Abstract
Remodeling of the pulmonary arteries is a common feature among the heterogeneous disorders that cause pulmonary hypertension. In these disorders, the remodeled pulmonary arteries often demonstrate inflammation and an accumulation of pulmonary artery smooth muscle cells (PASMCs) within the vessels. Adipose tissue secretes multiple bioactive mediators (adipokines) that can influence both inflammation and remodeling, suggesting that adipokines may contribute to the development of pulmonary hypertension. We recently reported on a model of pulmonary hypertension induced by vascular inflammation, in which a deficiency of the adipokine adiponectin (APN) was associated with the extensive proliferation of PASMCs and increased pulmonary artery pressures. Based on these data, we hypothesize that APN can suppress pulmonary hypertension by directly inhibiting the proliferation of PASMCs. Here, we tested the effects of APN overexpression on pulmonary arterial remodeling by using APN-overexpressing mice in a model of pulmonary hypertension induced by inflammation. Consistent with our hypothesis, mice that overexpressed APN manfiested reduced pulmonary hypertension and remodeling compared with wild-type mice, despite developing similar levels of pulmonary vascular inflammation in the model. The overexpression of APN was also protective in a hypoxic model of pulmonary hypertension. Furthermore, APN suppressed the proliferation of PASMCs, and reduced the activity of the serum response factor–serum response element pathway, which is a critical signaling pathway for smooth muscle cell proliferation. Overall, these data suggest that APN can regulate pulmonary hypertension and pulmonary arterial remodeling through its direct effects on PASMCs. Hence, the activation of APN-like activity in the pulmonary vasculature may be beneficial in pulmonary hypertension.
Keywords: pulmonary hypertension, pulmonary artery smooth muscle cells, metabolism, adiponectin
CLINICAL RELEVANCE.
Our research provides mechanistic insights into the beneficial effects of adiponectin on pulmonary arterial remodeling in pulmonary hypertension. These data establish that adiponectin can directly suppress remodeling via effects on pulmonary artery smooth muscle cells. In addition, the data provide insights into how metabolism and obesity may affect pulmonary vascular disease.
Pulmonary hypertension is an important pulmonary disorder that leads to significant morbidity and mortality and can occur in association with multiple diseases, many of which share a common pathologic appearance characterized by pulmonary arterial inflammation associated with an abnormal accumulation of pulmonary artery smooth muscle cells (PASMCs) in the pulmonary vasculature (1, 2). Accumulating evidence suggests that pulmonary vascular inflammation is an important stimulus for the pathologic changes seen in various types of pulmonary hypertension in both human and animal models (1–5). A role for inflammation in the pathogenesis of pulmonary hypertension was suggested by studies demonstrating the presence of increased concentrations of cytokines in patients with pulmonary hypertension (6, 7) and the presence of leukocytes in and around the remodeled vasculature of the lung (8–10). Furthermore, in animal models, pulmonary vascular inflammation induces arterial remodeling and pulmonary hypertension (3, 11–14). It has been suggested that inflammatory cells release mediators that stimulate remodeling of the vessel wall, in part by directly promoting the proliferation of PASMCs (3, 5, 15–17).
Recent experimental evidence suggests that adipose tissue may contribute to the pathogenesis of inflammatory vascular diseases such as atherosclerosis through the secretion of multiple bioactive mediators (adipokines) that influence energy homeostasis, inflammation, and tissue remodeling (18–20). One of the most important adipokines is adiponectin (APN), which has a wide range of metabolic, anti-inflammatory, and antiproliferative activities (21). Interestingly, persons with obesity have lower amounts of circulating APN compared with lean individuals, suggesting that decreased concentrations of APN may contribute to the increased incidence of vascular diseases associated with obesity. Links between APN and pulmonary vascular disease are not fully defined. However, recent data from murine models of pulmonary hypertension suggest that APN deficiency can increase the severity of pulmonary vascular inflammation, pulmonary arterial remodeling, and pulmonary hypertension (4, 17, 22, 23). In our previous study, APN-deficient (APN−/−) mice developed increased eosinophil recruitment into the lungs and increased pulmonary vascular remodeling after the induction of allergic vascular inflammation (17). This increased remodeling was largely secondary to the proliferation of PASMCs within the pulmonary arteries. Although APN deficiency may have exacerbated the pulmonary vascular disease in this model indirectly via its effects on vascular inflammation, other data suggest that APN may also directly inhibit pulmonary arterial remodeling, independent of its effects on inflammation (4, 24, 25). Based on these data, we hypothesize that APN could suppress pulmonary arterial remodeling via direct suppressive effects on PASMC proliferation (25). In the data presented here, we demonstrate that APN can suppress pulmonary arterial remodeling and pulmonary hypertension independently of its effects on pulmonary inflammation. Additional in vitro data show that APN can directly suppress the proliferation of PASMCs, and that APN decreases the activity of the serum response factor–serum response element (SRF-SRE) pathway, a critical signaling pathway for smooth muscle cell (SMC) proliferation (26–31). These data support a potential role for APN in the pathogenesis of pulmonary hypertension by modulating pulmonary arterial remodeling. Some of the results of this study were previously reported as an abstract (32).
MATERIALS AND METHODS
Murine Experiments
APN−/− mice and ΔGly-APN mice were backcrossed more than seven generations onto a C57BL/6 background (17, 33). Wild-type C57BL/6 control mice were obtained from the National Cancer Institute (Bethesda, MD). Male mice were used at age 6–8 weeks. All protocols were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital.
