Abstract
γ−Amino butyric acid (GABA) is a primary inhibitory neurotransmitter in the central nervous system, and is classically released by fusion of synaptic vesicles with the plasma membrane or by egress via GABA transporters (GATs). Recently, a GABAergic system comprised of GABAA and GABAB receptors has been identified on airway epithelial and smooth muscle cells that regulate mucus secretion and contractile tone of airway smooth muscle (ASM). In addition, the enzyme that synthesizes GABA, glutamic acid decarboxylase, has been identified in airway epithelial cells; however, the mechanism(s) by which this synthesized GABA is released from epithelial intracellular stores is unknown. We questioned whether any of the four known isoforms of GATs are functionally expressed in ASM or epithelial cells. We detected mRNA and protein expression of GAT2 and -4, and isoforms of glutamic acid decarboxylase in native and cultured human ASM and epithelial cells. In contrast, mRNA encoding vesicular GAT (VGAT), the neuronal GABA transporter, was not detected. Functional inhibition of 3H-GABA uptake was demonstrated using GAT2 and GAT4/betaine–GABA transporter 1 (BGT1) inhibitors in both human ASM and epithelial cells. These results demonstrate that two isoforms of GATs, but not VGAT, are expressed in both airway epithelial and smooth muscle cells. They also provide a mechanism by which locally synthesized GABA can be released from these cells into the airway to activate GABAA channels and GABAB receptors, with subsequent autocrine and/or paracrine signaling effects on airway epithelium and ASM.
Keywords: vesicular γ–amino butyric acid transporter, 3H–γ–amino butyric acid uptake, immunoblot, RT-PCR
CLINICAL RELEVANCE.
Asthma is an increasingly common respiratory disease, with significant morbidity and health care costs. No major additions to the pharmacological armamentarium to treat this disease have occurred in several decades. The GABAergic (γ–amino butyric acid [GABA]) pathway has recently emerged as a potential therapeutic target in asthma. We describe a mechanism by which endogenous GABA may be secreted by airway epithelium and smooth muscle, contributing to our understanding of the GABA pathway in the airway and identifying a novel therapeutic target.
γ–Amino butyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian central nervous system (CNS). GABA acts at two types of receptors: pentameric ligand-gated ionotropic (GABAA) channels, and G protein–linked metabotropic (GABAB) receptors. GABA is classically released by neuronal vesicular synaptic release, and its action is terminated by reuptake via GABA transporters (GATs) present on synaptic neurons and glia in the CNS (1).
There are four known isoforms of the nonvesicular GAT, which belongs to the SLC-6 genome family, with species-specific nomenclature. In the present article, human/rat nomenclature is used. These monoamine transporters have been most extensively characterized in the CNS where GAT1 is the most predominant (2), and subtype-specific differential expression occurs in different anatomic regions. GATs are more heavily expressed in intrasynaptic regions of neurons, where they serve to regulate extracellular concentrations of monoamines (3). GAT4/betaine–GABA transporter (BGT) 1 may play a role in osmotic regulation in renal cells (4), neural cells (5), and at the blood–brain barrier (6).
Recent studies suggest an emerging autocrine–paracrine GABAergic cell signaling system in the airway, both in airway smooth muscle (ASM) and airway epithelium. GABAA channels and GABAB receptors (7, 8) have been identified in the airway in both smooth muscle and epithelium, functioning to modulate epithelial mucus production (9) and ASM tone (10, 11). These data suggest a physiologic and possible pathophysiologic role for a GABAergic system in the airway. In addition, the enzyme that synthesizes GABA (glutamic acid decarboxylase [GAD]) is expressed in airway epithelium (7, 11, 12). Moreover, in previous studies in airway epithelium (7), we noted immunostaining for GAD in ASM, suggesting that ASM may be an additional source of endogenous airway GABA. Thus, a cellular source and receptor targets for the endogenous ligand GABA have been identified in airways, but the mechanism(s) by which this locally synthesized GABA can be released from epithelial or smooth muscle cells is unknown.
GABAA channels are widely expressed in neuronal tissue, and modulate an inward chloride current that causes membrane hyperpolarization. Hyperpolarization of ASM cells favors relaxation (13). Activation of GABAA channels present in ASM cells facilitates ASM relaxation (10, 13, 14), suggesting that endogenous GABAA channels may be a potential therapeutic target in the treatment of airway diseases, including asthma and chronic obstructive pulmonary disease.
Airway epithelium plays a crucial role in airway function and disease, such as asthma (15, 16). The accumulation of mucus in the asthmatic airway is well known, and is associated with goblet cell hypertrophy and hyperplasia (17). Defective mucus clearance has been observed in asthma, notably in fatal asthma (18). Xiang and colleagues (9) have shown that GABAA channels and the synthetic enzyme, GAD, are expressed in human airway epithelium, and have reported that GABAA activation enhanced airway epithelial proliferation and mucus production.
The emerging evidence of a role for both GABAA channels and GABAB receptors in ASM (8, 10, 14, 19) and epithelium (7, 9) led us to question the mechanism(s) by which synthesized GABA could be released from airway cells, and whether cells other than epithelial cells (i.e., smooth muscle) could be a source of GABA in the airway. Specifically, we questioned whether ASM also expresses GAD, and whether airway epithelium and smooth muscle cells functionally express the vesicular GAT (VGAT) or any of the four known GATs.
