enhanced transcriptions of these genes (19, 20). Using inhibitors as well as the transfection with dominant negative (dn) mutants of signaling molecules, we have found that the signaling pathways involved in TCDD-induced MUC5AC expression differ from that involved in CYP1A1 induction. For TCDD-induced MUC5AC, it occurs via an AhR-independent EGFR/extracellular signal–regulated kinase (ERK)/p38 signaling pathway–activated/Sp1-based transcriptional mechanism.
MATERIALS AND METHODS
Cell Culture
Human airway tissues were obtained from National Disease Research Interchange Inc. (Philadelphia, PA) with informed consent. The protocol was reviewed and approved periodically by University California at Davis (Davis, CA) Human Subjects Review Committee. Well differentiated primary normal HBE (NHBE) cells were prepared from these airway tissues and cultured under air–liquid interface conditions as previously described (21–23). An identical culture condition was performed for an immortalized NHBE cell line, HBE1, obtained from Dr. Yankaskas at the University of North Carolina (24). For treatments, TCDD and chemicals were added to air–liquid interface cultures in both apical and basal media.
Western Blot Analysis
Western blots (50–150 μg protein/lane) were performed as described previously (19, 20, 23), and probed with monoclonal antibodies specific for phosphorylated and unphosphorylated c-Jun N-terminal kinase (JNK), p38, ERK, EGFR, and Sp1 proteins (Cell Signaling, Danvers, MA), β-tubulin, and MUC5AC (clone 45M1; Santa Cruz Biotechnology, Santa Cruz, CA). Expression was normalized against the loading control protein β-tubulin and band intensities were quantitatively measured using a densitometer with multi-gauge software (Fujifilm, FTPAL, Palo Alto, CA).
Inhibitor and Transfection Studies
Inhibitors used in the study were from Sigma-Aldrich (St. Louis, MO), and were: mithramycin A, a specific Sp1 transcription factor inhibitor; AG1478, a specific EGFR inhibitor; SP600125, a specific JNK inhibitor; SB203580, a specific p38 inhibitor; and PD98059, a specific inhibitor of MAPK (also known as ERK) kinase−1. AhR antagonist, CH-223191, was obtained from Calbiochem (San Diego, CA). These chemicals and control vehicle were added to cultures 30 minutes before TCDD treatment.
Expression vectors for the dn mutants of JNK1 (APF), JNK2, and p38 (AGF) were kindly provided by Roger Davis (University of Massachusetts, Worcester, MA), and have been described previously (20, 25). dnERK1 (Lys71 → Arg) and dnERK2 (Lys52 → Arg) mutants were generously provided by Dr. Melanie Cobb (University of Texas Southwestern Medical Center at Dallas, Dallas, TX). Transfections were performed using fugene 6 (Roche Diagnostics Inc., Indianapolis, IN) in Opti-MEM (Invitrogen Inc., Carlsbad, CA), as described previously (20, 21).
The MUC5AC 3.7-kb promoter–firefly luciferase reporter construct was kindly provided by Dr. J. D. Li at the Department of Microbiology and Immunology, Rochester University Medical Center (Rochester, NY) (26, 27). Luciferase promoter studies were performed as described previously (19, 20).
RNA Isolation and Real-Time RT-PCR
RNA isolation and real-time RT-PCR quantification of gene expression were performed as described previously (19, 20, 23). The primer sequences for the genes are: β-actin, forward, 5′-AGTCGGTTGGAGCGAGCAT-3′, and reverse, 5′-AAAGTCCTCGGCCACATTGT-3′; CYP1A1, forward, 5′- ACCCTCCAGAGACAACAGGTAA-3′, and reverse, 5′-TAAGGACCTCCTAACCCTAGCA-3′; MUC5AC, forward, 5′-GGAACTGTGGGGACAGCTCTT-3′, and reverse, 5′- GTCACATTCCTCAGCGAGGTC-3′ and MUC5B forward, 5′-GCTGCTGCTACTCCTGTGAGG-3′, and reverse, 5′-AGGTGATGTTGACCTCGGTCTC-3′. The data are presented as fold induction after normalization to β-actin compared with the control condition.
Statistical Analysis
Data are expressed as mean (±SD). Experiments were conducted in triplicate, and repeated in at least three independent primary cultures and different passages. Statistical evaluation of data was performed by using the two-tailed Student's t test for unpaired values. Differences were considered statistically significant at a P value less than 0.05.
