Abstract
Chlorine (Cl2) gas exposure poses an environmental and occupational hazard that frequently results in acute lung injury. There is no effective treatment. We assessed the efficacy of antioxidants, administered after exposure, in decreasing mortality and lung injury in C57BL/6 mice exposed to 600 ppm of Cl2 for 45 minutes and returned to room air. Ascorbate and deferoxamine were administered intramuscularly every 12 hours and by nose-only inhalation every 24 hours for 3 days starting after 1 hour after exposure. Control mice were exposed to Cl2 and treated with vehicle (saline or water). Mortality was reduced fourfold in the treatment group compared with the control group (22 versus 78%; P = 0.007). Surviving animals in the treatment group had significantly lower protein concentrations, cell counts, and epithelial cells in their bronchoalveolar lavage (BAL). Lung tissue ascorbate correlated inversely with BAL protein as well as with the number of neutrophils and epithelial cells. In addition, lipid peroxidation was reduced threefold in the BAL of mice treated with ascorbate and deferoxamine when compared with the control group. Administration of ascorbate and deferoxamine reduces mortality and decreases lung injury through reduction of alveolar–capillary permeability, inflammation, and epithelial sloughing and lipid peroxidation.
Keywords: acute lung injury, oxidative stress, survival, aerosols, antioxidants
CLINICAL RELEVANCE.
Chlorine (Cl2) gas exposure poses an environmental and occupational hazard that frequently results in acute lung injury. Our studies show that administration of ascorbate and deferoxamine reduced mortality and decreased lung injury through reduction of alveolar–capillary permeability, inflammation, and epithelial sloughing and lipid peroxidation.
Chlorine (Cl2) is a corrosive gas that is produced in vast quantities globally due to its industrial applications and domestic usage (1, 2). It is a very reactive, irritating gas. A recent train derailment that occurred in South Carolina resulted in the death of 9 people and emergency care for over 500 (3). Out of 71 patients that had to be hospitalized, 12 met the criteria for acute lung injury (ALI) and 29 for acute respiratory distress syndrome (ARDS) (4). During the last few years, Cl2 cylinders have been bundled with traditional explosives, raising significant concerns regarding the possible re-emergence of this agent as a chemical weapon against both combatants and civilians (1). Thus, Cl2 is recognized as a significant public health hazard due to the potential for industrial, occupational, and environmental exposures.
The damaging effects of Cl2 depend on the concentration and duration of exposure (2, 5). The occurrence of symptoms ranges from minutes to hours. Severe exposure leads to ALI and death from ARDS. Treatment is mainly supportive with no specific antidote available to date (6).
Reactive oxygen and nitrogen species are known to contribute to the pathogenesis of ARDS (7, 8). For this reason, there have been numerous attempts to use a variety of antioxidants (which reduce the concentrations of reactive species) to decrease morbidity and mortality in patients with ARDS. Intravenous administration of the antioxidant N-acetylcysteine has been tested in patients with ARDS, with contradictory results regarding survival (9–11). On the other hand, oral and intravenous administration of ascorbic acid has been shown to decrease the severity of ALI in patients with severe burns (12). A decreased incidence of organ failure in surgical patients with sepsis has been observed upon coadministration of ascorbate and vitamin E (13). Similarly, combined administration of ascorbate and vitamin E improved 28-day survival in patients with sepsis (14).
A rigorous study documenting the beneficial effects of ascorbate on animal models of primary ARDS, induced by inhalation injury, and identifying putative mechanisms of action, has not been performed. In this study, we exposed mice to short-term, high-concentration Cl2 (600 ppm for 45 min) and demonstrated, for the first time, that in the absence of supplemental oxygen or respiratory support, after-exposure administration of a mixture containing ascorbate and deferoxamine via intramuscular injections, combined with aerosolized delivery, improved survival and decreased inflammation, lipid peroxidation, and the influx of plasma proteins to the lung surface compartment.
MATERIALS AND METHODS
Animals
Pathogen-free C57BL/6 male mice (20–25 g body weight) were purchased from Charles River Laboratories (Wilmington, MA). Experimental protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Exposure of Mice to Cl2
Mice were exposed to Cl2 (600 ppm for 45 min) in environmental chambers, as previously described (5, 15, 16), and returned to room air. Continuous measurements of Cl2 concentrations during the exposure were monitored with an Interscan Corporation (model RM34-1000 m) Cl2 detector, connected to a data logger for data storage. Additional information is shown in the online supplement.
Administration of Ascorbate and Deferoxamine
Mice were injected intramuscularly with 25 μl of endotoxin-free sterile saline containing 2 mg of ascorbic acid (American Regent, Shirley, NY) and 0.3 mg of deferoxamine (deferoxamine mesylate; Hospira, Lakeforest, IL) starting at 1 hour after exposure and every 12 hours thereafter up to 60 hours after exposure. In addition, mice received aerosolized ascorbate and deferoxamine via nose-only inhalation for 20 minutes at 1.5, 24, and 48 hours after exposure. Descriptions of the aerosol delivery system and the physical characteristics of aerosols are shown in the online supplement. Control mice were exposed to Cl2 and received intramuscular injections of saline and aerosolized endotoxin-free sterile water, as described previously here.
