Abstract
The extent by which early postnatal lung injury contributes to the development of chronic obstructive pulmonary disease (COPD) in the adult is unclear. We hypothesized that exposure to hyperoxia during early postnatal life can augment lung changes caused by adult chronic cigarette smoke (CS) exposure. C57BL/6J mice (1 d old) were exposed to hyperoxia (O2) for 5 days. At 1 month of age, half of the O2–exposed mice and half of the control mice were placed in a CS chamber for 6 months. After exposure to CS, mice underwent quasi-static pressure–volume curve and mean chord length measurements; quantification of pro–Sp-c expression; and measurement of lung IL-8/ KC, CXCR2/IL8Rα, TNF-α, and IL-6 mRNA by real-time PCR. Adult mice exposed to O2+CS had significantly larger chord length measurements (P < 0.02) and lung volumes at 35 cm H2O (P < 0.05) compared with all other groups. They also had significantly less pro–Sp-c protein and surfactant protein C mRNA expression (P < 0.003). Mice exposed to O2+CS and CS-only mice had significantly higher lung resistance and longer mean time constants (P < 0.01), significantly more inflammatory cells in the bronchoalveolar lavage fluid (P < 0.03), and significantly higher levels of lung CXCR2/IL8Rα mRNA compared with mice not exposed to smoke (P < 0.02). We conclude that exposure to early postnatal hyperoxia contributed additively to CS-induced COPD changes in adult mice. These results may be relevant to a growing population of preterm children who sustained lung injury in the newborn period and may be exposed to CS in later life.
Keywords: early postnatal hyperoxia, airspace abnormalities, chronic cigarette smoke exposure, chronic obstructive pulmonary disease
Clinical Relevance
Exposure to neonatal hyperoxia contributed additively to cigarette smoke–induced chronic obstructive pulmonary disease changes in adult mice. These results may be relevant to a growing population of preterm children who sustained lung injury in the newborn period and may be exposed to cigarette smoke in later life.
Exposure to hyperoxia, poor nutrition, or initiation of positive pressure ventilation during early postnatal life can increase the risk of chronic respiratory symptoms in adult life (1, 2). Perinatal stresses, such as hyperoxia, have been shown to induce p53 and p21. These genes act as checkpoint regulators in the cell cycle and, when induced by stress, can lead to cell cycle growth arrest (3). Induction of these genes during a critical period of postnatal lung growth can cause alveolar growth inhibition, resulting in enlarged, simplified, and fewer alveoli in the mature lung. In contrast to the changes that occur in the lung with smoke-induced chronic obstructive lung disease (COPD), hyperoxia-induced alveolar growth inhibition has not been associated with significant matrix breakdown or cell death (3–5). Similar to the COPD lung, however, lung parenchymal changes due to early postnatal hyperoxia exposure have been shown to be associated with altered lung mechanics and decreased lung elastance in adult mice (6). Many preterm children are exposed to hyperoxia during life-saving interventions at birth, and a subgroup of these children develops chronic respiratory symptoms in adult life (7, 8). It is not always apparent why certain at-risk children develop COPD symptoms as young adults. Environmental exposure to airborne pollution or cigarette smoke (CS) may increase the likelihood of developing early-onset COPD in children who sustained lung injury in early life. If children who are at increased risk for CS-induced COPD can be identified, preventative strategies could be used to attenuate respiratory morbidity in this vulnerable subgroup of individuals.
As many as 25% of people in the United States are active smokers (9). It is well known that exposure to CS can cause COPD and lung cancer in adults. However, the lung disease phenotype caused by CS exposure is variable, suggesting that other factors can modify disease expression, including genetic and environmental factors (10–14). Alpha-1 antitrypsin deficiency has been shown to increase the risk of COPD in early adulthood, and CS exposure can hasten disease onset in these individuals (15). Alternatively, genetic polymorphisms in the promoter sequence of MMP-12 have been shown to reduce the risk of COPD in adults that smoke (16).