RESULTS
AhR-Independent and -Dependent Stimulation of MUC5AC and CYP1A1 Gene Expression
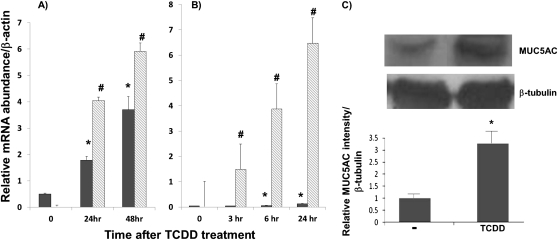
TCDD (10 nM) stimulated both MUC5AC and CYP1A1 expression in a time-dependent fashion in both primary NHBE and HBE1 cultures (Figures 1A and 1B, respectively). In the case of MUC5AC, significant up-regulation was observed at 24 and 48 hours (three- to sevenfold, respectively). This up-regulation was also observed at the protein level (Figure 1C). In the case of CYP1A1, maximal stimulation (97- to 254-fold induction) occurred between 3 and 6 hours. In contrast, TCDD has no stimulatory effect on MUC5B expression in these studies (data not included).
Figure 1.
Time course–dependent, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)–induced MUC5AC and cytochrome p450 1A1 (CYP1A1) gene expression. Primary normal human bronchial epithelial (NHBE) cells (A) and HBE1 cell line (B) were treated with 10 nM TCDD, and RNA was isolated at the various time points, as indicated, followed by the quantitative real-time RT-PCR analysis. Filled and shaded bars indicate relative MUC5AC and CYP1A1 messages after normalization with β-actin, respectively. (C) Western blot analysis of MUC5AC glycoprotein in cell extracts of NHBE cells after TCDD (10 nM) treatment. After normalization with β-tubulin band, the relative intensity of MUC5AC protein band is expressed in the graph below the Western blot. Values are expressed as the mean (±SD) from triplicate samples from one representative experiment. *P < 0.05, compared with the control condition (DMSO) for MUC5AC expression; #P < 0.05, compared with the control condition (DMSO) for CYP1A1 expression.
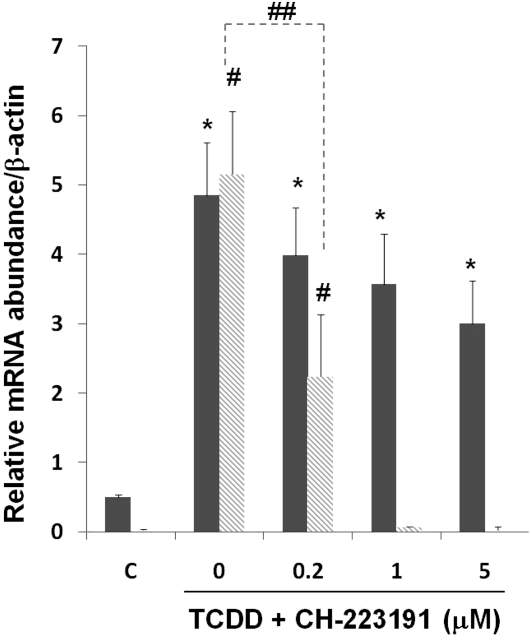
To examine if TCDD-mediated gene expression is through the classical AhR-dependent mechanism, cells were pretreated with an AhR antagonist, CH-223191. As shown in Figure 2, TCDD-induced CYP1A1 expression was drastically abrogated in the presence of the antagonist, whereas MUC5AC expression was slightly decreased with no statistical significance.
Figure 2.
Effects of aryl hydrocarbon receptor (AhR) antagonist in TCDD-induced gene expression. Primary NHBE cells were pretreated with the AhR antagonist, CH-223191, at different concentrations (μM) 30 minutes before TCDD (10 nM) treatment. RNAs were harvested 24 hours later, followed by quantitative RT-PCR. Filled and shaded bars are relative message levels of MUC5AC and CYP1A1, respectively. Values are expressed as the means (±SD) from triplicate samples from one representative experiment. *P < 0.05, compared with the control condition (DMSO) for MUC5AC messages; #P < 0.05, compared with the control condition (DMSO) for CYP1A1 messages; ##P < 0.05 between antagonist-treated and untreated conditions for TCDD-induced CYP1A1 expression.