Experimental Protocols
Mice were killed at 72 hours after exposure and their lungs were lavaged, as described in the online supplement. We measured total and differential cell counts, as well as the amount of protein in the cell-free bronchoalveolar lavage (BAL). Ascorbate, reduced glutathione, glutathione disulfide, and uric acid were quantified by HPLC using electrochemical detection, as described previously (5). Lipid peroxidation was assessed in the BAL and quantified with the OxiSelect Malondialdehyde Immunoblot Kit (Cell Biolabs, Inc., San Diego, CA) according the manufacturer's instructions.
Statistical Analysis
All values are presented as mean (±SEM) for the indicated number of mice. Data were square root transformed before analysis to meet the assumption of normal distribution. Statistical significance among multiple groups was checked with one- or two-way ANOVA followed by the Tukey and Bonferroni post hoc tests, respectively. Kaplan-Meier survival curves were analyzed by log-rank test. Correlations among nontransformed data were assessed by calculating the Spearman's rank correlation coefficient (ρ). Differences were deemed significant with a P value less than 0.05.
RESULTS
Ascorbate and Deferoxamine Treatment Reduces Mortality
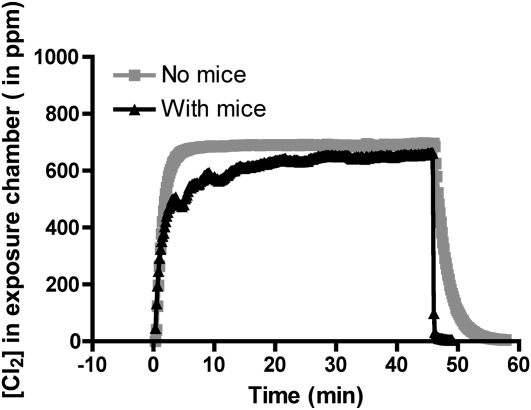
Continuous recordings of the chamber Cl2 concentrations in the absence and presence of mice are shown in Figure 1. As can be seen, Cl2 concentrations reached 600 ppm rapidly, and were maintained at that level throughout the exposures. All mice survived exposure to Cl2. Shortly after being returned to room air, mice developed labored breathing, with flaring of the nares and noticeable expiratory grunting, consistent with the presence of severe lung injury. Mice exposed to Cl2 had significantly lower respiratory rates than those of air-breathing mice (∼310 breaths/min), most likely due to activation of transient receptor ankyrin potential 1 channels (TRPA1) (17) (see Figure E2 in the online supplement).
Figure 1.
Continuous recordings of chlorine (Cl2) concentrations in the exposure chamber. Six mice were placed in the glass chamber and breathed air for approximately 10 minutes. At time zero, one of the mass flow controllers was connected to a Cl2 cylinder (1,000 ppm in air), while the other one remained connected to room air. The relative flow rates were adjusted to achieve a nominal concentration of 600 ppm. Cl2 concentrations were monitored continuously with an Interscan Corporation (model RM34-1000 m) Cl2 detector, connected to a data logger for data storage. After 45 minutes, the Cl2 cylinder was switched off and the compressed air flow rate was increased to 5 L/min. Differences in Cl2 profiles in the presence and absence of mice are probably due to adsorption and reaction of Cl2 with animal fur. Shown are typical measurements that were repeated six times with identical results.
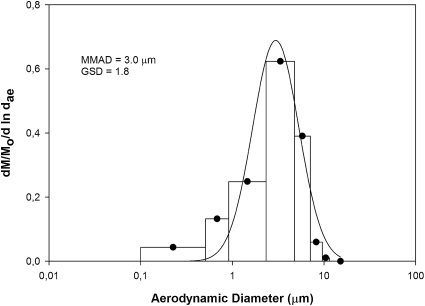
Particle size distribution data for aerosols are presented as mass median aerodynamic diameter, and geometric SD (GSD) (Figure 2). The mean mass median aerodynamic diameter and GSD for all exposures were 2.8 (±0.2) μm and 1.7 (±0.1), respectively. Because the GSD was greater than 1.2, the ascorbate aerosols were polydisperse; however, because the GSD was less than 2.0, the total amount of deposition within the respiratory tract of the mice probably did not differ substantially from that of a monodisperse aerosol with the same median diameter (18). Inhaled dose was calculated using aerosol concentration, murine minute ventilation, and exposure duration. Murine minute ventilation was calculated using data given by Flandre and colleagues (19). The mean ascorbic acid inhaled dose was 4.0 (±0.9) mg (n = 9). The ascorbic acid deposited in the distal lung regions was calculated as 5% of the inhaled dose (18) (0.22 ± 0.04 mg; n = 9). As discussed in the online supplement, because of the existence of other solutes (such as sodium bicarbonate) in the ascorbic acid solution, the amount of inhaled ascorbic acid was probably 15–25% less than the calculated value.
Figure 2.
Particle size distribution of aerosolized antioxidants. Cascade impactor samples were collected from the inhalation exposure plenum and analyzed using SigmaPlot software (see the online supplement for additional information). Particle size distribution is presented as mass median aerodynamic diameter (MMAD) and geometric SD (GSD) (both in microns). d ln dae, the change of the natural log of the aerodynamic equivalent diameter of the particles collected; d M/Mo, the change of mass fraction of particles collected on each stage of the impactor; Mo, total particle mass collected. Shown are results of a typical experiment that was repeated nine times with identical results.