Our study is based on the premise that early postnatal insults such as hyperoxia can increase the lung's susceptibility toward the development of CS-induced COPD (17). Few studies have examined the impact of early postnatal lung insults on adult lung exposed to chronic CS. We hypothesized that neonatal hyperoxia can augment changes in the adult lung caused by chronic CS exposure. To study this, we exposed newborn mice to 5 days of hyperoxia. These mice were then subjected to 6 months of chronic CS exposure starting at 1 month of age. We found that adult mice exposed to neonatal hyperoxia and chronic CS (O2+CS) developed significant structural and functional changes compared with control mice. This is the first study to demonstrate the impact of an early postnatal lung injury on the adult lung phenotype of mice exposed to chronic CS. Our findings suggest that exposure to neonatal hyperoxia may increase the severity of COPD changes in adults exposed to chronic CS.
Methods
Mice
Timed-pregnant C57BL/6J mice were obtained from the National Cancer Institute (Bethesda, MD). Experiments were conducted in accordance with the standards established by the United States Animal Welfare Acts set forth in NIH guidelines and the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee.
Hyperoxia Exposure
Pups (24 h old) from six timed-pregnant mice were placed in an 85 to 90% FiO2 hyperoxia chamber for 5 days. Excess CO2 was absorbed (#23001; Drierite, Xenia, OH). One-half of the pups were kept in room air.
CS Exposure
At 1 month of age, one half of the hyperoxia-exposed mice and one half of the room air–exposed mice were placed in a smoke chamber for 3 hours a day, 5 days a week for 6 months. The smoke machine (Model TE-10; Teague Enterprises, Davis, CA) burned two cigarettes at one time (2R4F reference cigarettes, 2.45 mg nicotine per cigarette; Tobacco Health Research Institute, University of Kentucky, Lexington, KY). The total particulate matter in the exposure chambers was 150 mg/m3.
Pulmonary Function Tests
Anesthetized animals (intraperitoneal ketamine/xylazine mixture; 100 and 15 mg/kg, respectively) were cannulated and ventilated with 100% O2. Baseline resistance and elastance using inspiratory occlusion was determined (18). The cannula was then occluded for 4 minutes for lung degassing. Complete degassing of the lung was based on previous visual inspection of the open thorax and an oxygen consumption rate of approximately 1 ml/min (19). Quasistatic pressure–volume curves were performed as previously reported (20).
Bronchoalveolar Lavage
Bronchoalveolar lavage samples were collected with 2 × 1 ml of sterile PBS through a tracheal cannula. Total cell counts were measured, and the cell differential was determined with Diff-Quik (Andwin Scientific, Tyron, NC).
Quantitative Real-Time PCR Analysis
RNA from total lung was processed with the SuperScript first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). Quantitative RT-PCR was performed using the Applied Biosystems (Foster City, CA) TaqMan assay system. PCR amplifications were performed on an ABI Prism 7700 Sequence Detection System using a fluorogenic 5′ nuclease assay (TaqMan probes). Probes and primers were synthesized by Applied Biosystems. Relative gene expressions were calculated by using the 2−ΔΔCt method (21). The GADPH gene was used an internal endogenous control.
Lung Fixation
Lungs were inflated with 1% (50–55°C) low-melt agarose at 30 cm H2O (22). Lungs were fixed overnight in 4% paraformaldehyde, paraffin embedded, cut, and stained with hematoxylin and eosin.
Lung Morphometry
Random lung sections were photographed with a 10× objective (Nikon Instruments Inc., Melville, NY). Chord length measurements (MCLs) were performed using an NIS-Elements AR (Nikon Instruments Inc.).
Immunohistochemistry
Anti–prosurfactant protein C (pro–Sp-c) (Santa Cruz Biotechnology, Santa Cruz, CA) and 4′,6-diamidino-2-phenylindole (DAPI) staining were performed. Antibodies were visualized using a Nikon E-800 fluorescent microscope. Five fields per lung were obtained, and images were digitally merged to identify pro–Sp-c staining cells. The pro–Sp-c staining+ cells were normalized to the number of DAPI+ cells (% dual-positive cells).