TCDD Induces EGFR and MAPK Signaling Pathway Activation and Their Role in Induced MUC5AC Expression
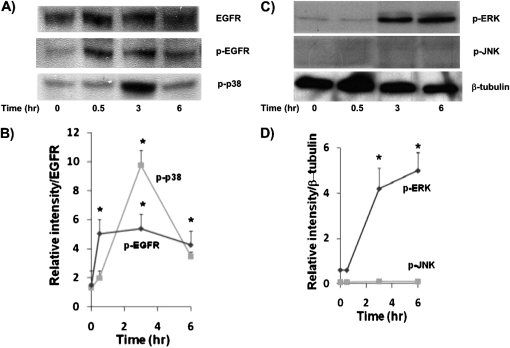
To determine if TCDD used a similar EGFR and MAPK pathways, and Sp1-based transcriptional mechanism, as reported previously (18, 20, 28, 29), NHBE cells were treated for 0.5, 3, 4, and 6 hours with 10 nM TCDD and Western blots were performed on resultant cell lysates. As shown in Figure 3, phosphorylation of EGFR was significantly increased after 0.5-hour TCDD treatment, whereas phosphorylation of ERK and p38 was observed at later time points, after 3 hours of TCDD treatment. In contrast to ERK phosphorylation, p38 phosphorylation was transient, diminishing at 6 hours. Interestingly, TCDD treatment did not induce JNK phosphorylation. Similar results were obtained with HBE1 cells treated with TCDD (data not shown).
Figure 3.
Western blot analysis of cell extracts from TCDD-treated NHBE cultures. Cell extracts were prepared from cultures after 0, 0.5, 3, and 6 hours after TCDD addition. (A) Antibodies specific to epidermal growth factor receptor (EGFR), phosphorylated EGFR (p-EGFR) and p-p38. (B) Densitometry analysis of these p-EGFR and p-p38 bands after normalization, with total EGFR band in the blot. (C) Antibodies specific to p–extracellular signal–regulated kinase (ERK), p–c-Jun N-terminal kinase (JNK), and β-tubulin. (D) Densitometry analysis of p-ERK and p-JNK protein bands normalized with β-tubulin. Experiments were repeated independently twice, with different primary NHBE cultures, as well as from HBE1 (data not included), with similar results. *P < 0.05, compared with 0-hour control.
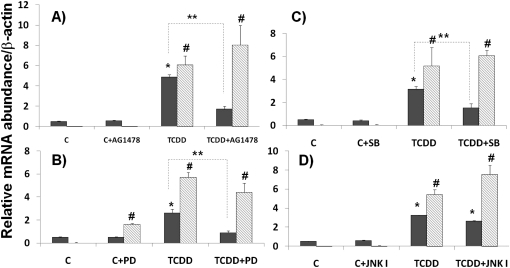
To address the functional roles of these pathways and Sp1 mechanism in TCDD-induced gene expression, an AhR antagonist and various inhibitors were used. As shown in Figure 4, TCDD-induced MUC5AC gene expression was significantly decreased with AG1478 (an EGFR inhibitor; Figure 4A), PD98059 (a MAPK inhibitor; Figure 4B), and SB203580 (a p38 inhibitor; Figure 4C) pretreatment, but not with SP600125 (a JNK inhibitor; Figure 4D) pretreatment, as compared with noninhibitor control. In contrast, pretreatments with inhibitors of EGFR, ERK, and p38 had no effect on TCDD-induced CYP1A1 message. For PD98059, there was a small but statistically significant increase in CYP1A1 expression without TCDD treatment. This may reflect the weak activation of AhR signaling by dioxin-like chemical structure of PD98059. These results demonstrate the dependence on EGFR, ERK, and p38 signaling, and the independence of AhR for TCDD-induced MUC5AC expression.
Figure 4.
Effects of EGFR and mitogen-activated protein kinase (MAPK) pathway inhibitors. Primary NHBE cells were treated with the inhibitors (A) AG1478 (EGFR inhibitor), (B) PD98058 (a MAPK kinase inhibitor), (C) SB203580 (a p38 inhibitor), and (D) SP600125 (a JNK inhibitor) 30 minutes before TCDD (10 nM) treatment. RNAs were harvested 24 hours later, followed by quantitative RT-PCR. Relative message abundances of MUC5AC (filled bars) and CYP1A1 (shaded bars) were normalized with β-actin message level. Values are expressed as the means (±SD) from triplicate samples from one representative experiment. *P < 0.05, compared with the control condition (DMSO) with no TCDD and inhibitor treatment for MUC5AC message; **P < 0.05 between inhibitor-treated and untreated conditions for TCDD-induced MUC5AC expression; #P < 0.05, compared with the control condition (DMSO) with no TCDD and inhibitor treatment for CYP1A1 message.