MATERIALS AND METHODS
Reagents
3H-GABA (35 Ci/mmol) was obtained from MP Biomedicals (Irvine, CA). NNC 05-2090 hydrochloride was obtained from Tocris (Ellisville, MO). All other chemicals were obtained from Sigma (St. Louis, MO), unless otherwise stated.
For discussion of methods concerning cell culture and native tissue isolation, RT-PCR (Table 1), laser capture microdissection and RT-PCR, and immunoblotting, see the online supplement.
TABLE 1.
SEQUENCE OF GLUTAMIC ACID DECARBOXYLASE AND γ-AMINO BUTYRIC ACID TRANSPORTER PRIMERS
| mRNA Product Size |
gDNA Product Size |
Genbank Accession No. (Human) | ||
|---|---|---|---|---|
| cDNA Name | Primer Sequence (5′–3′) Sense Antisense | (bp) | (bp) | |
| Human GAD67 | GAC AAT GTG ATT TTG ATA AAG TGC AAT GAA | 375 | 8,146 | NM_000817 |
| CAT CTG GTT GCA TCC TTG GAG TAT ACC CT | ||||
| Human GAD65 | GAG TGG AGT GGA GAG GGC CAA CTC TGT GAC | 488 | 19,840 | NM_000818 |
| TTG TGG TTC CAT ACT CCA TCA TTC TGG CTT TAA TC | ||||
| Human GAT1 (SLC6A1) | GCA TCA TCT CCT ACC TGA TCG GTC TCT CTA ACA TCA CTC | 248 | 4,431 | NM_003042 |
| GCC CGC CAC AAT GAT TGG TGT GAA GAA AG | ||||
| Human GAT2 (SLC6A13) | GGG CCT GCT GTT TCT TCT TCA TGG T | 289 | 1,317 | NM_016615 |
| TAA ACC CAA GCC ACA CAG AGG GAC T | ||||
| Human GAT3 (SLC6A11) | CTT GTC TGT TAT CTC CTA TTT TCT GGG CCT CGT GAT GTT A | 441 | 5,926 | NM_014229 |
| GTC GTC AAC TTC TGG AGT TTC TCG GGC AGT GT | ||||
| Human GAT4/BGT1 (SLC6A12) | GGA TGG ATG CGG GCA CCC AGA | 184 | 582 | NM_003044 |
| CTC ATG AAG CCC AGG ATG GAG AAG ACA ACA AA | ||||
| Human sequence used for guinea pig GAT4/BGT1 | CTT GCC TGG GCT CTC TTC TAC CTG TT | 294 | 2,162 | NM_003044 |
| GGA CTT GAC CCC CTT CCA GAT GCA GA | ||||
| Human VGAT (SLC32A1) | CCA GGG CCT GCA GAT GGA CAT CCT GAA A | 339 | 2,676 | NM 080552 |
| GCG ATG AGG ATC TTG CCG GTG TAG CAG C |
Definition of abbreviations: BGT, betaine–GABA transporter; GAT, γ–amino butyric acid transporter; VGAT, vesicular GAT; gDNA, genomic DNA.
3H-GABA Uptake Assay
Confluent, cultured, immortalized human ASM or epithelial cells (BEAS-2B [CRL-9609]; ATCC, Manassas, VA) in 24-well plates were incubated in growth supplement–free and serum-free media overnight. Duplicate wells from 24-well plates were averaged within each assay, and “n” refers to the number of averaged duplicate values. The cells were washed once in GABA uptake assay buffer (10 mM glucose, 141 mM NaCl, 3 mM KCl, 2.8 mM CaCl2 1 mM Mg SO4, 10 mM Hepes [pH 7.4]) at 37°C. All cells were preincubated in GABA uptake assay buffer containing 10 μM phaclofen and 200 μM gabazine (to block 3H-GABA binding to GABAB and GABAA channels, respectively) with or without 10 mM GABA (to define specific uptake of 3H-GABA), and with or without varying concentrations of GAT inhibitors at 37°C for 15 minutes to generate dose–response curves. 3H-GABA (4 μCi/ml) was then added for a 30-minute incubation at 37°C. The concentration ranges chosen for the GAT inhibitors were based on previously published half maximal (50%) inhibitory concentration (IC), or IC50 concentrations (20–22) (Table 2). The reaction was terminated by placing the cells on ice and washing four times with GABA uptake assay buffer at 4°C. The cells were lysed with 300 μl 1 N NaOH per well for a minimum of 1 hour. An aliquot of 300 μl 1 M HCl was then added to each well to neutralize the pH, and 500 μl of the lysate was quantified by liquid scintillation in 5 ml Ecolite(+) Liquid Scintillation cocktail (MP Biomedicals, Solon, OH) in a Tri-Carb 2100TR Liquid Scintillation Analyzer (Packard, Ramsey, MN).
TABLE 2.
PUBLISHED AFFINITIES OF SUBTYPE-SPECIFIC γ–AMINO BUTYRIC ACID TRANSPORTER INHIBITOR DRUGS
| SKF 89976A IC50 (21) |
β-Alanine IC50 (21) |
Flufenamic Acid IC50 (22) |
NNC 05-2090 IC50 (20) |
|
|---|---|---|---|---|
| GABA Transporter Subtype | (μM) | (μM) | (μM) | (μM) |
| Human GAT1 | 0.13 ± 0.01 | 5690 ± 1890 | >500 | 19 ± 2 |
| Human GAT2 | 550 ± 225 (rat) | 19 ± 7 | 41 ± 11 | |
| Human GAT3 | 944 ± 259 | 58 ± 3 | ∼30 | 15 ± 4 |
| Human GAT4/BGT-1 | 7210 ± 3630 | 1320 ± 224 | 1.4 ± 0.3 |
Definition of abbreviations: BGT, betaine–GABA transporter; GABA, γ–amino butyric acid; GAT, GABA transporter; IC50, half maximal (50%) inhibitory concentration; SKF 89976A, 1-(4,4-Diphenyl-3-butenyl)-3-piperidinecarboxylic acid hydrochloride.