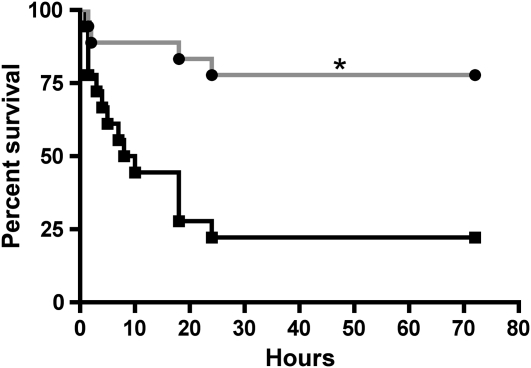
As shown in Figure 3, approximately 78% of mice exposed to Cl2 and treated with saline died within the first 24 hours of being returned to room air. Aerosolized and intramuscular ascorbate and deferoxamine significantly reduced the mortality by about fourfold (Figure 3). It is important to note that, immediately upon return to room air, mice treated with antioxidants had similar respiratory rates to those treated with saline (95.1 ± 8.1 breaths/min for saline versus 120.4 ± 10.4 breaths/min for the antioxidants; n = 18 in each case), indicative of similar levels of injury. Normal values for C57BL/6 values are approximately 300 breaths/min (see Figure E2).
Figure 3.
After-exposure antioxidants increase survival of Cl2-exposed mice returned to room air. Mice were exposed to 600 ppm of Cl2 for 45 minutes and returned to room air. Each data point shows the number of mice that were alive at the indicated time after Cl2 exposure. The experimental group (gray line, solid circles; n = 18) received intramuscular injections of ascorbate (2 mg) and deferoxamine (0.3 mg) in saline starting at 1 hour after exposure and every 12 hours thereafter up to 60 hours after exposure. They also received aerosols of ascorbate (150 mg/ml) and deferoxamine (0.3577 mg/ml) at 1.5, 24, and 48 hours after exposure in sterile water, as described in the online supplement. The control group (black line, solid squares; n = 18) received vehicle (saline for intramuscular injections; sterile water for aerosols) instead of antioxidants using identical protocols. A total of 14 mice were alive at 72 hours after exposure in the antioxidant group, and 4 in the saline group, respectively. Data points were fitted with Kaplan-Meier survival curves and compared with the log-rank test (P = 0.0007).
Ascorbate and Deferoxamine Treatment Ameliorates Alveolar–Capillary Barrier Damage
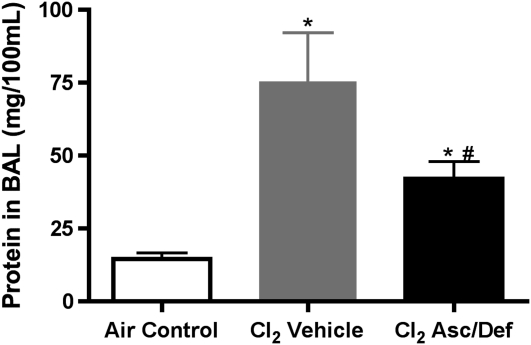
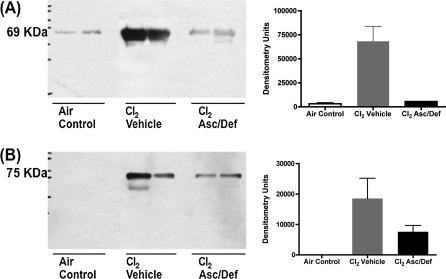
As shown in Figure 4, Cl2-exposed mice treated with antioxidants and killed at 72 hours after exposure had significantly lower levels of BAL protein as compared with vehicle controls (antioxidants, 42.06 ± 5.88 mg/100 ml; vehicle, 75.35 ± 16.76 mg/100 ml; X ± SE; n = 9 and 4, respectively; P < 0.05). However, protein in the antioxidant group was still significantly higher than air controls (14.64 ± 1.94 mg/100 ml; n = 18; P < 0.001). Western blot analyses of the same BAL samples showed that albumin (69 kD) and IgM (75 kD) were 10- and 2-fold higher in Cl2-exposed mice treated with saline as compared with the antioxidants (Figure 5). These findings indicate that after-exposure administration of antioxidants decreased, but did not eliminate, Cl2-induced injury to the blood gas barrier of mice.
Figure 4.
After-exposure antioxidants decreased total bronchoalveolar lavage (BAL) protein in mice exposed to Cl2. Mice were exposed to 600 ppm of Cl2 for 45 minutes and returned to room air and treated with either ascorbate and deferoxamine (Cl2 Asc/Def) or vehicle (Cl2 Vehicle), as described in Materials and Methods. Surviving mice were killed at 72 hours after Cl2 exposure and used for these measurements. A third group of mice was exposed to air (Air Control) and did not receive antioxidants. Protein concentrations in cell-free BAL were measured by the BCA Protein Assay. Values are means (±SE). *P < 0.001 compared with the Air Control group; #P < 0.05 compared with the Cl2 vehicle. Statistical analysis was performed after square root transformation of the data to meet assumption of normal distribution, followed by one-way ANOVA and Tukey multiple comparisons post test. The number of animals in each group was: air control, 18; Cl2 Asc/Def, 9; Cl2 vehicle, 4.
Figure 5.