TCDD-Induced MUC5AC Promoter–Reporter Gene Expression
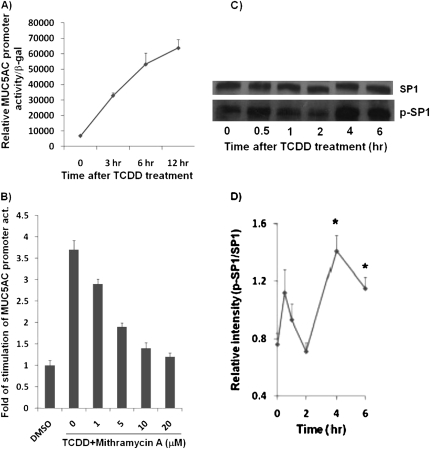
To confirm a direct transcriptional regulation of MUC5AC, pGL3-MUC5AC promoter–luciferase chimeric construct–transfected HBE1 cells were treated with either 10 nM TCDD or DMSO for 3, 6, or 12 hours. As shown in Figure 5A, MUC5AC transcriptional promoter activity was stimulated by TCDD in a time-dependent manner, with maximum stimulation occurring at 6–12 hours. Furthermore, the induced promoter activity is sensitive to mithramycin A treatment (Figure 5B). Mithramycin A is a potent inhibitor of Sp1-based transcriptional mechanism (19, 20). We had previously used this chemical to demonstrate phorbol 12-myristate 13-acetate (PMA)-mediated Sp1-dependent MUC5AC transcription at these doses without causing cellular cytotoxicity (determined by trypan blue dye exclusion) (20). In support of a role for this transcriptional mechanism, TCDD treatment was able to enhance the phosphorylation of nuclear Sp1 protein at 4–6 hours after the treatment (Figure 5C). These results suggest a direct transcriptional regulation of MUC5AC by TCDD.
Figure 5.
Transcriptional regulation of MUC5AC promoter–reporter gene activity by TCDD and specificity protein 1 (Sp1). (A) Effects of TCDD on the MUC5AC promoter–reporter gene expression activity. HBE1 cells were transiently transfected with the MUC5AC-pGL3 construct. After 24 hours, the cells were treated with 10 nM of TCDD for 3, 6, or 12 hours. The relative luciferase activity was normalized with β-gal to control for transfection efficiency. The values are expressed as the means (±SD) of triplicate samples from one representative experiment. *P < 0.05 compared with control. (B) Effects of mithramycin A on TCDD-induced MUC5AC promoter–reporter gene activity. Different amounts of mithramycin A (μM) were added to transfect cultures 30 minutes before TCDD treatment. At 6 hours after 10-nM TCDD treatment, cells were harvested for the reporter gene assays. The relative luciferase activity was normalized with β-gal to control for transfection efficiency, and the results are expressed as fold changes in activation relative to the control condition with no TCDD and mithramycin A treatments. The values are expressed as the means (±SD) of triplicate samples from one representative experiment. *P < 0.05 compared with control; #P < 0.05 for mithramycin A–treated conditions as compared with the TCDD-induced MUC5AC transcription. (C) HBE1 cells were treated with 10 nM TCDD for 0.5, 1, 2, 4, or 6 hours, and protein was isolated. A total of 500 μg of protein was immunoprecipitated with an agarose-conjugated anti-phosphoserine antibody overnight. A membrane was probed with total SP1 antibody. Total SP1 from the same cell lysate was also determined to assure equal expression in each condition. (D) Densitometry analysis of p-SP1 with total SP1 protein in the blot. *P < 0.05, compared with 0 hour control.
TCDD-Induced MUC5AC Promoter Activity Is Regulated by EGFR and MAPK Signaling Pathways
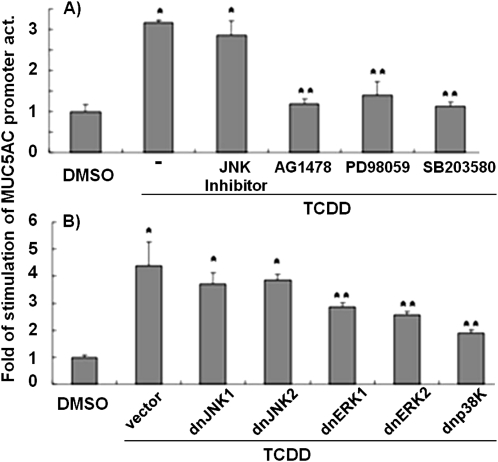
Inhibitor studies further confirm the significant roles of EGFR, ERK, and p38, but not JNK, in the regulation of MUC5AC promoter activity in response to TCDD (Figure 6A). A similar conclusion could be reached by transfecting cells with dn mutants of the various MAPKs. As shown in Figure 6B, TCDD-induced MUC5AC promoter activity was significantly inhibited by the presence of dnERK1, dnERK2, and dn-p38, but not with dnJNK1 or dnJNK2, compared with TCDD treatment plus control vector transfection. These results further confirm that MUC5AC induction by TCDD is transcriptionally mediated by EGFR, ERK, and p38.