Initial studies implicated functional expression of GAT2 and GAT4/BGT1. To determine whether the GAT2 or GAT4/BGT1 transporter was more functionally dominant, 3H-GABA uptake assays were performed in the absence or presence of 300 μM β-alanine (a saturating block of GAT2) and in the absence or presence of 5 μM NNC 05-2090. This concentration of β-alanine (300 μM) is 15 times the IC50 value of β-alanine at the human GAT2 (19 μM), but is well below the IC50 value of β-alanine for human GAT4/BGT1 (1,320 μM) (20). NNC 05-2090 (5 μM) is four times the IC50 value of NNC 05-2090 at the human GAT4/BGT-1 (1.4 μM), but is well below the IC50 value of NNC 05-2090 for human GAT2 (41 μM) (21).
See the online supplement for 3H-GABA uptake assay methods performed after cell membrane depolarization and in the absence of sodium and chloride ions, and for 3H-GABA release assay.
Statistical Analysis
In all RNA or immunoblot studies in native tissues, “n” refers to the number of individual patients or animals from which RNA and protein was extracted. In all RNA or protein studies from cultured cells, “n” refers to the number of individual flasks from which RNA or protein was extracted. Duplicate wells on 24-well plates were averaged for functional assays of 3H-GABA uptake or release, and “n” refers to the number of averaged duplicate values. Curve fitting analyses of GAT inhibitor dose–response curves determined by the 3H-GABA uptake assay were performed using nonlinear regression analyses with a sigmoidal dose–response function. Other 3H-GABA uptake and release assays were analyzed using one-way ANOVA with selected Bonferroni post tests or unpaired and paired t tests, as appropriate. All data were analyzed using Prism 4.0 software (GraphPad, San Diego, CA).
RESULTS
mRNA Expression of GAT and GAD Isoforms in Human ASM and Airway Epithelium
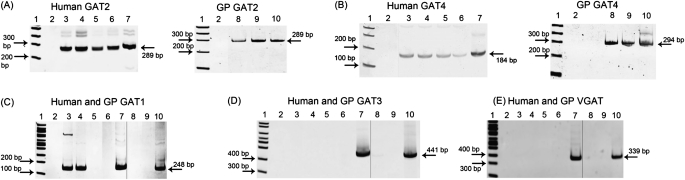
mRNA for GAT2 and GAT4 was detected in both native and cultured human ASM and epithelium, and in native guinea pig ASM and epithelium (Figure 1) (n = 2–3 individual human or guinea pig native tissues or individual culture flasks). mRNA for GAT1 and GAT3, as well as the classic neuronal VGAT, was not found despite successful detection of these transcripts in human and guinea pig brain controls (Figure 1) (n = 2–3). Although mRNA for GAT1 was detected in native human ASM and native human airway epithelium, it was not detected by RT-PCR analysis of pure populations of these tissues obtained from laser capture microdissection (Table 3) (n = 2–3). In addition, GAT1 protein was not detected by immunoblot and functional assays (data not shown), suggesting that it is not present or functional in these tissues. Therefore, we postulate that the mRNA detected in our whole-tissue RT-PCR for GAT1 detected mRNA originating from small amounts of neural tissue.
Figure 1.
Representative gel images of RT-PCR of γ–amino butyric acid (GABA) transporter (GAT) subtypes from RNA from freshly dissected human and guinea pig (GP) tissues and cultured human airway smooth muscle (ASM) and epithelial cells. mRNA for (A) GAT2 and (B) GAT4 is present in native human ASM and epithelium, cultured human ASM and epithelium (A and B, left panels), and native guinea pig ASM and epithelium (A and B, right panels). (C) mRNA for GAT1 is present in native human ASM and epithelium, and may represent contaminating neural structures in these tissues. mRNA for (D) GAT3 and (E) vesicular GAT (VGAT) is not present in human or guinea pig ASM or epithelium. Images are representative of experiments performed on RNA isolated from two to three different native guinea pig or human tissues, or two to three cultured cell flasks. 1, 100 base pair ladder; 2, water, which denotes the negative control (no cDNA input); 3, native human ASM tissue; 4, native human airway epithelial tissue; 5, cultured human ASM cells; 6, cultured human airway epithelial cells; 7, human brain; 8, native guinea pig ASM tissue; 9, native guinea pig airway epithelial tissue; and 10, guinea pig brain. Separate gel images are denoted by a solid demarcating line.
TABLE 3.