After-exposure antioxidants decrease BAL albumin and IgM in mice exposed to Cl2. Mice were exposed to 600 ppm of Cl2 for 45 minutes and returned to room air and treated with either ascorbate and deferoxamine (Cl2 Asc/Def) or vehicle (Cl2 Vehicle), as described in Materials and Methods. Surviving mice were killed 72 hours after Cl2 exposure and used for these measurements. A third group of mice was exposed to air (Air Control) and did not receive antioxidants. Equal volumes (40 μl) of BAL were separated by 10% SDS-PAGE and transferred polyvinylidene difluoride (PVDF) membranes and blotted for (A) albumin and (B) IgM. Bands were digitized and mean values are shown in C and D. Values of densitometry units are expressed as means (±SE). Shown are results of a typical experiment.
Ascorbate and Deferoxamine Treatment Decrease Cl2-Induced Lung Inflammation and Airway Epithelial Sloughing
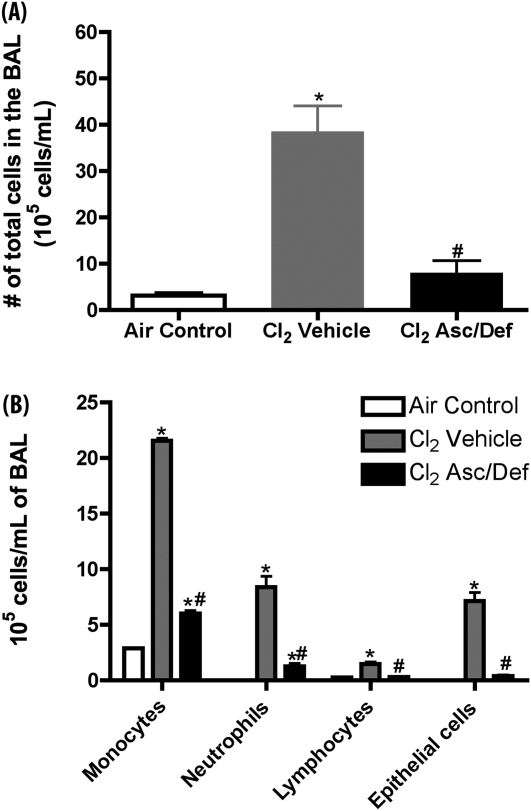
Lung inflammation was assessed by measurements of total and differential cell counts in the BAL in mice killed at 72 hours after exposure. Treatment with antioxidants decreased the number of total cells from 38.5 (±5.6) × 105 to 8 (±2.7) × 105 cells/ml, which was not significantly different from the corresponding value in air controls (3.17 ± 0.6 × 105 cells/ml; Figure 6A). As shown in Figure 6B, administration of antioxidants decreased all classes of inflammatory cells as well as the number of epithelial cells sloughed in the BAL.
Figure 6.
After-exposure antioxidants decrease number of cells in BAL in mice exposed to Cl2. Mice were exposed to 600 ppm of Cl2 for 45 minutes, returned to room air, and treated with either ascorbate and deferoxamine (Cl2 Asc/Def) or vehicle (Cl2 Vehicle), as described in Materials and Methods. Surviving mice were killed 72 hours after Cl2 exposure and used for these measurements. A third group of mice was not exposed to Cl2, and did not receive any treatment serving as baseline. After being killed, the lungs of mice were lavaged and BAL samples were spun at 300 × g for 10 minutes at 4°C to pellet cells. (A) Total number of cells in BAL; (B) the indicated types of inflammatory and epithelial cells. Values are means (±SEM); number of animals in each group: (A) Air Control, 18; Cl2 Asc/Def, 9; Cl2 Vehicle, 4; (B) Air Control, 7; Cl2 Asc/Def, 9; Cl2 Vehicle, 4. Statistical analysis was performed after square root transformation of the data to meet assumption of normal distribution, followed by one-way ANOVA and Tukey multiple comparisons post test (A), and two-way ANOVA and Bonferroni post test. Values are expressed as means (±SE). *P < 0.001 compared with air control; #P < 0.001 compared with Cl2 vehicle.
Levels of Ascorbate in the BAL Correlate Inversely with Various Indices of Lung Injury and Inflammation
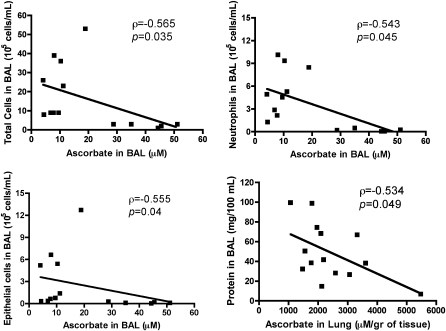
BAL and lung tissue ascorbate levels in mice killed 72 hours after exposure were measured by HPLC, as described previously (5). As shown in Figure 7, there were significant inverse correlations among ascorbate levels in the BAL with total cell counts (Figure 7A), number of epithelial cells or neutrophils in the BAL (Figures 7B and 7C), and between lung tissue ascorbate and total protein concentration in BAL (Figure 7D).
Figure 7.