Figure 6.
Effects of EGFR and MAPK inhibitors and dominant negative (dn) mutants on TCDD-induced MUC5AC promoter–reporter gene activity. (A) Effects of inhibitors. HBE1 cells were transiently transfected with the MUC5AC promoter–reporter construct, as described in Figure 5. After 24 hours, cells were pretreated with various inhibitors 30 minutes before 10-nM TCDD treatment. (B) Effects of dn mutant overexpression. HBE1 cells were cotransfected with MUC5AC promoter–reporter construct, and these dn mutant constructs or corresponding empty vector constructs. After 24 hours, cells were treated with 10 nM TCDD. Cells were harvested for reporter gene assays 6 hours later. For each transfection, relative luciferase activities were normalized with β-gal activities, which were then compared to controls with no inhibitor and empty vector transfection (2nd column). The values are expressed as the means (±SD) of triplicate samples from one representative experiment. *P < 0.05, compared with control; **P < 0.05, compared with TCDD treatment alone with no inhibitor (A) or transfection with the control vector (B).
DISCUSSION
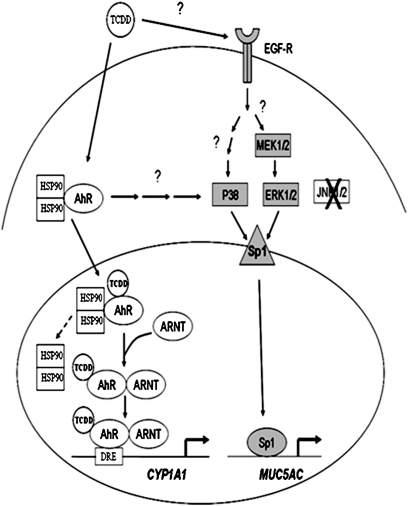
Many studies have linked environmental contaminants, including cigarette smoke and acreolin, with enhanced expression and overproduction of MUC5AC by airway epithelial cells (29, 30). A recent study demonstrated TCDD induction of MUC5AC in the human adenocarcinoma cell line, NCI-H441 in vitro, and in C57/BL6 mice in-vivo (31). However, the effect of TCDD on MUC5AC expression in normal human airway epithelial cells is unknown, as is the signal transduction pathway involved in this effect. Epidemiological studies have associated TCDD exposure with an increased incidence of COPD (3–6). Given that goblet cell hyperplasia is a cardinal feature of COPD, we hypothesized that TCDD treatment of human airway epithelial cells would increase MUC5AC expression. Our results demonstrate that TCDD-mediated MUC5AC up-regulation is independent of the conventional AhR pathway, and instead requires activation of EGFR, ERK, p38, and the transcription factor, Sp1 (Figure 7). Interestingly, TCDD did not alter MUC5B up-regulation, showing a selective effect of TCDD-induced mucin gene expression.
Figure 7.
Schematic diagrams of the signal transduction in TCDD-induced MUC5AC gene expression and the conventional AhR pathway in the activation of CYP1A1 expression. The shaded region indicates the signaling pathway of TCDD-induced MUC5AC gene expression. ARNT, AhR nuclear translocator; DRE, dioxin response element; HSP, heat shock protein; MEK, MAPK kinase.
Classically, TCDD functions through the AhR. However, several recent studies have demonstrated that TCDD can also activate the EGFR, leading to the activation of MAPK signaling pathways. The exact mechanism of this pathway remains undefined (14, 15, 32). Likewise, studies using cigarette smoke and PMA have shown that MUC5AC up-regulation involves the EGFR/RAS/RAF/ERK/SP1 pathway (20, 28–30). Therefore, we focused our study on the role of the EGF/MAPK pathway on in TCDD mediated up-regulation of MUC5AC (Figure 7). Our results demonstrate that TCDD activates EGFR within 30 minutes, and activates ERK and p38 within 3–4 hours, followed by enhanced Sp1 phosphorylation at 4–6 hours. TCDD treatment did not lead to JNK activation. Consistent with these results, chemical inhibitors of EGFR, ERKs, p38, and Sp1 attenuated TCDD-induced MUC5AC promoter activity and MUC5AC mRNA expression. In contrast, JNK inhibitors had no effect. Furthermore, an AhR antagonist (33) did not attenuate TCDD-induced MUC5AC gene expression, despite its ability to attenuate AhR-dependent CYP1A1 expression. These results further demonstrate that MUC5AC induction is independent of the AhR signaling.