SUMMARY OF CONVENTIONAL AND LASER CAPTURE MICRODISSECTION RT-PCR RESULTS FOR γ–AMINO BUTYRIC ACID TRANSPORTER AND GLUTAMIC ACID DECARBOXYLASE ISOFORMS IN HUMAN AND GUINEA PIG AIRWAY SMOOTH MUSCLE AND EPITHELIUM
| Method of tissue dissection: | GAT1 |
GAT2 |
GAT3 |
GAT4 |
VGAT | GAD 65 | GAD 67 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gross Dissection | LCM | Gross Dissection | LCM | Gross Dissection | LCM | Gross Dissection | LCM | Gross Dissection | Gross Dissection | Gross Dissection | |
| Native HASM | + | − | + | + | − | − | + | + | − | + | |
| Cx HASM | − | + | − | + | − | + | |||||
| Native HEpi | + | − | + | + | − | − | + | + | − | + | |
| Cx HEpi | − | + | − | + | − | + | |||||
| Native GPASM | − | + | − | + | − | + | |||||
| Native GPEpi | − | + | − | + | − | + | |||||
| GPBr | + | + | + | + | + | + | + | ||||
| HBr | + | + | + | + | + | + | + | ||||
Definition of abbreviations: Cx, cultured; GAT, γ–amino butyric acid transporter; GPASM, guinea pig airway smooth muscle; GPBr, guinea pig brain; GPEpi, guinea pig airway epithelium; HASM, human airway smooth muscle; HBr, human brain; HEpi, human airway epithelium; LCM, laser capture microdissection; VGAT, vesicular GAT.
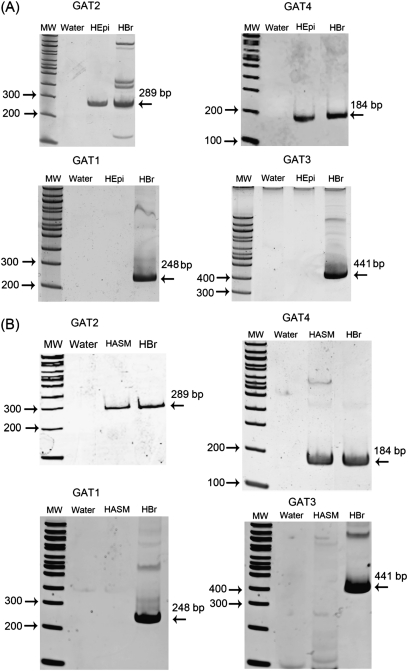
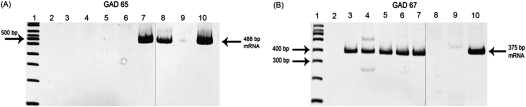
RT-PCR analyses of RNA isolated from human airway epithelial and smooth muscle cells obtained by laser capture microdissection confirmed the presence of mRNA for GAT2 and GAT4, but not GAT1 or GAT3 (Figure 2) (n = 2–3 tissues from individual patients). RT-PCR analyses demonstrated that native and cultured human ASM express mRNA encoding GAD67, but not GAD65, and confirmed the presence of mRNA encoding GAD67, but not GAD65, in native human and cultured airway epithelium (Figure 3) (n = 2–3 tissues obtained from individual patients).
Figure 2.
Representative gel images of RT-PCR of RNA isolated by laser capture microdissection for GAT subtypes 1–4. (A) Human airway epithelium from tracheal rings. (B) Human ASM from tracheal rings. Encoding mRNA for GAT subtypes 2 and 4 were detected in RNA isolated from pure human airway epithelium and smooth muscle cells, whereas mRNA for GAT1 and GAT3 were not. Images are representative of experiments performed on RNA isolated from two to three different native guinea pig or human tissues. HASM, human ASM from tracheal ring; HBr, human brain (positive control); HEpi, human airway epithelium from tracheal ring; MW, 100 base pair ladder. Water denotes the negative control (no cDNA input).
Figure 3.
Representative gel images of RT-PCR of glutamic acid decarboxylase (GAD) isoforms GAD65 and GAD67 from RNA from freshly dissected native human and guinea pig ASM and epithelial tissue, and cultured human ASM and epithelial cells. mRNA for GAD65 is present in guinea pig ASM, whereas mRNA for GAD67 is present in native and cultured human ASM and epithelium. Images are representative of experiments performed on RNA isolated from two to three different native guinea pig or human tissues or two to three cultured cell flasks. 1, 100 base pair ladder; 2, water, which denotes the negative control (no cDNA input); 3, native human ASM; 4, native human airway epithelium; 5, cultured human ASM cells; 6, cultured human epithelial cells; 7, human brain; 8, native guinea pig ASM tissue; 9, native guinea pig airway epithelial tissue; and 10, guinea pig brain. Separate gel images are denoted by a solid demarcating line.
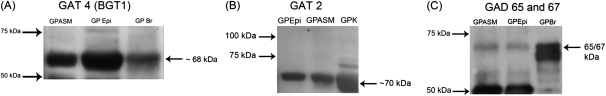
Immunoblot Analysis of GAT and GAD Isoforms in Guinea Pig ASM and Airway Epithelium
GAT2 and GAT4/BGT1 proteins were detected in native guinea pig ASM and epithelium at approximate molecular masses of 70 and 68 kD, respectively (Figure 4) (n = 2–3 individual guinea pig native tissues). An antibody recognizing both isoforms of GAD, GAD 65/67, was used for immunoblot analysis, and detected an immunoreactive band in native guinea pig ASM and epithelium. GAT1 and GAT3 proteins were not found in these tissues (data not shown). Immunoblots processed in the absence of primary antibodies confirmed the absence of specific bands in the molecular mass regions of interest.
Figure 4.
Representative immunoblot images of GAT and GAD isoforms. (A) GAT4/betaine–GABA transporter (BGT) 1 and (B) GAT2 proteins are present in native guinea pig ASM and epithelium. Guinea pig brain and guinea pig kidney are positive controls for GAT4/BGT1 and GAT2, respectively. (C) GAD protein is present in native guinea pig airway epithelium and smooth muscle and guinea pig brain. GPASM, guinea pig ASM; GPBr, guinea pig brain; GPEpi, guinea pig airway epithelium; GPK, guinea pig kidney. Images are representative of experiments performed on protein isolated from two to three different native guinea pig tissues.