BAL indices of inflammation and lung injury depend on ascorbate. Mice were exposed to 600 ppm of Cl2 for 45 minutes and returned to room air and treated with either ascorbate and deferoxamine (Cl2 Asc/Def) or vehicle (Cl2 Vehicle) as described in Materials and Methods. Surviving mice were killed 72 hours after Cl2 exposure and used for these measurements. Ascorbate levels in BAL and lung tissues were measured by HPLC as described previously (5). No statistical significant differences were found in either BAL or lung tissue ascorbate between these two groups of mice because of large variations. Therefore data were grouped and the presence of significant correlations among total cells (top left), neutrophils (top right), epithelial cell (bottom left), and total protein (bottom right) in the BAL and BAL ascorbate were examined using Spearman's rank correlation coefficient (ρ), derived from the actual data. Values of ρ and its P values are shown in each panel.
Ascorbate and Deferoxamine Treatment Decreases Lipid Peroxidation in BAL
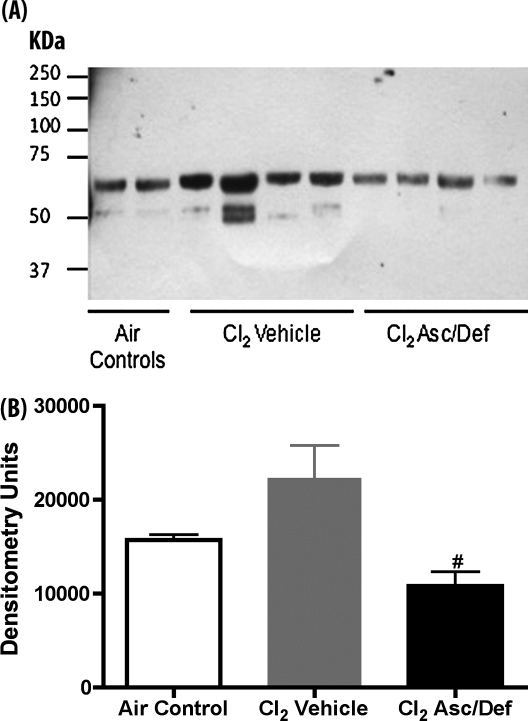
Generalized assessment of oxidative damage was estimated by detection of advanced lipid peroxidation end products, the protein adducts of malondialdehyde formed by lipid peroxidation. As shown in Figure 8, there was a twofold decrease in the antioxidant group compared with the vehicle group (10,813 ± 1,516 vs. 22,319 ± 3,457 densitometry units; n = 4; P < 0.05).
Figure 8.
Ascorbate and deferoxamine treatment decreased BAL lipid peroxidation in Cl2-exposed mice. Mice were exposed to 600 ppm of Cl2 for 45 minutes, returned to room air, and treated with either ascorbate and deferoxamine (Cl2 Asc/Def) or vehicle (Cl2 Vehicle), as described in Materials and Methods. Surviving mice were killed 72 hours after Cl2 exposure and were used for these measurements. A third group of mice was exposed to air and did not receive any treatment. Equal amounts of proteins (5 μg) from cell-free BAL were separated by 10% SDS-PAGE and transferred to PVDF membranes. Lipid peroxidation was detected using a primary rabbit anti-malondialdehyde (MDA) antibody. Values of densitometry units are expressed as means (±SE). #P < 0.05 versus Cl2 saline. Statistical analysis was performed after square root transformation of the data to meet assumption of normal distribution, followed by one-way ANOVA and Tukey multiple comparisons post test. Number of animals in each group was: Air Control, 2; Cl2 Asc/Def, 4; Cl2 Vehicle, 4.
DISCUSSION
Cl2 exposure is a global public health issue due to transportation and other accidents and intentional releases, thus enhancing the potential for mass casualty situations (1, 3, 4). In addition to significant mortality, individuals surviving the initial insult may experience reduced residual volume, forced vital capacity, and reactive airway disease syndrome (16, 20, 21).
Inhaled Cl2 dissolves in the epithelial lung lining fluid (ELF), where it reacts with low–molecular weight antioxidants (such as ascorbate, reduced glutathione, and urate), with rate constants exceeding its rate constant for hydrolysis to hypochlorous acid (HOCl) (2). Once resident low–molecular weight scavengers are depleted, Cl2 reacts with water to generate HOCl (and its conjugated base hypochlorite [OCl−] and hydrochlorous acid [HCl]). The latter is mainly buffered by bicarbonate, which exists in millimolar concentrations in the ELF (22). Cl2, HOCl, and OCl− react with functional groups of proteins and amino acids, such as cysteine (23, 24), methionine, side-chain amino groups in amino acids, terminal amino groups, yielding the corresponding chloramines (25), and aromatic amino acids (such as tyrosine), yielding 3-chlorotyrosine (26–28) and amino-phospholipids, such as phosphatidylethanolamine and phosphatidylserine that are present in the lining fluid layers covering the airways and alveolar spaces (2). Chloramines are toxic, and both contribute to and compound the injurious effects of Cl2/HOCl/OCl− to biological tissues (29–32). For example we have recently shown that chloramines, formed by the action of HOCl on taurine and lysine damage epithelial sodium channels located in the apical surfaces of distal lung epithelial cells, which result in decreased levels of vectorial Na+ transport across distal lung spaces (15). Here, we demonstrate the presence of products of lipid peroxidation in the BAL of mice exposed to Cl2 and returned to room air for 72 hours.