Surprisingly, we found that TCDD induced p38 activation, which was important for MUC5AC up-regulation. A role for p38 activation in MUC5AC expression by other environmental contaminants has not been previously described. The requirement of p38 MAPK for up-regulation of MUC5AC expression is consistent with a past publication that showed a p38-dependent MUC5AC induction in human HeLa and HM3 cell lines by nontypeable Haemophilus influenzae (34). A subsequent study in NCIH292 cell line has also demonstrated the requirement of p38 MAPK activation for IL-1β– and TNF-α–induced MUC5AC expression (35). Fujisawa and colleagues (36) have recently demonstrated the involvement of p38 MAPK pathway in IL-13–induced mouse Muc5ac expression in the mouse cell system. Despite these findings, the authors are unable to determine the downstream transcriptional factor(s) affected by p38 MAPK. We have shown previously that PMA-induced JNK and p38 kinases activations are required for Sp1-based MUC5B gene expression (19). A similar mechanism may exist in TCDD-induced MUC5AC expression. A possible explanation for this discrepancy is that p38 activation by TCDD could be independent of the EGFR pathway. In an article by Weiss and coworkers (37), TCDD induced c-Jun expression through a nonclassical p38–MAPK pathway that was independent of AhR. Thus, we speculate that the TCDD-activated EGFR is only associated with downstream activation of ERK, and not with p38 or JNK. Activation of both p38 and JNK is needed for MUC5B up-regulation (19, 20). That TCDD fails to mediate JNK activation may explain the lack of MUC5B induction in this study. This result is consistent with the notion that both mucin genes, MUC5AC and MUC5B, are regulated by EGFR-dependent and -independent mechanisms, respectively (19, 20).
A question arises as to how TCDD activates EGFR. According to Vogel and coworkers (38), TCDD increases the level of cellular src, which subsequently increases the production of transforming growth factor (TGF)–α and activates EGFR. However, we did not detect a significant increase in either cellular src or TGF-α gene expression after TCDD treatment (data not shown). Alternatively, previous reports demonstrate that TCDD generates reactive oxygen species (ROS) by inducing the CYP1 enzymes (39, 40). In turn, a different study shows that ROS can activate TNF-α–converting enzyme, which cleaves pro–TGF-α and activates EGFR (41). This cleavage may occur only at the membrane level, and results in no TGF-α release to the medium. Thus, we speculate that TCDD activates EGFR through the generation of ROS, which in turn activates TNF-α–converting enzyme and cleaves pro–TGF-α. Further examination into this pathway is needed.
In this study, we have concurrently measured CYP1A1 gene expression as a positive control and compared activation of the AhR pathway with TCDD-induced MUC5AC gene expression. As expected, TCDD induced up-regulation of CYP1A1 gene expression in human airway epithelial cells. However, there was no correlation between CYP1A1 and MUC5AC gene expression in regard to sensitivity to chemical inhibitors. For example, PD98059 up-regulated CYP1A1, whereas SP600125 attenuated its expression. Previous studies have demonstrated that PD98059 and SP600125 are antagonists for AhR (42, 43). Thus, whereas attenuation of CYP1A1 by SP600125 was expected, the up-regulation by PD98059 was not. However, previous studies have shown that flavonoids, such as PD98059, can have both antagonistic and agonistic effects on the AhR (44, 45). Therefore, we speculate that PD98059 operates more consistently as an AhR agonist rather than as an antagonist in airway epithelial cells.
In summary, we have shown that TCDD induces MUC5AC and CYP1A1 gene expression, but not MUC5B, in both primary NHBE cells and in the HBE cell line, HBE1. Second, MUC5AC protein is also elevated in TCDD-treated primary NHBE cells. Third, we demonstrate that TCDD mediates MUC5AC up-regulation independent of the conventional AhR pathway, and uses the activation of EGFR, MAPK kinase, ERK, p38, and the transcription factor, Sp1. Finally, we demonstrate that TCDD-induced MUC5AC gene expression is potentially regulated at the promoter level. Detailed studies, including TCDD activation of EGFR and the association between AhR and p38 in airway epithelial cells, will be the subject of future investigations.
This work was supported in part by National Institutes of Health grants HL077902, HL077315, HL096373, ES00628, and ES07685, and California Tobacco-Related Disease Research Program grant 16RT-0127. Y.C.L. and S.V. were supported by T32 HL07013.