Functional Analysis of GAT via 3H-GABA Uptake Assay
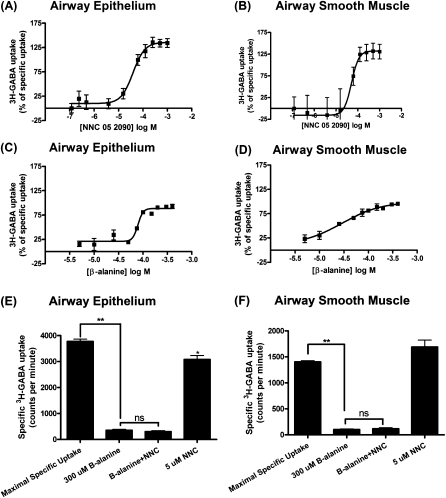
3H-GABA uptake assay was performed on cultured immortalized human airway epithelial and smooth muscle cells grown to confluence. The following compounds are selective for the specified GAT subtypes: SKF89976A is GAT1 selective (23); β-alanine is GAT2 selective; flufenamic acid is GAT3 selective (22); and NNC 05-2090 is GAT4/BGT1 selective (21). The IC50 for the GAT2-selective inhibitor, β-alanine, in human airway epithelial and smooth muscle cells was 79 and 28 μM, respectively (Figure 5) (n = 4). The IC50 for the GAT4/BGT1–selective inhibitor, NNC 05-2090, in human airway epithelial and smooth muscle cells was 39 and 51 μM, respectively (Figures 5A–5D) (n = 4). These values are closer to the affinity of NNC 05-2090 for the GAT2 rather than the GAT4/BGT1 transporter (21). Thus, β-alanine and NNC 05-2090 yield IC50 values consistent with predominant function of GAT2. Inhibitors of GAT1 and GAT3 (SKF 89976A and flufenamic acid, respectively) blocked 3H-GABA uptake only at high, nonspecific concentrations, consistent with the failure to detect their mRNA and protein expression in human airway epithelial and smooth muscle cells (data not shown).
Figure 5.
3H-GABA uptake and release assays. (A–D) Dose–response curves using GAT isoform inhibitors in human airway epithelial and smooth muscle cells. Human airway epithelial cell dose–response curves with (A) NNC 05-2090, a selective GAT4/BGT-1 inhibitor (half maximal [50%] inhibitory concentration or IC50, 39 μM) and (C) β-alanine, a selective GAT2 inhibitor (IC50, 79 μM). Human ASM cell dose–response curves with (B) NNC 05-2090, a selective GAT4/BGT-1 inhibitor (IC50, 51 μM) and (D) β-alanine, a selective GAT2 inhibitor (IC50, 28 μM). Dose–response curves are representative of four experiments. Data are presented as means (±SEM). (E) Effect of GAT2 and GAT4 inhibitors alone or in combination on 3H-GABA uptake in human bronchial epithelial cells. The GAT2 inhibitor, β-alanine, significantly decreases specific 3H-GABA uptake (n = 6; **P < 0.001), and the addition of the GAT4 inhibitor, NNC 05-2090, does not further decrease specific 3H-GABA uptake. Addition of 5 μM NNC 05-2090 alone induced a small but significant decrease in specific 3H-GABA uptake (n = 6; *P < 0.05). (F) Effect of GAT2 and GAT4 inhibitors alone or in combination on 3H-GABA uptake in human ASM cells. The GAT2 inhibitor, β-alanine, significantly decreases specific 3H-GABA uptake (n = 12; **P < 0.001), and the addition of the GAT4 inhibitor, NNC 05-2090, does not further decrease specific 3H-GABA uptake. Data are presented as means (±SEM).
To demonstrate that GAT2 is the predominant functional GAT in human ASM and epithelium, despite the detection of the GAT4 protein, we repeated 3H-GABA uptake assay studies in the presence of 300 μM β-alanine (∼15 times the IC50 for GAT2, but below the IC50 for the GAT4/BGT-1 [1,320 uM]) (20) and 5 μM NNC 05-2090 (∼four times the IC50 for GAT4, but below the IC50 of NNC 05-2090 for GAT2 [41 μM]) (21) to determine the contribution of GAT2 and GAT4 to GABA transport function in human airway epithelial and smooth muscle cells. β-Alanine (300 μM) greatly inhibited 3H-GABA uptake in both human ASM (n = 12; P < 0.001) and epithelial cells (n = 6; P < 0.001), whereas the addition of 5 μM NNC 05-2090 did not result in an additional inhibition of uptake (Figures 5E and 5F). Low concentrations of NNC 05-2090 (5 μM) inhibited 3H-GABA uptake in human airway epithelium to a small but significant degree (n = 6; P < 0.05), while being ineffective in human ASM cells (n = 12; P > 0.05) (Figures 5E and 5F). Taken together, these results suggest that GAT2 is the predominant GAT in these human ASM and human airway epithelial cells.
In neuronal cells, GAT requires the cotransport of sodium and chloride, along with GABA (24). To confirm classical functional requirements of GAT in airway epithelial and smooth muscle cells, 3H-GABA uptake assays were performed in the absence of either sodium or chloride ions. Elimination of either of these ions from the assay buffer virtually eliminated specific 3H-GABA uptake, confirming the necessity of sodium and chloride for GAT function in human airway epithelial (n = 20; ***P < 0.001) and smooth muscle cells (n = 8–16; ***P < 0.001) (Figures 6A and 6B).