The sites of actions of Cl2 depend on its inhaled concentration. When inhaled at concentrations less than 50–100 ppm, Cl2 causes reversible bronchospasm and increased airway resistance (33, 34). At concentrations higher than 100 ppm (likely to be encountered during industrial accidents and terrorist attacks), Cl2 causes both airway and alveolar injury (1, 5, 15, 16). Previous studies by our group highlighted the pivotal role of antioxidant defenses in Cl2 induced injury. Significant decreases of rat lung BAL and tissue ascorbate after exposure to either 187 or 400 ppm Cl2 for 30 minutes and recovery in air for 1 and 24 hours were noted (5). Depression of antioxidant levels has also been demonstrated in humans with ALI (8, 35). Prophylactic systemic administration of ascorbate, deferoxamine, and N-acetyl-cysteine in rats exposed to Cl2 prevented the decrease of ascorbate in their BAL and mitigated the onset of hypoxemia and higher protein levels in the BAL (5). The current study demonstrates that after-exposure administration of antioxidants in mice exposed to lethal concentrations of Cl2 improved survival with concomitant diminution of inflammation and blood gas barrier permeability, as evidenced by BAL protein measurements.
The fact that administration of these two antioxidants reversed Cl2-induced lipid peroxidation in the BAL suggests that their mode of action likely involves scavenging of reactive species generated after Cl2 exposure. Ascorbate reduces oxidants (such as HOCl, generated by the hydrolysis of Cl2) in the lung ELF and tissues by donating one or two electrons (1, 2), while decreasing the generation of reactive intermediates by inhibiting endothelial cell NADPH oxidase (36) reduces lipid peroxidation that leads to the initiation of local and systemic inflammation (37–39). Ascorbate-mediated reduction of vitamin E radicals (generated during reduction of lipid hydroperoxides or peroxy radicals [40]) also contributes to global antioxidant activities. Ascorbate is known to protect microvascular dysfunction in critically ill patients by rapid uptake from the endothelial cells, scavenging reactive oxygen species, and stimulating endothelial nitric oxide synthase (41). It also prevents apoptosis of endothelial cells by TNF-α in vitro (42) and in congestive heart failure in vivo (37). Deferoxamine chelates free iron and prevents the generation of reactive intermediates via Fenton reactions. At the same time, it decreases the pro-oxidant capacity of ascorbate due to its ability to reduce Fe+3 and oxygen to superoxide and hydrogen peroxide (43). Our findings indicating that after-Cl2 administration of ascorbate and deferoxamine decreased lipid peroxidation in mice treated with antioxidants are in agreement with these findings. This provides a putative mechanism by which ascorbate and deferoxamine may protect the blood gas barrier from the toxic effects of Cl2 intermediates generated during and after the initial insult.
The reported contribution of reactive intermediates in the pathogenesis of ARDS (1, 7, 8, 44), has led to considerable interest in assessing the beneficial effects of antioxidants in reducing morbidity and mortality in patients with these disorders. Randomized, double-blind, placebo-controlled studies concluded that intravenous administration of N-acetyl-cysteine in patients with ARDS reduced the need for ventilatory support and improved systemic oxygenation, but failed to reduce mortality (9, 11). More recent studies using higher concentrations of N-acetyl-cysteine in patients with ALI reported that reduction of mortality depended on polymorphisms in the glutathione-S-transferase gene (45). Thus, beneficial effects of N-acetyl-cysteine in human ARDS remain in dispute.
On the other hand, several human studies documented the beneficial effects of ascorbate in improving survival and limiting multiorgan failure in patients with ARDS. Intravenous ascorbic acid administration decreased the severity of ALI in patients with severe burns (12); intravenous coadministration of ascorbate with vitamin E decreased the incidence of organ failure in patients with sepsis (13); finally, enteral coadministration of ascorbate and vitamin E improved 28-day survival in patients with sepsis (14).
We opted to administer antioxidants via both routes for the following reasons. First, reactive Cl2 and its reactive intermediates are known to activate inflammatory cytokine pathway and genes and inflammatory cells, which will contribute to oxidant stress both in the pulmonary and extrapulmonary spaces (21, 46). In addition, activation of TRPA1 ion channels, located in a subset of airway sensory neurons by Cl2 and HOCl, will contribute to the onset and propagation of systemic inflammation (17, 47). Finally, damage to skin and ocular epithelia by Cl2 may increase systemic oxidant load by a variety of mechanisms. Thus, there is accumulating evidence that the deleterious effects of inhaled Cl2, as well as other oxidant gases, such ozone (48), may extend to extrapulmonary sites, resulting in long-term systemic abnormalities (49). In our study, the concentration of ascorbate used is within the range used in the previous studies (12–14), and the concentration of deferoxamine is two times lower than what has been used in clinical practice (50, 51), meaning that it would be well tolerated by humans. The delivery of aerosolized antioxidants to airway and alveolar epithelia in patients with primary ARDS due to smoke and other oxidant gas inhalation, as well as aspiration of gastric contents, is of considerable benefit.
In conclusion, the coadministration of ascorbate and deferoxamine intramuscularly and via aerosols in C57BL/6 mice after exposure to lethal concentrations of Cl2 resulted in fourfold increase of survival when they were returned to room air. Significantly less pulmonary edema, lung inflammation, oxidative damage, and airway epithelial sloughing was observed in animals treated with antioxidants than those treated with vehicle. The observed effects are attributed to the protective effects of ascorbate and deferoxamine on the integrity of the alveolar capillary barrier and reduction of advanced lipid peroxidation end products and lung inflammation. These findings establish the rational basis for additional bench and clinical trials to establish the efficacy of aerosolized and systemic antioxidants in the mitigation of lung injury after inhalation of oxidant gases. It is therefore possible that this regimen could be considered as a treatment for civilians that are accidentally exposed to Cl2. However, it should be stressed that the regimen of antioxidants we used in this study mitigated, but did not eliminate, the Cl2-induced increase in alveolar permeability to plasma proteins. Thus, ascorbate and deferoxamine may need to be administered more frequently, by additional routes (i.e., intravenously), or combined with other antioxidants to increase efficacy and further decrease mortality.