Originally Published in Press as DOI: 10.1165/rcmb.2010-0313OC on October 22, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Van den Berg M, Birnbaum L, Bosveld AT, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 1998;106:775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnbaum LS. The mechanism of dioxin toxicity: relationship to risk assessment. Environ Health Perspect 1994;102:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C, Pesatori AC. Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol 2001;153:1031–1044. [DOI] [PubMed] [Google Scholar]
- 4.Steenland K, Piacitelli L, Deddens J, Fingerhut M, Chang LI. Cancer, heart disease, and diabetes in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Natl Cancer Inst 1999;91:779–786. [DOI] [PubMed] [Google Scholar]
- 5.Pesatori AC, Zocchetti C, Guercilena S, Consonni D, Turrini D, Bertazzi PA. Dioxin exposure and non-malignant health effects: a mortality study. Occup Environ Med 1998;55:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consonni D, Pesatori AC, Zocchetti C, Sindaco R, D'Oro LC, Rubagotti M, Bertazzi PA. Mortality in a population exposed to dioxin after the Seveso, Italy accident in 1976: 25 years of follow up. Am J Epidemiol 2008;167:847–858. [DOI] [PubMed] [Google Scholar]
- 7.Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem 1988;263:13802–13805. [PubMed] [Google Scholar]
- 8.Denis M, Cuthill S, Wikstrom AC, Poellinger L, Gustafsson JA. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun 1988;155:801–807. [DOI] [PubMed] [Google Scholar]
- 9.Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X–associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol 1998;18:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (aryl hydrocarbon) receptor. J Biol Chem 1999;274:13519–13524. [DOI] [PubMed] [Google Scholar]
- 11.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 2003;43:309–334. [DOI] [PubMed] [Google Scholar]
- 12.Sun YV, Boverhof DR, Burgoon LD, Fielden MR, Sacharewski TR. Comparative analysis of dioxin response elements in human, mouse, and rat genomic sequences. Nucleic Acids Res 2004;32:4512–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol 1996;12:55–89. [DOI] [PubMed] [Google Scholar]
- 14.Madhukar BV, Brewster DW, Matsumura F. Effects of in vivo–administered 2,3,7,8-tetrachlorodibenzo-p-dioxin on receptor binding of epidermal growth factor in the hepatic plasma membrane of rat, guinea pig, mouse, and hamster. Proc Natl Acad Sci USA 1984;81:7407–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SJ, Yoon WK, Kim HJ, Son HY, Cho SW, Jeong KS, Kim TH, Kim SH, Kim SR, Ryu SY. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activates ERK and p38 mitogen-activated protein kinases in RAW 264.7 cells. Anticancer Res 2005;25:2831–2836. [PubMed] [Google Scholar]
- 16.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. [DOI] [PubMed] [Google Scholar]
- 17.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol 2006;34:661–665. [DOI] [PubMed] [Google Scholar]
- 18.Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol 2008;70:405–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu DY, Wu R, Chen Y, Tarasova N, Chang MM. PMA stimulates MUC5B gene expression through an Sp1-based mechanism in airway epithelial cells. Am J Respir Cell Mol Biol 2007;37:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YCD, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase–independent and –dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am J Pathol 2007;170:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu R, Martin WR, Robinson CB, St George JA, Plopper CG, Kurland G, Last JA, Cross CE, McDonald RJ, Boucher R. Expression of mucin synthesis and secretion in human tracheobronchial epithelial cells grown in culture. Am J Respir Cell Mol Biol 1990;3:467–478. [DOI] [PubMed] [Google Scholar]
- 22.Wu R, Zhao YH, Chang MM. Growth and differentiation of conducting airway epithelial cells in culture. Eur Respir J 1997;10:2398–2403. [DOI] [PubMed] [Google Scholar]
- 23.Fujisawa T, Velichko S, Thai P, Hung L-Y, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1β and IL-17A; the NF-κB paradigm. J Immunol 2009;183:6236–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, Paradiso AM, Rinehart CA Jr, Sarkadi B, Schlegel R, Boucher RC. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol 1993;264:C1219–C1230. [DOI] [PubMed] [Google Scholar]