Figure 6.
Effect of elimination of sodium, elimination of chloride, or depolarization with potassium chloride on 3H-GABA uptake. Specific 3H-GABA uptake is significantly decreased in (A) human airway epithelial cells (n = 20; ***P < 0.001) and (B) human ASM cells (n = 8–16; ***P < 0.001) in the absence of sodium or chloride ions. Specific 3H-GABA uptake is significantly decreased in (C) human bronchial epithelial cells (n = 6; **P < 0.01) and (D) human ASM cells (n = 20; *P < 0.05) treated with 80 mM KCl to induce cell membrane depolarization. Data are presented as means (±SEM).
Classically, GATs display directionality of transport favoring uptake of GABA from the synaptic cleft. These transporters can be reversed in neuronal cells by depolarization (25). Depolarization favors efflux of GABA from the cell, which would yield a lower specific 3H-GABA uptake. Indeed, depolarization with 80 mM KCl resulted in decreased specific 3H-GABA uptake in both cultured, immortalized human airway epithelial (n = 6; **P < 0.01) and cultured, immortalized human ASM cells (n = 20; *P < 0.05) (Figures 6C and 6D).
Functional Analysis of GAT via 3H-GABA Release Assay
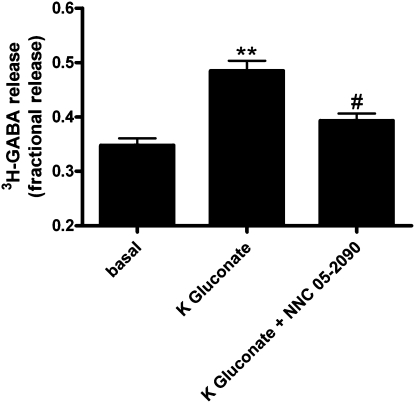
3H-GABA release assay was performed on cultured, immortalized human airway epithelial cells grown to confluence. We demonstrated 3H-GABA release in these cells, and determined the effects of cell membrane depolarization and GAT inhibition on this release. Under conditions of cell membrane depolarization, cultured human airway epithelial cells released significantly more 3H-GABA as compared with basal release in 28 mM sodium chloride buffer (Figure 7) (n = 8; P < 0.001). Depolarization (80 mM potassium gluconate)-stimulated release was significantly blocked by 500 μM NNC, a high and nonselective dose to inhibit all isoforms of GATs (Figure 7) (n = 8; P < 0.001).
Figure 7.
Effect of cell membrane depolarization on fractional 3H-GABA release by cultured human airway epithelial cells. Fractional release of 3H-GABA by cultured human airway epithelial cells in a basal low–sodium chloride buffer (28 mM) is significantly increased in the presence of potassium gluconate (80 mM) to induce cell membrane depolarization (n = 8; **P < 0.001). GAT blockade by a high concentration (500 μM) of NNC 05-2090, that inhibits all subtypes of GATs, inhibits the action of potassium gluconate (n = 8; **P < 0.001). In the presence of the GAT inhibitor, NNC 05-2090, potassium gluconate does not significantly increase basal fractional release of 3H-GABA (n = 8; #P > 0.05). Data are presented as means (±SEM).
DISCUSSION
The primary finding of the present study is that GAT2 is the predominant functional GAT expressed in native and cultured human airway epithelium and smooth muscle, and identifies the mechanism by which GABA synthesized within these cells can be released into the airway. Although we also demonstrate that mRNA and protein for GAT4 is present in ASM and epithelial cells, functional studies with selective inhibitors indicate that GAT2 is the primary functional subtype of GAT in both airway epithelium and smooth muscle. The mRNAs encoding GAT1, GAT3, and VGAT were not detected in airway epithelium or ASM. These RNA analyses were confirmed in RNA samples isolated by laser capture microdissection from human airways, a method that allows pure selection of cell types from native tissues.
Inhibitors of GAT1 or GAT3 (SKF 89976A and flufenamic acid, respectively), used at concentrations selective for these subtypes, did not significantly inhibit 3H-GABA uptake, which agrees with our molecular studies in which the mRNA and protein for these transporter subtypes was not detected. In contrast, IC50 values for the GAT2-selective inhibitor, β-alanine, and the GAT4-selective inhibitor, NNC 05-2090, in 3H-GABA uptake assays demonstrate that it is likely that GAT2 is the functionally predominant GAT in both airway epithelium and smooth muscle. Moreover, 3H-GABA uptake studies using a GAT2-selective concentration of β-alanine alone (300 μM or five times the IC50 for GAT2), a GAT4-selective concentration of NNC 05-2090 alone (5 μM or four times the IC50 for GAT4), or these two inhibitors together, demonstrated that the GAT2 subtype is the functionally predominant subtype. These concentrations of β-alanine and NNC 05-2090 were chosen to maximize the specificity of the GABA subtype blockade, while decreasing the likelihood of nonspecific blockade of other GAT subtypes. It is possible that GAT4/BGT1, although present in these cells, either has a different IC50 value in airway epithelial and smooth muscle cells than in neuronal cells, or serves a different function, such as transport of betaine. In renal cells, the transport of betaine is implicated in maintenance of intracellular osmotic pressure, and it may play a similar role in astrocytes (4, 5, 26). The role of GAT4/BGT1 in airway epithelial and smooth muscle cells is not clear.