Supplementary Material
Acknowledgments
The authors thank Mr. Kyle Bohannon for help in aerosol delivery and Ms. Teri Potter for editorial assistance.
Originally Published in Press as DOI: 10.1165/rcmb.2010-0432OC on December 3, 2010
This work was supported by National Institute of Environmental Health Sciences grants 5U54ES017218 and 5U01ES015676 (S.M.) and a European Respiratory Society Fellowship (LTRF58-2010) (S.G.Z.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author Disclosure: G.L.S. has received a sponsored grant from Philip Morris in 2000–2003 as a coinvestigator. S.M. has received consultancy payment from Sepracor, Inc., has received industry-sponsored grants from Talecris, Inspire Pharmaceuticals, and Sepracor, Inc., has patents received/pending with UAB Research Foundation (U.S. Provisional Patent Application no. 60/573,558: “Methods for using pyrimidine synthesis inhibitors to increase airway epithelial cell fluid uptake.” (Filed May 21, 2004; Inventors: Dr. Ian C. Davis, Dr. Wayne Sullender, and Dr. Sadis Matalon) converted to international PCT application (no. PCT/US2005/017939); May 2005; his spouse has patents received/pending with Purdue University (U.S. Provisional Patent Application no. 61/052,414: “DPPC Formulations and Methods for Using” (Filed May 12, 2008; Inventors: Dr. Elias I. Franses, Dr. Sook Heun Kim, Yoonjee Park, and Dr. Sadis Matalon), and has received fees from Elsevier for editing a book on free radical effects on membranes. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc 2010;7:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol 2010;299:L289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenck MA, Van SD, Drociuk D, Belflower A, Youngblood C, Whisnant MD, Taylor R, Rudnick V, Gibson JJ. Rapid assessment of exposure to chlorine released from a train derailment and resulting health impact. Public Health Rep 2007;122:784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van SD, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, et al. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 2009;27:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low–molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 2008;295:L733–L743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kales SN, Christiani DC. Acute chemical emergencies. N Engl J Med 2004;350:800–808. [DOI] [PubMed] [Google Scholar]
- 7.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 8.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant–antioxidant balance in acute lung injury. Chest 2002;122:314S–320S. [DOI] [PubMed] [Google Scholar]
- 9.Suter PM, Domenighetti G, Schaller MD, Laverriere MC, Ritz R, Perret C. N-acetylcysteine enhances recovery from acute lung injury in man: a randomized, double-blind, placebo-controlled clinical study. Chest 1994;105:190–194. [DOI] [PubMed] [Google Scholar]
- 10.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants n-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest 1997;112:164–172. [DOI] [PubMed] [Google Scholar]
- 11.Bernard GR, Lucht WD, Niedermeyer ME, Snapper JR, Ogletree ML, Brigham KL. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J Clin Invest 1984;73:1772–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg 2000;135:326–331. [DOI] [PubMed] [Google Scholar]
- 13.Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, Radella F, Garcia I, Maier RV. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 2002;236:814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crimi E, Liguori A, Condorelli M, Cioffi M, Astuto M, Bontempo P, Pignalosa O, Vietri MT, Molinari AM, Sica V, et al. The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double-blind, placebo-controlled trial. Anesth Analg 2004;99:857–863. [DOI] [PubMed] [Google Scholar]
- 15.Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem 2010;285:9716–9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Post exposure administration of a β2-agonist decreases chlorine induced airway hyper-reactivity in mice. Am J Respir Cell Mol Biol (In press) [DOI] [PMC free article] [PubMed]
- 17.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 2008;118:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans: a review. J Toxicol Environ Health 1985;15:197–214. [DOI] [PubMed] [Google Scholar]
- 19.Flandre TD, Leroy PL, Desmecht DJ. Effect of somatic growth, strain, and sex on double-chamber plethysmographic respiratory function values in healthy mice. J Appl Physiol 2003;94:1129–1136. [DOI] [PubMed] [Google Scholar]
- 20.Tuck SA, Ramos-Barbon D, Campbell H, McGovern T, Karmouty-Quintana H, Martin JG. Time course of airway remodelling after an acute chlorine gas exposure in mice. Respir Res 2008;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 2003;168:568–574. [DOI] [PubMed] [Google Scholar]
- 22.Nielson DW. Electrolyte composition of pulmonary alveolar subphase in anesthetized rabbits. J Appl Physiol 1986;60:972–979. [DOI] [PubMed] [Google Scholar]
- 23.den Hartog GJ, Haenen GR, Vegt E, van der Vijgh WJ, Bast A. Efficacy of HOCl scavenging by sulfur-containing compounds: antioxidant activity of glutathione disulfide? Biol Chem 2002;383:709–713. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 2003;25:259–274. [DOI] [PubMed] [Google Scholar]