Murine Models of Pulmonary Hypertension
The high-dose ovalbumin (OVA) model of pulmonary hypertension was performed as described (3). PBS was used to challenge control mice. Mice were analyzed 24 hours after the last challenge. The hypoxic model of pulmonary hypertension was performed as previously described (17, 34). We also developed an additional high-dose OVA model that induced pulmonary hypertension at normoxia in wild-type mice. Additional information is available in the online supplement.
Murine Analysis
Bronchoalveolar lavage (BAL), removal of the lungs, and analysis were performed as described (17, 35). Additional information is available in the online supplement.
Histologic Analyses
Histopathologic and quantitative measurements of pulmonary artery wall thickness were performed as previously described (17). Additional information is available in the online supplement.
Hemodynamic Studies
Right-ventricular systolic pressure (RVSP) was measured as previously described (17). Additional information is available in the online supplement.
Quantification of Gene and Protein Expression
RNA was purified from the lung and analyzed by quantitative RT-PCR (QPCR), as previously described (35). Additional information is available in the online supplement. Lung extracts and serum were collected, diluted 1:1,000 and 1:10,000, respectively, and used in a commercial ELISA kit to measure protein concentrations of mouse adiponectin (B-Bridge International, Mountain View, CA).
APN Binding Assay
Information regarding the APN binding assay is available in the online supplement.
Preparation of Lung Protein Extracts
Lungs were homogenized in PBS containing protease inhibitors (Roche, Indianapolis, IN). Crude lysates were centrifuged at 900 × g to remove debris, and the supernatant was filtered and collected. Additional information is available in the online supplement.
Culture and Proliferation Assay of PASMCs
PASMCs were isolated from the main pulmonary arteries of male C57BL/6 mice, as previously described (36). Cells were used in experiments after passages 3–6. For proliferation assays, PASMCs were seeded in 96-well plates at 5,000 cells per well. PASMCs were starved in 0.1% BSA medium overnight. Purified APN (ALEXIS-Enzo Life Sciences, Farmingdale, NY) or 18 μg of lung protein extracts were added, and cells were incubated for 72 hours. Proliferation was assayed using the CyQUANT NF Cell Proliferation Assay Kit (Invitrogen, Carlsbad, CA).
SRE Activity in PASMCs
For the determination of SRE activity, PASMCs were transfected with SRE–luciferase and Renilla–luciferase (10:1), using Lipofectamine 2000 (Invitrogen) (38). The next day, cells were serum-starved for 2 hours and then stimulated with serum, serum plus APN, or lung protein extracts for 6 hours. SRE activity was assessed using a dual-luciferase reporter assay system (Promega, Madison, WI), as previously described (37).
Statistical Analysis
Results are shown as mean ± SEM. Groups were compared using a Student's t test. Between-group comparisons of means were performed by 2-way ANOVA. P < 0.05 was regarded as significant.
RESULTS
APN Inhibits Pulmonary Arterial Remodeling In Vivo
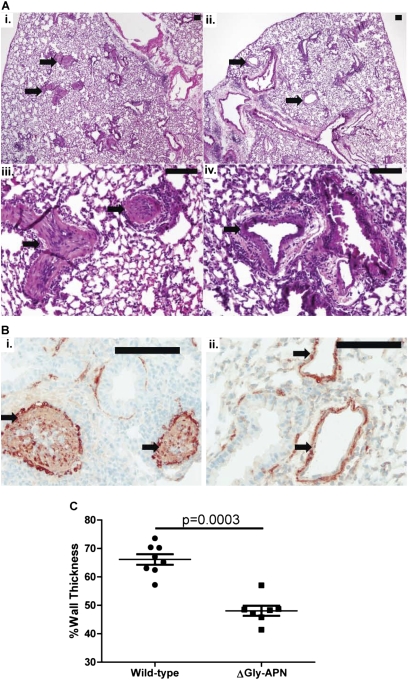
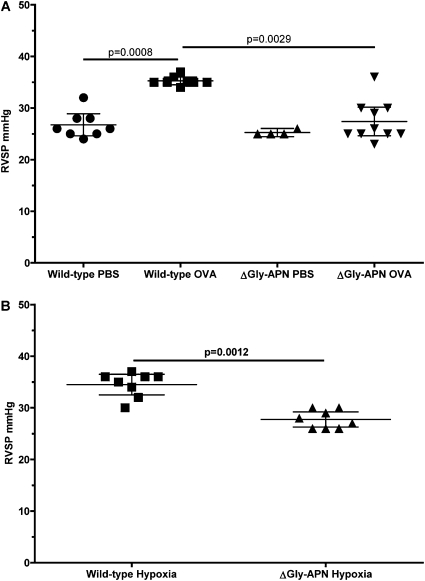
We hypothesized that APN can suppress pulmonary arterial remodeling via its direct effects on the proliferation of PASMCs. Thus we sought to determine if elevations in the level of APN would exert effects on pulmonary vascular remodeling, independent of its effects on inflammation. For these experiments, we used a transgenic strain of mice reported to have 2- to 3-fold higher APN concentrations in serum (ΔGly-APN mice) compared with wild-type mice (33). These mice were produced by the transgenic insertion of a mutant form of APN. Unexpectedly, these mice exhibited increased expression and secretion of native APN, without secretion of the mutant form. These mice were protected from high fat diet–induced insulin resistance and had a modified profile of fat deposition, but were otherwise normal and of equal size and weight to age-matched and sex-matched wild-type mice (the ΔGly-APN mice were backcrossed nine generations onto a C57BL/6 background). We confirmed that the ΔGly-APN mice had increased concentrations of APN in plasma compared with wild-type mice, as demonstrated previously (data not shown) (33). Next we used male ΔGly-APN and wild-type control mice in the high-dose OVA model of pulmonary hypertension (3). Only male mice were used for these experiments, because of their more dramatic phenotype in the OVA model of lung inflammation. As seen in previous studies, PBS challenges of wild-type mice did not result in detectable inflammation or pulmonary arterial remodeling (3, 17). Moreover, no inflammation or pulmonary arterial remodeling was detectable in the PBS-challenged ΔGly-APN mice (data not shown). OVA-challenged wild-type mice developed prominent eosinophilic vascular inflammation, associated with pulmonary