Airway epithelial cells possess the synthetic enzyme for GABA, GAD isoforms 65 and 67 (7, 9, 12). We show that GAD mRNA is also present in human and guinea pig ASM, and confirm its previously reported expression in airway epithelial cells (9, 12). Furthermore, we detected GAD protein in native guinea pig airway epithelium and ASM, implicating the ASM as a source of airway GABA in addition to airway epithelium.
In neurons, GATs are electrogenic in that they cotransport two sodium ions and one chloride ion, along with one molecule of GABA, in the same direction (24). To demonstrate that our 3H-GABA uptake assay was truly measuring GAT function, and to confirm that GATs in airway cells also have a cotransport requirement for sodium and chloride, we show that the elimination of extracellular sodium or chloride totally abolishes 3H-GABA uptake in both human airway epithelial and smooth muscle cells.
Although, traditionally, GAT function has been thought of as an uptake phenomenon to terminate the action of GABA in the synaptic cleft, it is now accepted that in the CNS, GATs can reverse direction under pathological and physiological conditions (including cell membrane depolarization) to release GABA (25, 27). GATs may function not only to transport GABA into the neuron (thereby decreasing synaptic GABA levels), but may also play a role in maintenance of a tonic neural inhibition in the CNS by maintaining a tonic level of GABA via GAT “reversal.” (27) Although the normal physiological direction of GABA transport in airway epithelium and smooth muscle is not known, our 3H-GABA assay is designed to measure 3H-GABA uptake by these cells. We demonstrate that GAT function measured by 3H-GABA uptake can be decreased with cell membrane depolarization, similar to GAT in the CNS (25). These findings are of particular interest in the ASM, because depolarizing stimuli favor contraction of ASM, and we have previously shown that activation of GABAA channels can contribute to hyperpolarization and the subsequent relaxation of ASM (11, 14, 19). We further demonstrate that GATs in cultured human airway epithelial cells release 3H-GABA under ionically favorable conditions, and that this release is significantly increased by cell membrane depolarization. Thus, cell membrane depolarization induces GABA release through GATs. This released GABA could then be available to activate GABAA channels on ASM to counter-act the depolarizing contractile stimulus.
GAT cotransports two sodium ions and one chloride ion, along with GABA, and an ionic sodium chloride gradient affects GABA transport (3). Preliminary studies determined the optimal external sodium chloride concentration to allow 3H-GABA release via GATs. 3H-GABA release assays were therefore performed in a low (28 mM) sodium chloride buffer to allow for a favorable ionic gradient and optimal 3H-GABA release. Cell membrane depolarization was performed with potassium gluconate, instead of the more commonly used potassium chloride, to maintain the chloride ionic gradient, while allowing for cell membrane depolarization. β-Alanine does not sterically block GAT (28), but, instead, is a substrate for transport by GAT2 (29). In 3H-GABA uptake assays, β-alanine blocked 3H-GABA uptake by preferential transport into the cell over 3H-GABA. However, β-alanine was not an optimal choice for use as a GAT2 inhibitor for 3H-GABA release studies. Because β-alanine does not sterically block GAT2, an extracellular application of this compound did not provide an effective blockade of 3H-GABA release by GAT2. We used NNC 05-2090, a lipophilic aromatic compound (28), at a high, nonselective dose (500 μM) to block all GATs after loading of human airway epithelial cells with 3H-GABA. This allowed investigation of the effects of cell membrane depolarization and GAT blockade on 3H-GABA release. We show that human airway epithelial cells release significantly more 3H-GABA with cell membrane depolarization by 80 mM potassium gluconate, and this increased 3H-GABA release is blocked by 500 μM NNC 05-2090, a concentration expected to block all subtypes of GATs.
Our findings describe, for the first time, a mechanism by which airway epithelial and smooth muscle cells may release GABA synthesized locally within airway epithelium or ASM cells. This GABA can be a source of airway GABA that acts on GABAA channels (9, 10) and GABAB receptors (7, 8) in airway epithelium and smooth muscle, which has implications for epithelial mucus production, goblet cell hyperplasia, and ASM relaxation. The presence and function of GAT in the airway suggests a novel target for therapeutic intervention. Airway GABA production and release via GATs, as well as epithelial GABAA channels, may be a potential target for down-regulation to decrease mucus production and epithelial goblet cell hyperplasia and increase ASM relaxation. This carries implications for disease states with increased mucus secretion, such as asthma, cystic fibrosis, and chronic obstructive pulmonary disease.
In summary, these studies identify GAT2 as the predominant functional GAT in human airway epithelium and smooth muscle, identify the expression of the synthetic enzyme for GABA (GAD) in human ASM, and identify a mechanism of release of GABA from ASM and airway epithelial cells via classical GATs.