- 25.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. evidence for hypochlorous acid generation. J Clin Invest 1982;70:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crow JP. Measurement and significance of free and protein-bound 3-nitrotyrosine, 3-chlorotyrosine, and free 3-nitro-4-hydroxyphenylacetic acid in biologic samples: a high-performance liquid chromatography method using electrochemical detection. Methods Enzymol 1999;301:151–160. [DOI] [PubMed] [Google Scholar]
- 27.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest 1996;98:1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 1997;99:2075–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foote CS, Goyne TE, Lehrer RI. Assessment of chlorination by human neutrophils. Nature 1983;301:715–716. [DOI] [PubMed] [Google Scholar]
- 30.Pattison DI, Hawkins CL, Davies MJ. Hypochlorous acid-mediated protein oxidation: how important are chloramine transfer reactions and protein tertiary structure? Biochemistry 2007;46:9853–9864. [DOI] [PubMed] [Google Scholar]
- 31.Robaszkiewicz A, Bartosz G, Soszynski M. N-chloroamino acids cause oxidative protein modifications in the erythrocyte membrane. Mech Ageing Dev 2008;129:572–579. [DOI] [PubMed] [Google Scholar]
- 32.Song W, Wei S, Shou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid and chloramines. J Biol Chem 2010;285:9716–9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris JB, Wilkie WS, Shusterman DJ. Acute respiratory responses of the mouse to chlorine. Toxicol Sci 2005;83:380–387. [DOI] [PubMed] [Google Scholar]
- 34.Bonetto G, Corradi M, Carraro S, Zanconato S, Alinovi R, Folesani G, Da DL, Mutti A, Baraldi E. Longitudinal monitoring of lung injury in children after acute chlorine exposure in a swimming pool. Am J Respir Crit Care Med 2006;174:545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowler RP, Velsor LW, Duda B, Chan ED, Abraham E, Ware LB, Matthay MA, Day BJ. Pulmonary edema fluid antioxidants are depressed in acute lung injury. Crit Care Med 2003;31:2309–2315. [DOI] [PubMed] [Google Scholar]
- 36.Wu F, Schuster DP, Tyml K, Wilson JX. Ascorbate inhibits NADPH oxidase subunit P47phox expression in microvascular endothelial cells. Free Radic Biol Med 2007;42:124–131. [DOI] [PubMed] [Google Scholar]
- 37.Aguirre R, May JM. Inflammation in the vascular bed: importance of vitamin C. Pharmacol Ther 2008;119:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol 2008;20:783–793. [DOI] [PubMed] [Google Scholar]
- 39.Hoyle GW, Hoyle CI, Chen J, Chang W, Williams RW, Rando RJ. Identification of triptolide, a natural diterpenoid compound, as an inhibitor of lung inflammation. Am J Physiol Lung Cell Mol Physiol 2010;298:L830–L836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballinger CA, Cueto R, Squadrito G, Coffin JF, Velsor LW, Pryor WA, Postlethwait EM. Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic Biol Med 2005;38:515–526. [DOI] [PubMed] [Google Scholar]
- 41.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2009;2:103–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saeed RW, Peng T, Metz CN. Ascorbic acid blocks the growth inhibitory effect of tumor necrosis factor-alpha on endothelial cells. Exp Biol Med (Maywood) 2003;228:855–865. [DOI] [PubMed] [Google Scholar]
- 43.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res 1996;145:532–541. [PubMed] [Google Scholar]
- 44.Chow CW, Herrera Abreu MT, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol 2003;29:427–431. [DOI] [PubMed] [Google Scholar]
- 45.Moradi M, Mojtahedzadeh M, Mandegari A, Soltan-Sharifi MS, Najafi A, Khajavi MR, Hajibabayee M, Ghahremani MH. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine. Respir Med 2009;103:434–441. [DOI] [PubMed] [Google Scholar]
- 46.Midwinter RG, Vissers MC, Winterbourn CC. Hypochlorous acid stimulation of the mitogen-activated protein kinase pathway enhances cell survival. Arch Biochem Biophys 2001;394:13–20. [DOI] [PubMed] [Google Scholar]
- 47.Bessac BF, Jordt SE. Sensory irritation by toxic gases-mechanisms, health effects and countermeasures. Proc Am Thorac Soc 2010;7:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chuang GC, Yang Z, Westbrook DG, Pompilius M, Ballinger CA, White CR, Krzywanski DM, Postlethwait EM, Ballinger SW. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am J Physiol Lung Cell Mol Physiol 2009;297:L209–L216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honavar J, Samal AA, Bradley K, Brandon A, Balanay J, Squadrito GL, MohanKumar K, Maheshwari A, Postlethwait EM, Matalon S, et al. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting ENOS-dependent signaling. Am J Respir Cell Mol Biol 2010;Dec 5 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 50.Collins AF, Fassos FF, Stobie S, Lewis N, Shaw D, Fry M, Templeton DM, McClelland RA, Koren G, Olivieri NF. Iron-balance and dose–response studies of the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1) in iron-loaded patients with sickle cell disease. Blood 1994;83:2329–2333. [PubMed] [Google Scholar]
- 51.Olivieri NF, Koren G, Hermann C, Bentur Y, Chung D, Klein J, St LP, Freedman MH, McClelland RA, Templeton DM. Comparison of oral iron chelator L1 and desferrioxamine in iron-loaded patients. Lancet 1990;336:1275–1279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.