Statistics
Student's t tests and ANOVA (STATA 11; Stata Corp., College Station, TX) were used for statistical analysis. Statistical significance was accepted at P < 0.05.
Results
Weights by Sex and CS Exposure
Male mice exposed to chronic CS weighed significantly less than male mice not exposed to CS (P < 0.0001), and female mice exposed to chronic CS weighed significantly less than female mice not exposed to CS (P < 0.001). Mice exposed to neonatal hyperoxia and CS (O2+CS) weighed less than control (Ctr) mice (P < 0.04), but their weights were not significantly different compared with the other groups of mice (Table 1).
TABLE 1.
MEAN WEIGHTS BY SEX AND CIGARETTE SMOKE EXPOSURE
| Population | Groups | n | Female (%) | Weight (g) |
| By Exposure | Control | 4 | 50 | 31 ± 7.8* |
| O2 | 6 | 66 | 27 ± 6.6 | |
| CS | 5 | 60 | 23 ± 2.5 | |
| O2+CS | 8 | 37.5 | 24 ± 1.7† | |
| By sex and CS exposure | Non—CS-exposed male mice | 4 | 36.5 ± 2.6 | |
| CS-exposed male mice | 7 | 25 ± 1.0‡ | ||
| Non—CS-exposed female mice | 6 | 23.2 ± 0.4 | ||
| CS-exposed female mice | 6 | 21.5 ± 0.8§ |
Definition of abbreviations: CS, cigarette smoke; O2 hyperoxia.
Mean ± SEM.
The O2+CS mice weighed significantly less than the control mice (P < 0.04).
The CS-exposed male mice weighed significantly less than the CS-exposed male mice (P < 0.0001).
The CS-exposed female mice weighed significantly less than the non–CS-exposed female mice (P < 0.001).
Increased Structural and Cellular Lung Changes in Mice Exposed to Neonatal O2 and Chronic CS
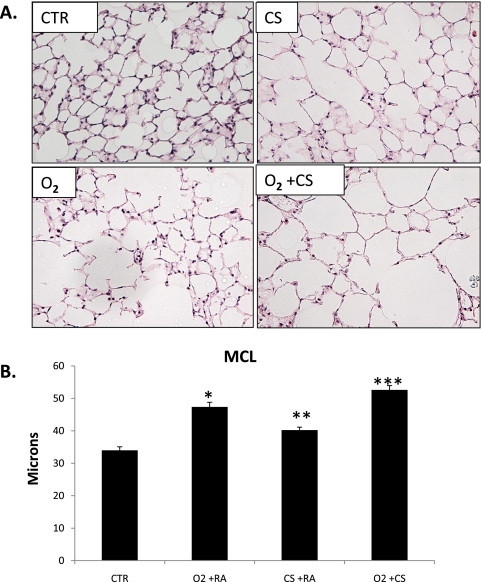
Exposure to early postnatal hyperoxia has been shown to alter alveolar growth, causing simplified and enlarged alveoli and decreased lung surface area (4, 23). In our study, we found that the lungs of adult O2+CS mice had larger MCLs compared with all other groups of mice (P < 0.02) (Figures 1A and 1B,). The MCLs of mice exposed to neonatal O2 only were significantly larger than the MCLs of CS-only mice and Ctr mice (P < 0.002). The differences in MCL between the O2+CS mice and the O2-only and CS-only mice indicated an additive interaction between hyperoxia and chronic CS exposure. A subanalysis of female and male mice did not show a difference in MCL between female and male mice in the O2+CS group. The mean MCL of the male mice was 49.48 ± 4.3 (n = 5), and the mean MCL of the female mice was 50.58 ± 3.48 (P < 0.72; n = 3). Because C57BL/6J mice were first exposed to CS at 4 weeks of age, we were interested in determining if alveolar genesis was complete at that time. We therefore calculated lung surface area measurements from 4-week-old and 6-month-old control C57BL/6J mice. We found no significant difference between the lung surface areas of the 4-week-old mice and the 6-month-old mice (P < 0.132), indicating that postnatal alveolar genesis was complete by 4 weeks of age in the C57BL/6J mice (see Table E1 in the online supplement).