arterial remodeling (Figures 1Ai and 1Aiii). However, OVA-challenged ΔGly-APN mice exhibited less pulmonary arterial remodeling than wild-type mice, despite the development of eosinophilic vascular inflammation (Figures 1Aii and 1Aiv). Staining for α-SMC actin confirmed that cells within the vasculature were SMCs (Figure 1B), and image analysis indicated a significant reduction in pulmonary arterial wall thickness in ΔGly-APN mice compared with vessels in wild-type mice (Figure 1C). Although this high-dose OVA model produces extensive pulmonary artery remodeling, it does not produce elevated pulmonary artery pressures in wild-type mice unless the mice are rendered hypoxic during measurements (3). Consequently, we developed a modified model with more frequent dosing of OVA for a longer period. Wild-type mice in this model developed similar extensive pulmonary arterial remodeling, but also demonstrated elevations in pulmonary artery pressure, as assessed by RVSP, while breathing room air (Figure 2A). Consistent with our remodeling data, ΔGly-APN mice had reduced right ventricular pressures compared with wild-type mice, with concentrations similar to those in PBS-challenged mice (Figure 2A). Other hemodynamic measurements, including systemic blood pressure, heart rate, and right ventricular diastolic pressure, were not different between the two genotypes after either PBS or OVA challenges (data not shown).
Figure 1.
Overexpression of adiponectin (APN) reduces pulmonary vascular remodeling. (A) Representative hematoxylin and eosin–stained lung sections from wild-type mice (i, ×40 magnification; iii, ×200 magnification) and ΔGly-APN mice (ii, ×40 magnification; iv, ×200 magnification) after ovalbumin (OVA) immunization and challenge (n = 7–8 mice per group). Arrows indicate pulmonary arteries. Bars, 100 μm. (B) Representative α-smooth muscle cell actin staining of a lung section from a wild-type mouse (i, ×200 magnification) and ΔGly-APN mouse (ii, ×400 magnification) after OVA immunization and challenge. Arrows indicate pulmonary arteries. Bars, 100 μm. (C) Vessel medial wall thickness (percentage of total) in medium and small pre-acinar blood vessels in lung sections from wild-type (circles) and ΔGly-APN (squares) mice after OVA immunization and challenge (n = 7–8 mice per group).
Figure 2.
APN overexpression inhibits the development of pulmonary hypertension. (A) Right ventricular systolic pressure (RVSP) in wild-type and ΔGly-APN mice measured after OVA immunization and OVA or PBS challenges. Circles, squares, triangles and inverse triangles indicate data from wild-type PBS, wild-type OVA, ΔGly-APN PBS, and ΔGly-APN OVA mice, respectively (n = 4–10 mice per group). (B) RVSP in wild-type and ΔGly-APN mice measured after 3 weeks of hypoxia. Squares and triangles indicate data from wild-type hypoxia mice and ΔGly-APN hypoxia mice, respectively (n = 8 mice per group).
To provide further evidence for the effects of APN on the pulmonary vasculature and to investigate whether the effects of adiponectin were dependent on allergic inflammation, we used ΔGly-APN mice in the hypoxic model of pulmonary hypertension. After 3 weeks of continuous exposure to 10% oxygen, ΔGly-APN mice manifested lower RVSP than wild-type mice (Figure 2B). Control mice maintained in normoxia did not have elevated RVSPs, and the systemic hemodynamics were not different between the two genotypes with either normoxia or hypoxia (data not shown). These data demonstrate that APN can modulate pulmonary hypertension in two different models of disease.
APN Does Not Affect Pulmonary Vascular Inflammation In Vivo
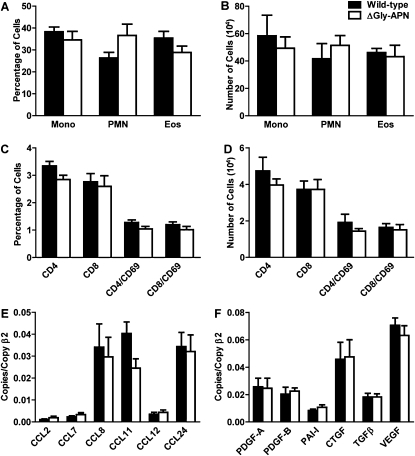
We also quantified the inflammatory response in ΔGly-APN and wild-type mice in the high-dose OVA model of pulmonary hypertension. As already stated, we did not observe significant inflammation in the PBS-challenged wild-type and ΔGly-APN mice, as expected (data not shown). Surprisingly, the numbers of inflammatory cells around the pulmonary vessels (Figure 1A) and in BAL fluid were not different between OVA-challenged ΔGly-APN mice and wild-type mice (Figures 3A and 3B). Lymphocyte recruitment and activation were also unaffected by the overexpression of APN (Figures 3C and 3D). In addition, we saw no effects of increased APN concentrations on the lung RNA levels of a panel of chemokines that had been upregulated in APN−/− mice in a model of pulmonary hypertension (17), or of a panel of growth factors that could regulate the proliferation of PASMCs in response to inflammation in the lungs (Figures 3E and 3F). As shown previously (17), PBS-challenged mice did not develop increased concentrations of chemokines or growth factors (data not shown). Thus, despite the prominent effects of APN on pulmonary arterial remodeling, we saw no inhibition of inflammation because of increased concentrations of APN. These data suggest that APN exerts direct effects on the remodeling response, independent of its effects on inflammation.