Supplementary Material
This work was supported by National Institutes of Health General Medical Sciences grant GM065281 and National Center for Research Resources grant ULI RR 024,156 (C.W.E.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0177OC on November 5, 2010
Author Disclosure: W.G. has received sponsored grants from the National Institutes of Health (NIH) (more than $100,001). C.W.E. has received sponsored grants from the National Institutes of Health (NIH) (more than $100,001). R.A.P. has served as a scientific consultant for AstraZeneca, Merck, and GlaxoSmithKline, has served on the board for BioMarck, Cytokinetics, and Epigenesis, and has received industry-sponsored grants from Immune Control ($10,001–$50,000), AstraZeneca ($10,001–$50,000), and NIH (more than $100,001). J.R.S. has served as a consultant and lecturer for Covidean. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol 2005;94:2073–2085. [DOI] [PubMed] [Google Scholar]
- 2.Bragina L, Marchionni I, Omrani A, Cozzi A, Pellegrini-Giampietro DE, Cherubini E, Conti F. GAT-1 regulates both tonic and phasic GABA(A) receptor-mediated inhibition in the cerebral cortex. J Neurochem 2008;105:1781–1793. [DOI] [PubMed] [Google Scholar]
- 3.Kanner BI. Structure and function of sodium-coupled GABA and glutamate transporters. J Membr Biol 2006;213:89–100. [DOI] [PubMed] [Google Scholar]
- 4.Kempson SA, Parikh V, Xi L, Chu S, Montrose MH. Subcellular redistribution of the renal betaine transporter during hypertonic stress. Am J Physiol Cell Physiol 2003;285:C1091–C1100. [DOI] [PubMed] [Google Scholar]
- 5.Olsen M, Sarup A, Larsson OM, Schousboe A. Effect of hyperosmotic conditions on the expression of the betaine–GABA–transporter (BGT-1) in cultured mouse astrocytes. Neurochem Res 2005;30:855–865. [DOI] [PubMed] [Google Scholar]
- 6.Takanaga H, Ohtsuki S, Hosoya K, Terasaki T. GAT2/BGT-1 as a system responsible for the transport of gamma-aminobutyric acid at the mouse blood–brain barrier. J Cereb Blood Flow Metab 2001;21:1232–1239. [DOI] [PubMed] [Google Scholar]
- 7.Mizuta K, Osawa Y, Mizuta F, Xu D, Emala CW. Functional expression of GABAB receptors in airway epithelium. Am J Respir Cell Mol Biol 2008;39:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osawa Y, Xu D, Sternberg D, Sonett JR, D'Armiento J, Panettieri RA, Emala CW. Functional expression of the GABAB receptor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2006;291:L923–L931. [DOI] [PubMed] [Google Scholar]
- 9.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, et al. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med 2007;13:862–867. [DOI] [PubMed] [Google Scholar]
- 10.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA Jr, Yang J, Emala CW Sr. GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2008;294:L1206–L1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallos G, Gleason NR, Virag L, Zhang Y, Mizuta K, Whittington RA, Emala CW. Endogenous gamma-aminobutyric acid modulates tonic guinea pig airway tone and propofol-induced airway smooth muscle relaxation. Anesthesiology 2009;110:748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G., Wang R., Hackett N.R., and Crystal R.G. Enhanced expression of the glutamate decarboxylase 67 in human small airway epithelium in response to cigarette smoking [abstract]. Am J Respir Crit Care Med 2009;179:A1904. [Google Scholar]
- 13.Kotlikoff MI, Kamm KE. Molecular mechanisms of beta-adrenergic relaxation of airway smooth muscle. Annu Rev Physiol 1996;58:115–141. [DOI] [PubMed] [Google Scholar]
- 14.Gallos G, Gleason NR, Zhang Y, Pak SW, Sonett JR, Yang J, Emala CW. Activation of endogenous GABAA channels on airway smooth muscle potentiates isoproterenol-mediated relaxation. Am J Physiol Lung Cell Mol Physiol 2008;295:L1040–L1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J 2007;30:134–155. [DOI] [PubMed] [Google Scholar]
- 16.Warner SM, Knight DA. Airway modeling and remodeling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol 2008;8:44–48. [DOI] [PubMed] [Google Scholar]
- 17.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol 1960;13:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992;101:916–921. [DOI] [PubMed] [Google Scholar]
- 19.Gleason NR, Gallos G, Zhang Y, Emala CW. The GABAA agonist muscimol attenuates induced airway constriction in guinea pigs in vivo. J Appl Physiol 2009;106:1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol 1994;269:219–224. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen C, Sorensen PO, Egebjerg J. 1-(3-(9H-carbazol-9-yl)-1-propyl)-4-(2-methoxyphenyl)-4-piperidinol, a novel subtype selective inhibitor of the mouse type II GABA-transporter. Br J Pharmacol 1997;120:983–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karakossian MH, Spencer SR, Gomez AQ, Padilla OR, Sacher A, Loo DD, Nelson N, Eskandari S. Novel properties of a mouse gamma-aminobutyric acid transporter (GAT4). J Membr Biol 2005;203:65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krause S, Schwarz W. Identification and selective inhibition of the channel mode of the neuronal GABA transporter 1. Mol Pharmacol 2005;68:1728–1735. [DOI] [PubMed] [Google Scholar]
- 24.Masson J, Sagne C, Hamon M, El MS. Neurotransmitter transporters in the central nervous system. Pharmacol Rev 1999;51:439–464. [PubMed] [Google Scholar]
- 25.Wu Y, Wang W, Richerson GB. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J Neurosci 2001;21:2630–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lammers PE, Beck JA, Chu S, Kempson SA. Hypertonic upregulation of betaine transport in renal cells is blocked by a proteasome inhibitor. Cell Biochem Funct 2005;23:315–324. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Wang W, Díez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron 2007;56:851–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hog S, Greenwood JR, Madsen KB, Larsson OM, Frolund B, Schousboe A, Krogsgaard-Larsen P, Clausen RP. Structure-activity relationships of selective GABA uptake inhibitors. Curr Top Med Chem 2006;6:1861–1882. [DOI] [PubMed] [Google Scholar]
- 29.Tiedje KE, Stevens K, Barnes S, Weaver DF. Beta-alanine as a small molecule neurotransmitter. Neurochem Int 2010;57:177–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.