Figure 1.
(A) Representative lung sections from control (Ctr), O2, cigarette smoke (CS), and O2+CS exposed mice. (B) Mean chord length measurements of Ctr, O2, CS, and O2+CS mice. O2 was significantly larger than CS and Ctr (*P < 0.002). CS was significantly larger than Ctr (**P < 0.05). O2+CS was significantly larger than all other groups (***P < 0.02) by one-way ANOVA. Error bars represent SEM (n = 3–8 per group).
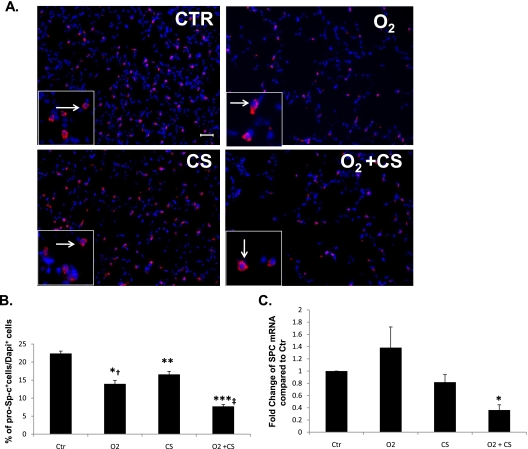
Yee and colleagues reported that exposure to early postnatal hyperoxia resulted in fewer pro–Sp-c+ type 2–expressing epithelial cells in the adult lung (6). Using immunohistochemistry, a semiquantitative technique, we measured pro–Sp-c expression in the lungs of all groups of mice. We found fewer pro–Sp-c+ cells in the lungs of O2-only, CS-only, and O2+CS mice compared with Ctr mice (P < 0.0001, P < 0.03, and P < 0.0001, respectively) (Figures 2A and 2B). The O2+CS lungs had significantly less surfactant protein C (SPC) mRNA by real-time PCR compared with all other groups (P < 0.002). No difference in SPC mRNA levels was found between the O2-only, CS–only, and Ctr mouse lungs (Figure 2C).
Figure 2.
(A) Representative examples of pro–Sp-c (pink staining, white arrows) and DAPI (blue) staining in adult lungs (original magnification: ×20). (B) Ctr lung had significantly more pro–Sp-c protein than O2, CS, and O2+CS lung (*P < 0.0001; **P < 0.005; ***P < 0.0001). O2 lung had significantly more pro–Sp-c protein than O2+CS lung (†P < 0.003), and CS lung had significantly more than O2+CS lung (‡P < 0.001). n = 3 for each group. (C) Surfactant protein C mRNA was significantly decreased in O2+CS lung compared with lung from Ctr, O2, and CS mice using real-time PCR from lung homogenates (*P < 0.002). Error bars represent SEM (n = 3–8 per group).
Increased Lung Volumes and Compliance in Mice Exposed to Neonatal O2 and Chronic CS
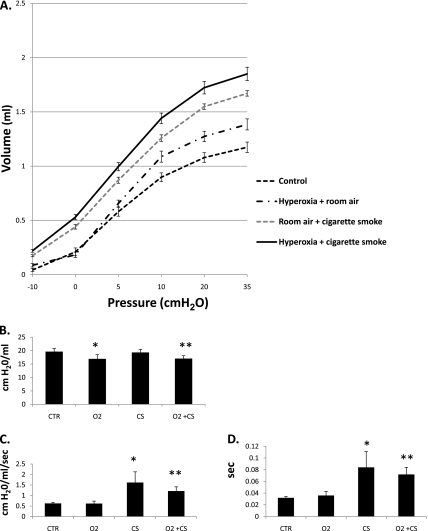
Quasistatic pressure–volume measurements were used to detect changes in lung volumes at fixed pressures. Volumes were recorded at pressures of −10, 0, 5, 10, 25, and 35 cm H2O in all mice (Figure 3A). Volumes were significantly different among all groups except for volumes at −10, 0, and 5 cm H2O between the Ctr and O2 mice (Table 2). When corrected for weight, compliance was significantly greater in the CS-only and O2+CS mice compared with Ctr mice at pressures of 0 to 5, 5 to 10, 10 to 20, and 20 to 35 cm H2O (P < 0.02). The O2+CS mice had greater compliance than the CS-only mice at 0 to 5 and 5 to 10 cm H2O (P < 0.005). The O2+CS mice had greater compliance than the O2-only mice at 10 to 20 and 20 to 35 cm H2O (P < 0.005).