Figure 3.
APN overexpression does not affect inflammation. (A and B) Percentage and number of mononuclear cells (Mono), neutrophils (PMN), and eosinophils (Eos) in bronchoalveolar lavage (BAL) fluid of wild-type and ΔGly-APN mice after OVA immunization and challenge (n = 8 mice per group). (C and D) Percentage and number of lymphocyte subsets in BAL fluid of wild-type and ΔGly-APN mice after OVA immunization and challenge (n = 8 mice per group). Chemokine (E) and growth factor (F) RNA expression in lungs of wild-type and ΔGly-APN mice after OVA immunization and challenge, expressed as copy number of indicated transcripts, divided by number of copies of the housekeeping gene β2-microglobulin (n = 8 mice per group). CCL, chemokine (C-C motif) ligand; PDGF-A, platelet-derived growth factor isoform A; PDGF-B, platelet-derived growth factor isoform B; PAI-I, plasminogen activator inhibitor 1; CTGF, connective tissue growth factor; TGFβ, transforming growth factor beta; EGF, vascular endothelial growth factor.
APN Suppresses the Proliferation of PASMCs In Vitro
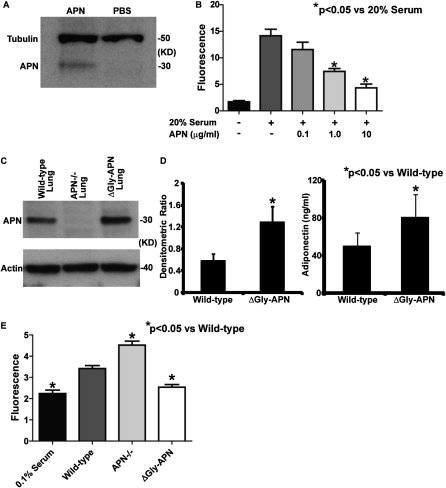
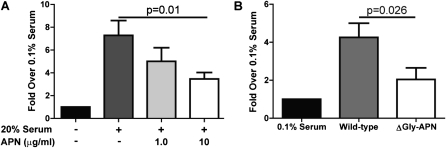
Our in vivo data suggest a direct suppressive effect of APN on pulmonary arterial remodeling. Therefore, we reasoned that APN can directly influence the proliferation of PASMCs. To address this question, we isolated and cultured PASMCs from wild-type mice (36), and used QPCR to measure the expression of the known APN receptors AdipoR1, AdipoR2, T-cadherin, and calreticulin. Both AdipoR1 and AdipoR2 were detected in RNA isolated from cultured PASMCs, but not the other receptors (data not shown). To demonstrate that APN binds to PASMCs, we incubated PASMCs with purified APN on ice for 30 minutes, washed the cells with cold PBS, and isolated the cellular proteins. Western blotting of the protein extracts with an antibody to APN demonstrated the presence of APN, consistent with the binding of APN to PASMCs (Figure 4A). We then stimulated cultured PASMCs with serum and increasing concentrations of APN, and measured proliferation after 72 hours. As shown by others (25), APN suppressed the proliferation of PASMCs in a dose-dependent manner (Figure 4B).
Figure 4.
APN reduces the proliferation of pulmonary artery smooth muscle cells (PASMCs). (A) Western blot of protein isolated from PASMCs after incubation with APN or PBS and staining with the indicated antibodies. Data shown are from one of three independent experiments. (B) Proliferation of PASMCs after 72 hours of stimulation under the indicated conditions, as measured by a fluorescent assay. Each condition was performed in triplicate. PASMCs were prepared from three wild-type mice (*P < 0.05). (C) Western blot of lung protein extracts prepared from OVA-challenged wild-type, ΔGly-APN, and APN-deficient (APN−/−) mice, and stained with the indicated antibodies. The experiment was repeated three times. (D) Adiponectin protein concentrations in the lungs of OVA-challenged wild-type and ΔGly-APN mice were assayed by densitometry of Western blots (expressed as density ratio between APN band and α-actin band) and protein ELISA (n = 3–4 samples per group). (E) Proliferation of PASMCs after 72 hours of stimulation with lung protein extracts from OVA-challenged wild-type and ΔGly-APN mice, as measured by a fluorescent assay. Each condition was performed in triplicate, using four mice per group for lung samples. PASMCs were prepared from three wild-type mice (*P < 0.05).