- 25.Vuong H, Patterson T, Shapiro P, Kalvakolanu DV, Wu R, Ma WY, Dong Z, Kleeberger SR, Reddy SP. Phorbol ester–induced expression of airway squamous cell differentiation marker, SPRR1B, is regulated by protein kinase Cdelta /Ras/MEKK1/MKK1-dependent/AP-1 signal transduction pathway. J Biol Chem 2000;275:32250–32259. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Gallup M, Fan N, Szymkowski DE, Basbaum CB. Cloning of the amino-terminal and 5′-flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts. J Biol Chem 1998;273:6812–6820. [DOI] [PubMed] [Google Scholar]
- 27.Ha U, Lim JH, Jono H, Koga T, Srivastava A, Malley R, Pages G, Pouyssegur J, Li JD. A novel role for IkappaB kinase (IKK) alpha and IKKbeta in ERK-dependent up-regulation of MUC5AC mucin transcription by Streptococcus pneumoniae. J Immunol 2007;178:1736–1747. [DOI] [PubMed] [Google Scholar]
- 28.Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol 2004;344:683–695. [DOI] [PubMed] [Google Scholar]
- 29.Shao MX, Nakanaga T, Nadel JA. Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor–alpha–converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L420–L427. [DOI] [PubMed] [Google Scholar]
- 30.Borchers MT, Wert SE, Leikauf GD. Acrolein-induced MUC5ac expression in rat airways. Am J Physiol 1998;274:L573–L581. [DOI] [PubMed] [Google Scholar]
- 31.Wong PS, Vogel CF, Kokosinksi K, Matsumura F. Arylhydrocarbon receptor activation in NCI-H441 cells and C57/BL6 mice: possible mechanisms for lung dysfunction. Am J Respir Cell Mol Biol 2010;42:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheon H, Woo YS, Lee JY, Kim HS, Kim HJ, Cho S, Won NH, Sohn J. Signaling pathway for 2,3,7,8-tetrachlorodibenzo-p-dioxin–induced TNF-alpha production in differentiated THP-1 human macrophages. Exp Mol Med 2007;39:524–534. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, et al. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazophenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD–induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol 2006;69:1871–1878. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Lim DJ, Han J, Kim YS, Basbaum CB, Li JD. Novel cytoplasmic proteins of nontypeable Haemophilus influenzae up-regulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase–Akt pathway. J Biol Chem 2002;277:949–957. [DOI] [PubMed] [Google Scholar]
- 35.Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, Yoon JH. Interleukin-1β and tumor necrosis factor–α induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases–MSK1–CREB activation in human airway epithelial cells. J Biol Chem 2003;278:23243–23250. [DOI] [PubMed] [Google Scholar]
- 36.Fujisawa T, Ide K, Holtzman MJ, Suda T, Suzuki K, Kuroishi S, Chida K, Nakamura H. Involvement of the p38 MAPK pathway in IL-13–induced mucous cell metaplasia in mouse tracheal epithelial cells. Respirology 2008;13:191–202. [DOI] [PubMed] [Google Scholar]
- 37.Weiss C, Faust D, Dürk H, Kolluri SK, Pelzer A, Schneider S, Dietrich C, Oesch F, Göttlicher M. TCDD induces c-jun expression via a novel Ah (dioxin) receptor–mediated p38-MAPK–dependent pathway. Oncogene 2005;24:4975–4983. [DOI] [PubMed] [Google Scholar]
- 38.Vogel CF, Zhao Y, Wong P, Young NF, Matsumura F. The use of c-src knockout mice for the identification of the main toxic signaling pathway of TCDD to induce wasting syndrome. J Biochem Mol Toxicol 2003;17:305–315. [DOI] [PubMed] [Google Scholar]
- 39.Knerr S, Schaefer J, Both S, Mally A, Dekant W, Schrenk D. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induced cytochrome P450s alter the formation of reactive oxygen species in liver cells. Mol Nutr Food Res 2006;50:378–384. [DOI] [PubMed] [Google Scholar]
- 40.Park JY, Shigenaga MK, Ames BN. Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci USA 1996;93:2322–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao MX, Nadel JA. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha–converting enzyme. J Immunol 2005;175:4009–4016. [DOI] [PubMed] [Google Scholar]
- 42.Puppala D, Gairola CG, Swanson HI. Identification of kaempferol as an inhibitor of cigarette smoke–induced activation of the aryl hydrocarbon receptor and cell transformation. Carcinogenesis 2007;28:639–647. [DOI] [PubMed] [Google Scholar]
- 43.Joiakim A, Matieu PA, Palermo C, Gasiewicz TA, Reiners JJ Jr. The Jun N-terminal kinase inhibitor SP600125 is a ligand and antagonist of the aryl hydrocarbon receptor. Drug Metab Dispos 2003;31:1279–1282. [DOI] [PubMed] [Google Scholar]
- 44.Reiners JJ Jr, Lee JY, Clift RE, Dudley DT, Myrand SP. PD98059 is an equipotent antagonist of the aryl hydrocarbon receptor and inhibitor of mitogen-activated protein kinase kinase. Mol Pharmacol 1998;53:438–445. [DOI] [PubMed] [Google Scholar]
- 45.Chen S, Operaña T, Bonzo J, Nguyen N, Tukey RH. ERK kinase inhibition stabilizes the aryl hydrocarbon receptor: implications for transcriptional activation and protein degradation. J Biol Chem 2005;280:4350–4359. [DOI] [PubMed] [Google Scholar]