Figure 3.
(A) Expiratory limbs from pressure–volume curves were obtained from mice that underwent quasistatic pressure–volume pulmonary function tests. Significant differences were found between groups at all pressures, except at −10, 0, and 5 cm H2O between Ctr and O2 mice. (B) Mean lung elastance was significantly decreased in the O2 and O2+CS mice compared with Ctr and CS mice (*P < 0.02 and **P < 0.02, respectively). (C) Mean lung resistance was greater in the CS and O2+CS mice compared with Ctr and O2 mice (*P < 0.01 and **P < 0.0001, respectively). (D) Time constants were significantly longer in the CS and O2+CS mice compared with Ctr and O2 mice (*P < 0.01 and **P < 0.0001, respectively). Error bars represent SEM (n = 4–8 per group).
TABLE 2.
LUNG VOLUMES AT FIXED PRESSURES*
| Groups | Volume at −10 cm H2O (ml) | Volume at 0 cm H2O (ml) | Volume at 5 cm H2O (ml) | Volume at 10 cm H2O (ml) | Volume at 20 cm H2O (ml) | Volume at 35 cm H2O (ml) |
| Ctr | 0.04 ± 0.01 | 0.21 ± 0.04 | 0.57 ± 0.04 | 0.95 ± 0.04 | 1.07 ± 0.05 | 1.17 ± 0.05 |
| O2 | 0.09 ± 0.02 | 0.18 ± 0.03 | 0.65 ± 0.03 | 1.08 ± 0.05† | 1.27 ± 0.05† | 1.38 ± 0.05† |
| CS | 0.17 ± 0.01‡§ | 0.44 ± 0.02‡§ | 0.87 ± 0.03‡§ | 1.25 ± 0.03‡§ | 1.54 ± 0.03‡§ | 1.67 ± 0.03‡§ |
| O2+CS | 0.22 ± 0.01‡¶ | 0.53 ± 0.02‡¶ | 0.99 ± 0.04‡¶ | 1.44 ± 0.05‡¶ | 1.72 ± 0.06‡¶ | 1.85 ± 0.06‡¶ |
Definition of abbreviations: CS, cigarette smoke; Ctr, control; O2, hyperoxia.
Quasi-static pressure volume curves were performed, and volumes were measured at −10, 0, 5, 10, 20, and 35 cm H2O (± SEM).
Significant difference compared with control group (P < 0.05).
Significant difference compared with control group (P < 0.001).
Significant difference compared with O2 (P < 0.02).
Significant difference compared with O2 and CS (P < 0.05).
Mice exposed to neonatal hyperoxia (O2 and O2+CS mice) had significantly lower lung elastance compared with Ctr and CS mice (O2 and O2+CS versus Ctr mice: P < 0.02 and P < 0.003, respectively; O2 and O2+CS versus CS mice: P < 0.02 and P < 0.004, respectively) (Figure 3B). The CS and O2+CS mice had modest increases in lung resistance compared with Ctr mice (P < 0.006 and P < 0.0002, respectively) and O2-only mice (P < 0.001 and P < 0.0001, respectively) (Figure 3C). The mean time constants of the CS and O2+CS mice were significantly longer compared with O2-only and Ctr mice (Figure 3D). Statistical analyses for smoke and oxygen interactions were performed to determine if the combination of neonatal O2 and CS was additive or multiplicative. The combination of neonatal hyperoxia and chronic CS exposure was found to be additive when analyzed against elastance, resistance, and lung volume.