To provide a more relevant test of the situation in vivo, we also used protein isolated from the lungs of wild-type, ΔGly-APN, and APN−/− mice after high-dose OVA immunization and challenge in a proliferation assay. APN protein was detected in the lung extracts of wild-type and ΔGly-APN mice, but not in those of APN−/− mice, and lung APN concentrations were higher in ΔGly-APN mice than in wild-type mice, as measured by Western blotting and ELISA (Figures 4C and 4D). We incubated these lung extracts with cultured wild-type PASMCs for 72 hours. Compared with protein extracted from wild-type lungs, protein from the lungs of APN−/− mice led to an increased proliferation of PASMCs, whereas protein from the lungs of ΔGly-APN mice stimulated less proliferation of PASMCs (Figure 4D). These data suggest that the APN−/− mice in our model had a lung protein milieu that enhanced the proliferation of PASMCs relative to wild-type mice, whereas the overexpression of APN produced a lung protein profile that inhibited the proliferation of PASMCs. In addition, these data are consistent with our hypothesis that APN can directly regulate pulmonary arterial remodeling by suppressing the proliferation of PASMCs.
APN Suppresses SRE Activity in PASMCs
In response to various stimuli, SMCs can change their phenotype from contractile to highly proliferative and synthetic (38). This process is critical in the response of SMCs to physiologic stress, and is mediated in part via the SRF-SRE pathway. This pathway was demonstrated to play a central role in regulating many SMC-specific genes, and is essential for the development and proliferation of SMCs (28, 29, 31, 39–41). We hypothesized that APN may modulate PASMC proliferation in part via changes in SRF-SRE activity. To explore this possibility, we transfected PASMCs with an SRE–luciferase construct (37), and treated the cells with 20% serum and increasing concentrations of APN. SRE activity was then measured with a dual-luciferase reporter assay. As expected, SRE activity was increased in PASMCs with 20% serum treatment, but APN suppressed the SRE response to serum in a dose-dependent manner (Figure 5A). We also tested the effects of lung protein extracts taken from wild-type and ΔGly-APN mice after high-dose OVA immunization and challenge. Consistent with the proliferation data, protein from the lungs of ΔGly-APN mice induced less SRE activity than protein from the lungs of wild-type mice (Figure 5B). These data suggest that the antiproliferative effect of APN on PASMCs could be mediated in part via the suppression of SRF-SRE activity in these cells.
Figure 5.
APN suppresses activity of serum response element (SRE) in PASMCs. (A) Relative luciferase activity (ratio of SRE–luciferase to Renilla–luciferase in each condition, compared with activity in PASMCs in serum-free conditions) in PASMCs after cotransfection with SRE–luciferase and Renilla–luciferase constructs and 6 hours of stimulation under the indicated conditions. Each condition was performed in duplicate, and the experiment was repeated three times. PASMCs were prepared from six wild-type mice. (B) Relative luciferase activity of PASMCs after cotransfection with SRE–luciferase and Renilla–luciferase constructs and 6 hours of stimulation with lung protein extracts from OVA-challenged wild-type and ΔGly-APN mice. Each condition was performed in duplicate, and the experiment was repeated three times. PASMCs were prepared from six wild-type mice.
DISCUSSION
We provide evidence that APN can mitigate pulmonary arterial remodeling in vivo. Furthermore, data from in vitro studies confirm a direct suppressive effect of APN on the proliferation of PASMCs, and suggest that the effect may be mediated in part by a downregulation of the SRF-SRE pathway. These data complement findings from our previous study, which demonstrated that APN−/− mice in this model of pulmonary hypertension had increased arterial remodeling and elevated pulmonary artery pressures (17). Overall, these studies add to the growing evidence linking metabolism, inflammation, and pulmonary vascular disease (3–5, 16, 24, 25), and suggest a potential therapeutic role for the manipulation of adipokine activity in pulmonary hypertension.
The discovery and characterization of multiple bioactive mediators derived from adipose tissues that can influence immunity and tissue repair clearly establish a link between metabolism, vascular inflammation, and remodeling (42). Although most of the data are derived from studies of systemic vascular processes, an appreciation of the effects on pulmonary vascular disease has been increasing. Studies of human samples and animal models support a mechanistic role for insulin resistance, apolipoprotein E deficiency, and peroxisome proliferator–activated receptor-γ (PPAR-γ) activity in the pathogenesis of pulmonary hypertension (4, 24, 25, 43, 44). Furthermore, treatment with PPAR-γ agonists, such as rosiglitazone, was shown to mitigate pulmonary hypertension and pulmonary arterial remodeling in animal models, similar to the effects on systemic vascular remodeling (45–47). An increased incidence of pulmonary hypertension and pulmonary vascular remodeling in obesity is also evident (48–50). In light of the obesity-associated downregulation of APN expression along with its anti-remodeling activity, these data suggest that APN may play a mechanistic role, connecting obesity and metabolism with increased pulmonary arterial remodeling. In fact, Hansman and colleagues suggested that the elevation of APN by PPAR-γ agonists could explain the beneficial effects of this therapy on pulmonary hypertension (25). Our initial study was among the first to demonstrate a direct effect of APN on pulmonary hypertension (17). Two subsequent studies have reported data consistent with our experiments (22, 23). Together, these data strongly suggest that APN has a protective role in pulmonary vascular disease (4).
In our murine models of OVA-induced pulmonary hypertension (3, 17), allergic pulmonary inflammation was induced to stimulate pulmonary vascular remodeling and pulmonary hypertension. There is increasing evidence suggesting that perivascular inflammation may contribute to the pathogenesis of the obstructive arterial lesions seen in pulmonary hypertension (5, 51). This may be most relevant in pulmonary hypertension related to infections, such as schistosomiasis (the most common form of pulmonary hypertension worldwide) (52) and autoimmunity, but may also be important in other forms of pulmonary hypertension (51). Inflammation likely provides a direct stimulus for vascular remodeling, possibly via the release of growth factors and other mediators, or via metabolic changes such as focal hypoxia (53).
Eosinophils in particular are known as potent sources of growth factors that are mitogenic for SMCs (54), and were shown to be necessary for airway remodeling in models of chronic allergic airway inflammation (55, 56). Given that APN−/− mice exhibited increased eosinophil recruitment into the lung in our model of pulmonary hypertension, some of the increased remodeling seen in these mice may result from increased inflammation. However, ΔGly-APN mice, which exhibit reduced pulmonary arterial remodeling, did not show reduced inflammation, suggesting that APN directly suppresses the growth of PASMCs, independent of its effects on inflammation. We speculate that the very strong stimulus for inflammation in this model of pulmonary hypertension overwhelmed any anti-inflammatory effects of APN.
The anti-remodeling activities of APN are well-documented in the systemic vasculature, liver, lung, and heart (57–60). In vitro, APN suppresses the proliferation and migration of vascular SMCs (61), and in vivo, APN−/− mice exhibit an increased accumulation of SMCs after vascular mechanical injury (62). These data suggest that APN could also inhibit pulmonary arterial remodeling in pulmonary hypertension (specifically, the accumulation of SMCs in the pulmonary vasculature). Consistent with this idea, APN was shown to bind to the pulmonary vascular endothelium, and adenovirus-mediated overexpression of APN mitigated pulmonary vascular remodeling in a hypoxic model of pulmonary hypertension (22, 23). Thus, based on these data and the data presented here, APN is likely to suppress pulmonary vascular remodeling in pulmonary hypertension via its direct effects on PASMCs. However, the molecular mechanisms for this suppression remain unclear.
One of the most prominent features of vascular remodeling is an increased mass of SMCs. Unlike other muscle cells, SMCs remain plastic, and can alternate between a contractile state and a proliferative state in response to pathophysiologic stress. Thus, the increased numbers of muscle cells seen in pulmonary vascular remodeling are likely derived from existing SMCs or myofibrolasts that change into a highly proliferative and synthetic phenotype before forming new muscle (63, 64). We reasoned that one mechanism by which APN could affect the proliferation of PASMCs involves modulating the phenotype of PASMCs. Because the SRF-SRE pathway is one of the major regulators of the SMC phenotype, we hypothesized that this pathway could be a potential target of the antiproliferative activity of APN. Consistent with this, gene-expression profiling of laser-microdissected intrapulmonary arteries in mice with hypoxia-induced pulmonary hypertension demonstrated an upregulation of SRF, suggesting a role for this pathway in the pathogenesis of pulmonary hypertension (65).
SRF is a phylogenetically conserved, MADs-box transcription factor that mediates the rapid transcriptional response to growth and differentiation signals in SMCs. SRF-SRE controls the expression of more than 200 genes, including immediate early genes such as c-fos and Egr1, which are involved in cellular proliferation (30). The knockdown of SRF in primary vascular smooth muscle cells leads to cell-cycle arrest in G1 with impaired proliferation (31), and the inducible deletion of SRF in the SMCs of adult mice leads to a thinning of smooth muscle layers in the gut and bladder, with an associated dilation of the intestinal tract and the death of mice within 2–3 weeks of SRF deletion (66, 67). Thus, factors that affect the SRF-SRE pathway will likely exert a profound effect on the proliferative capacity of SMCs, and could influence vascular remodeling. Although our data clearly demonstrate an effect of APN on SRF activity, the exact molecular mechanisms behind this interaction remain unknown.
In addition to its effects on SRF, APN may modulate the growth and proliferation of PASMCs in pulmonary hypertension via other mechanisms. For example, APN was shown to inhibit the growth factor–mediated activation of the mammalian target of rapamycin (mTOR) via adenosine monophosphate-activated kinase, which increases the tuberous sclerosis complex–mediated suppression of mTOR (68). APN also directly binds growth factors such as platelet-derived growth factor isoform B homodimer, which could work to limit proliferation by sequestering it from its receptor in PASMCs (4, 69). Furthermore, APN inhibits Rho (Ras homologue) kinase signaling, which was shown to play a role in the development of pulmonary hypertension (70, 71).
In conclusion, our study adds to the growing body of evidence suggesting that metabolism may influence the pathogenesis of pulmonary hypertension. Specifically, we demonstrated that APN inhibits the development of pulmonary arterial remodeling and pulmonary hypertension in this model. Furthermore, we demonstrated that APN can inhibit the SRF-SRE pathway, which suggests a novel mechanism for our findings. These data have direct relevance to the pathogenesis of pulmonary hypertension, and may identify potential therapeutic targets in this important disorder.
Supplementary Material
Acknowledgments
The authors thank Barry Sandall and Carol Leary for their technical support.