Chronic CS, but Not Neonatal Hyperoxia, Increased Lung Inflammation in Mice
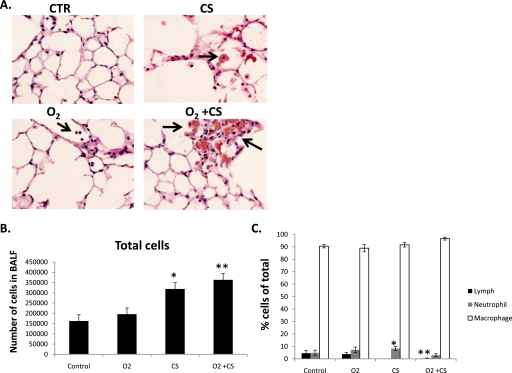
More inflammatory cells were found in the bronchoalveolar lavage fluid (BALF) of mice exposed to chronic CS than in mice not exposed to CS (Figures 4A–4C). The cell differentials were similar between the groups except for mice exposed to O2+CS. The O2+CS mice had a significantly lower percentage of lymphocytes (1.0 ± 0.3%) compared with Ctr mice (4.5 ± 2.3%) and O2 mice (7.2 ± 1.2%) (P < 0.03 and P < 0.001, respectively).
Figure 4.
(A) Enlarged macrophages were found in the airspaces of CS and O2+CS mice. Black arrows point to macrophages. (B) Cell differentials from BALF. (C) Total cell counts from CS and O2+CS BALF were significantly higher than O2 alone and control BALF (*P < 0.03 and **P < 0.01, respectively). Error bars represent SEM (n = 4–8 per group).
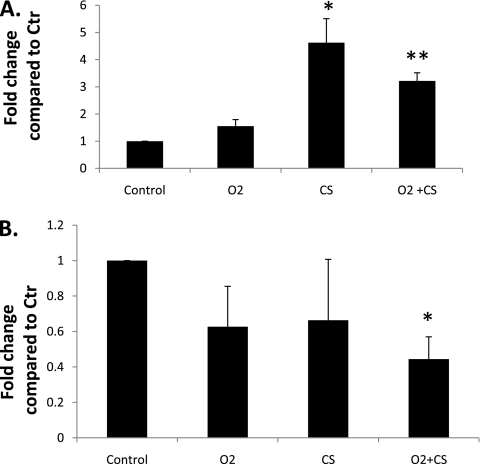
Real-time PCR was used to determine if inflammatory mediators were increased in the lung homogenate of mice exposed to O2 or CS. CXCR2/IL8Rα expression was significantly increased in the CS-only and in the O2+CS mice compared with the O2-only and Ctr lungs (P < 0.02 and P < 0.03 and P < 0.001 and P < 0.001, respectively) (Figure 5). No difference in IL-8/KC expression was found between any of the groups. We were also interested in determining if CS exposure decreased IL-6 expression in the lung. Meuronen and colleagues previously reported higher numbers of macrophages in the BALF of smokers but decreased IL-6 expression in the macrophages of smokers compared with nonsmokers (24). We found that IL-6 expression was 2.4-fold less in the O2+CS lung compared with Ctr lung (P < 0.01). No significant decrease in IL-6 was found in the CS-only lung; however, when an outlier was removed from analysis, a significant decrease in IL-6 expression was also found in the CS-only lung compared with Ctr lung (P < 0.02).
Figure 5.
Differences in lung cytokine/chemokine mRNA levels between the different exposure groups. (A) CXCR2/IL8Rα mRNA expression was significantly increased in CS and O2+CS lung compared with control and O2 lung (*P < 0.02 and **P < 0.01, respectively). (B) IL-6 mRNA expression was significantly decreased in the O2+CS lung compared with control lung (*P < 0.02)., Error bars represent SEM (n = 3–6 per group).
Discussion
In this study, we investigated the contribution of neonatal hyperoxia exposure on the lung phenotype of adult mice exposed to chronic CS. We found that exposure to neonatal hyperoxia contributed additively to the structural and functional changes found in adult mice exposed to chronic CS. These findings suggest that the consequences of a neonatal lung injury may be long term and that children who sustain an early postnatal lung injury may be at increased risk for early-onset and severe CS-induced COPD changes in adult life.