This work was supported by National Institutes of Health grants HL088297 (B.D.M.), T32 HL07874 (B.D.M. and M.W.), DK55758 (P.E.S.), and HL074352 (K.D.B.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0316OC on November 12, 2010
Author Disclosure: K.D.B. received sponsored grants from Ikaria LLC and the National Institutes of Health for more than $100,001 each. B.M. received sponsored grants from the Roche Organ Transplant Research Foundation for $50,001–$100,000 and the National Institutes of Health for more than $100,001. P.E.S. received sponsored grants from the National Institutes of Health and the Juvenile Diabetes Research Foundation for more than $100,001 each. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 2004;351:1655–1665. [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 2010;121:2045–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 2008;205:361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansmann G, Rabinovitch M. The protective role of adiponectin in pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol 2010;298:L1–L2. [DOI] [PubMed] [Google Scholar]
- 5.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2008;118:2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995;151:1628–1631. [DOI] [PubMed] [Google Scholar]
- 7.Fartoukh M, Emilie D, Le Gall C, Monti G, Simonneau G, Humbert M. Chemokine macrophage inflammatory protein–1alpha mRNA expression in lung biopsy specimens of primary pulmonary hypertension. Chest 1998;114:50S–51S. [DOI] [PubMed] [Google Scholar]
- 8.Dorfmuller P, Humbert M, Perros F, Sanchez O, Simonneau G, Muller KM, Capron F. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol 2007;38:893–902. [DOI] [PubMed] [Google Scholar]
- 9.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J 2005;26:1110–1118. [DOI] [PubMed] [Google Scholar]
- 10.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 11.Tormanen KR, Uller L, Persson CG, Erjefalt JS. Allergen exposure of mouse airways evokes remodeling of both bronchi and large pulmonary vessels. Am J Respir Crit Care Med 2005;171:19–25. [DOI] [PubMed] [Google Scholar]
- 12.Rydell-Tormanen K, Uller L, Erjefalt JS. Remodeling of extra-bronchial lung vasculature following allergic airway inflammation. Respir Res 2008;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rydell-Tormanen K, Johnson JR, Fattouh R, Jordana M, Erjefalt JS. Induction of vascular remodeling in the lung by chronic house dust mite exposure. Am J Respir Cell Mol Biol 2008;39:61–67. [DOI] [PubMed] [Google Scholar]
- 14.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009;104:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54(Suppl 1)S20–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009;54:S10–S19. [DOI] [PubMed] [Google Scholar]
- 17.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol 2009;41:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol 2005;115:925–927. [DOI] [PubMed] [Google Scholar]
- 19.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 2007;18:313–325. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772–783. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110:267–278. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa Y, Kishida K, Kihara S, Funahashi T, Shimomura I. Adiponectin ameliorates hypoxia-induced pulmonary arterial remodeling. Biochem Biophys Res Commun 2009;382:183–188. [DOI] [PubMed] [Google Scholar]
- 23.Summer R, Fiack CA, Ikeda Y, Sato K, Dwyer D, Ouchi N, Fine A, Farber HW, Walsh K. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol 2009;297:L432–L438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, et al. An antiproliferative BMP-2/PPARgamma/ApoE axis in human and murine smcs and its role in pulmonary hypertension. J Clin Invest 2008;118:1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator–activated receptor–gamma activation. Circulation 2007;115:1275–1284. [DOI] [PubMed] [Google Scholar]
- 26.Camoretti-Mercado B, Dulin NO, Solway J. Serum response factor function and dysfunction in smooth muscle. Respir Physiol Neurobiol 2003;137:223–235. [DOI] [PubMed] [Google Scholar]
- 27.Camoretti-Mercado B, Liu HW, Halayko AJ, Forsythe SM, Kyle JW, Li B, Fu Y, McConville J, Kogut P, Vieira JE, et al. Physiological control of smooth muscle–specific gene expression through regulated nuclear translocation of serum response factor. J Biol Chem 2000;275:30387–30393. [DOI] [PubMed] [Google Scholar]
- 28.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 2003;35:577–593. [DOI] [PubMed] [Google Scholar]
- 29.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol 2007;292:C70–C81. [DOI] [PubMed] [Google Scholar]
- 30.Posern G, Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol 2006;16:588–596. [DOI] [PubMed] [Google Scholar]
- 31.Werth D, Grassi G, Konjer N, Dapas B, Farra R, Giansante C, Kandolf R, Guarnieri G, Nordheim A, Heidenreich O. Proliferation of human primary vascular smooth muscle cells depends on serum response factor. Eur J Cell Biol 2010;89:216–224. [DOI] [PubMed] [Google Scholar]
- 32.Weng M, Raher MJ, Leyton P, Combs TP, Scherer PE, Bloch KD, Medoff BD. Adiponectin modulates pulmonary vascular remodeling by inhibiting pulmonary artery smooth muscle cell proliferation. Am J Respir Crit Care Med 2010;181:A1184. [Google Scholar]
- 33.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 2004;145:367–383. [DOI] [PubMed] [Google Scholar]
- 34.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 2004;287:L1241–L1247. [DOI] [PubMed] [Google Scholar]
- 35.Medoff BD, Seed B, Jackobek R, Zora J, Yang Y, Luster AD, Xavier R. Carma1 is critical for the development of allergic airway inflammation in a murine model of asthma. J Immunol 2006;176:7272–7277. [DOI] [PubMed] [Google Scholar]
- 36.Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA, Kawai N, Bloch KD. Bone morphogenetic protein (Bmp) Type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J Biol Chem 2008;283:3877–3888. [DOI] [PubMed] [Google Scholar]
- 37.Ishiguro K, Xavier R. Homer-3 regulates activation of serum response element in T cells via its EVH1 domain. Blood 2004;103:2248–2256. [DOI] [PubMed] [Google Scholar]
- 38.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004;84:767–801. [DOI] [PubMed] [Google Scholar]
- 39.Kumar MS, Owens GK. Combinatorial control of smooth muscle–specific gene expression. Arterioscler Thromb Vasc Biol 2003;23:737–747. [DOI] [PubMed] [Google Scholar]
- 40.Owens GK. Molecular control of vascular smooth muscle cell differentiation. Acta Physiol Scand 1998;164:623–635. [DOI] [PubMed] [Google Scholar]
- 41.Solway J, Forsythe SM, Halayko AJ, Vieira JE, Hershenson MB, Camoretti-Mercado B. Transcriptional regulation of smooth muscle contractile apparatus expression. Am J Respir Crit Care Med 1998;158:S100–S108. [DOI] [PubMed] [Google Scholar]
- 42.Krenning G, Moonen JR, Harmsen MC. Pleiotropism of adiponectin: inflammation, neovascularization, and fibrosis. Circ Res 2009;104:1029–1031. [DOI] [PubMed] [Google Scholar]
- 43.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator–activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 2003;92:1162–1169. [DOI] [PubMed] [Google Scholar]
- 44.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator–activated receptor–gamma in mice causes PDGF receptor–beta–dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol 2009;297:L1082–L1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crossno JT Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 2007;292:L885–L897. [DOI] [PubMed] [Google Scholar]
- 46.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 2010;42:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EK, Lee J-H, Oh Y-M, Lee Y-S, Lee S-D. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology 2010;15:659–668. [DOI] [PubMed] [Google Scholar]
- 48.Haque AK, Gadre S, Taylor J, Haque SA, Freeman D, Duarte A. Pulmonary and cardiovascular complications of obesity: an autopsy study of 76 obese subjects. Arch Pathol Lab Med 2008;132:1397–1404. [DOI] [PubMed] [Google Scholar]
- 49.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001;104:2797–2802. [DOI] [PubMed] [Google Scholar]
- 50.Taraseviciute A, Voelkel NF. Severe pulmonary hypertension in postmenopausal obese women. Eur J Med Res 2006;11:198–202. [PubMed] [Google Scholar]
- 51.Hamid R, Newman JH. Evidence for inflammatory signaling in idiopathic pulmonary artery hypertension: TRPC6 and nuclear factor–kappaB. Circulation 2009;119:2297–2298. [DOI] [PubMed] [Google Scholar]
- 52.Crosby A, Jones FM, Southwood M, Stewart S, Schermuly R, Butrous G, Dunne DW, Morrell NW. Pulmonary vascular remodeling correlates with lung eggs and cytokines in murine schistosomiasis. Am J Respir Crit Care Med 2010;181:279–288. [DOI] [PubMed] [Google Scholar]
- 53.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol 2010;184:4062–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masu K, Ohno I, Suzuki K, Okada S, Hattori T, Shirato K. Proliferative effects of eosinophil lysates on cultured human airway smooth muscle cells. Clin Exp Allergy 2002;32:595–601. [DOI] [PubMed] [Google Scholar]
- 55.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5–deficient mice. J Clin Invest 2004;113:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 57.Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, et al. Enhanced carbon tetrachloride–induced liver fibrosis in mice lacking adiponectin. Gastroenterology 2003;125:1796–1807. [DOI] [PubMed] [Google Scholar]
- 58.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia–reperfusion injury through AMPK- and COX-2–dependent mechanisms. Nat Med 2005;11:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, et al. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol 2008;294:L1035–L1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002;8:731–737. [DOI] [PubMed] [Google Scholar]
- 61.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB–binding protein and regulates growth factor–induced common postreceptor signal in vascular smooth muscle cell. Circulation 2002;105:2893–2898. [DOI] [PubMed] [Google Scholar]
- 62.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, et al. Role of adiponectin in preventing vascular stenosis: the missing link of adipo-vascular axis. J Biol Chem 2002;277:37487–37491. [DOI] [PubMed] [Google Scholar]
- 63.Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2004;1:176–183. [DOI] [PubMed] [Google Scholar]
- 64.Gizycki MJ, Adelroth E, Rogers AV, O'Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol 1997;16:664–673. [DOI] [PubMed] [Google Scholar]
- 65.Kwapiszewska G, Wilhelm J, Wolff S, Laumanns I, Koenig IR, Ziegler A, Seeger W, Bohle RM, Weissmann N, Fink L. Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension. Respir Res 2005;6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angstenberger M, Wegener JW, Pichler BJ, Judenhofer MS, Feil S, Alberti S, Feil R, Nordheim A. Severe intestinal obstruction on induced smooth muscle–specific ablation of the transcription factor SRF in adult mice. Gastroenterology 2007;133:1948–1959. [DOI] [PubMed] [Google Scholar]
- 67.Mericskay M, Blanc J, Tritsch E, Moriez R, Aubert P, Neunlist M, Feil R, Li Z. Inducible mouse model of chronic intestinal pseudo-obstruction by smooth muscle–specific inactivation of the SRF gene. Gastroenterology 2007;133:1960–1970. [DOI] [PubMed] [Google Scholar]
- 68.Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F. Adiponectin sensitizes insulin signaling by reducing P70 S6 kinase–mediated serine phosphorylation of IRS-1. J Biol Chem 2007;282:7991–7996. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 2005;280:18341–18347. [DOI] [PubMed] [Google Scholar]
- 70.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 2004;287:L656–L664. [DOI] [PubMed] [Google Scholar]
- 71.Man K, Ng KT, Xu A, Cheng Q, Lo CM, Xiao JW, Sun BS, Lim ZX, Cheung JS, Wu EX, et al. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin Cancer Res 2010;16:967–977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.