Similar to our findings, a previous study found that lung parenchymal changes from neonatal hyperoxia were not completely reversible with age (6). In the adult mouse, Yee and colleagues reported a positive correlation between airspace abnormalities and percentage of oxygen exposure in the perinatal period. Comparable to our study, adult mice exposed to high concentrations of oxygen during early postnatal life developed simplified, enlarged alveoli and decreased lung elastance in the absence of significant parenchymal destruction. Features of this lung phenotype resembled that of the aging lung. Mouse models of accelerated aging (including the SAMP8, SAMP2, and SMAP1 mice) have been shown to exhibit loss of lung elastic recoil, enlarged mean linear intercepts, and sensitivity to CS exposure (25). From these genetic models, it has been suggested that accelerated aging may be a significant risk factor for the development of COPD but that aging alone does not cause COPD changes (25). In our study, adult mice exposed to neonatal hyperoxia and chronic CS developed additive changes with respect to MCL and lung volume changes. Although the combination of the two exposures was additive, our findings suggest that a previous exposure to neonatal hyperoxia may, in part, influence severity and onset of CS-induced COPD changes in the adult.
In this study, we found that lungs from mice exposed to neonatal hyperoxia and chronic CS had fewer pro–Sp-c+ expressing type 2 epithelial cells. This finding indicates that environmental exposures, such as hyperoxia or CS, may alter the percentage of alveolar cell type in the lung. Yee and colleagues also found a decreased percentage of pro–Sp-c+ expressing type 2 epithelial cells in adult mice exposed to perinatal hyperoxia. Although their mice had fewer pro–Sp-c+ expressing type 2 epithelial cells, surfactant composition and activity were normal (6). This suggests that the changes in lung mechanics observed in our model are likely due to structural changes rather than surfactant dysfunction. This is in contrast to acute lung injury models, in which surfactant dysfunction is commonly found (26, 27). Surfactant dysfunction would also be expected to cause a more restrictive lung phenotype, whereas in our study the O2+CS mice had increased lung compliance, higher lung volumes, and decreased lung elastance, consistent with parenchymal changes rather than surfactant deficiency. Although fewer pro–Sp-c+ expressing cells correlate with the COPD changes in the O2+CS lung, the mechanism linking these two observations has not been elucidated. We speculate that a decrease in pro–Sp-c+ expressing type 2 epithelial cells may adversely influence lung reserve and response to environmental insults. Alternatively, a lower percentage of pro–Sp-c+ expressing type 2 epithelial cells may negatively affect the lung's ability to recover from an insult. Further studies are needed to address these issues.
Oxidative stress and inflammation have been shown to contribute to COPD lung changes in the adult (28). Recently, Vecchio and colleagues reported a decrease in HDAC2 expression and an increase in NF-κB activation in C57BL/6J macrophages exposed to CS extract (29). Our study supports an association between chronic CS exposure, inflammation, and COPD lung changes. In the CS-only and O2+CS mice, we found increased numbers of inflammatory cells in the BALF and increased expression of CXCR2/IL8Rα lung mRNA, suggesting a correlation between chronic CS exposure and increased lung inflammation. Induction of CXCR2/IL8Rα has previously been demonstrated in animal models of CS-induced COPD, Streptococcus pneumoniae, and rhinovirus-induced airway inflammation (30–33). We did not find an association between early postnatal hyperoxia exposure and increased inflammation in the adult lung. Furthermore, we did not find that prior exposure to hyperoxia potentiated CS-induced lung inflammation. This was surprising because a proinflammatory lung phenotype had been previously described in the preterm infant with bronchopulmonary dysplasia (34). Taken together, our results show that although neonatal hyperoxia exposure contributed to structural and functional lung changes in the adult O2+CS mice, these changes were not associated with lung inflammation. These findings further suggest that early postnatal hyperoxia and CS exposure contribute additively but separately to the COPD lung phenotype in adult mice.
There are limitations to our study. The C57BL/6J mice developed only mild lung changes in response to O2 and CS exposures. These mild changes, however, allowed us to discern differences in lung structure and function between mice exposed to O2+CS and mice exposed to early postnatal O2 and chronic CS alone. Alternatively, a shorter perinatal O2 exposure that had no effect on airspace development would have allowed us to determine if brief perinatal O2 exposure could affect lung phenotype in adult CS-exposed mice. Strain specificity may also determine the injury response to early postnatal hyperoxia or chronic CS exposure. If exposures had been performed in the CS-sensitive AKR/J mice, more pronounced changes in lung structure and function may have been appreciated. We also used male and female mice in our analysis, and gender differences may be a factor in COPD susceptibility. In our study, female and male mice had similar abnormalities in lung structure and function when exposed to neonatal O2 and chronic CS; however, a larger sample size may have uncovered more subtle differences. Our study also did not address whether decreased body weight and COPD lung changes in the CS-exposed mice had a common mechanism. Although mechanistic links between CS exposure, weight loss, and COPD changes have been suggested, calorie restriction alone may not contribute to COPD changes in the mouse lung. A recent study found that calorie restriction was not associated with COPD changes in mice. In their study, Bishai and colleagues found no evidence of COPD changes in calorie-restricted C57BL/6J and C3H/HeJ mice (not exposed to CS) (35). Additional studies are needed to identify other early life factors that may contribute to the adult COPD phenotype and to understand the link between pulmonary inflammation, early senescence, impaired alveolar growth, chronic CS exposure, and the COPD phenotype.
In summary, we found that neonatal hyperoxia caused additive structural and functional changes in adult mice exposed to chronic CS. This is the first study to describe the impact of neonatal hyperoxia on the lung phenotype of adult mice exposed to chronic CS. The results from our study indicate that exposure to early postnatal hyperoxia may increase the severity of COPD changes in adults exposed to CS.
Supplementary Material
Acknowledgments
The authors thank Dr. Donald Massaro for comments and insights regarding postnatal lung growth and development in mice.
Footnotes
This work was supported by a Flight Attendant Medical Research Institute Clinical Innovator Award (S.M.), COPD SCCOR grant P50HL084945 (R.W., S.B., and S.M.), and the Grace Anne Dorney Fund.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0259OC on January 14, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 1998;53:81–94 [DOI] [PubMed] [Google Scholar]
- 2.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729 [DOI] [PubMed] [Google Scholar]
- 3.McGrath SA. Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol 1998;18:179–187 [DOI] [PubMed] [Google Scholar]
- 4.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 1998;275:L110–L117 [DOI] [PubMed] [Google Scholar]
- 5.McGrath-Morrow SA, Stahl J. Apoptosis in neonatal murine lung exposed to hyperoxia. Am J Respir Cell Mol Biol 2001;25:150–155 [DOI] [PubMed] [Google Scholar]
- 6.Yee M, Chess PR, McGrath-Morrow SA, Wang Z, Gelein R, Zhou R, Dean DA, Notter RH, O'Reilly MA. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol 2009;297:L641–L649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baraldi E, Carraro S, Filippone M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum Dev 2009; 85(Suppl):S1–S3 [DOI] [PubMed] [Google Scholar]
- 8.Doyle LW, Anderson PJ. Long-term outcomes of bronchopulmonary dysplasia. Semin Fetal Neonatal Med 2009;6:391–395 [DOI] [PubMed] [Google Scholar]
- 9.McClave AK, Whitney N, Thorne SL, Mariolis P, Dube SR, Engstrom M. Adult tobacco survey: 19 states, 2003 –2007. MMWR Surveill Summ 2010;59:1–75 [PubMed] [Google Scholar]
- 10.Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax 2006;61:935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svartengren M, Engstrom G, Anderson M, Hallberg J, Edula G, de Verdier MG, Dahlback M, Lindberg CM, Forsman-Semb K, Nihlen U, et al. Twins studies as a model for studies on the interaction between smoking and genetic factors in the development of chronic bronchitis. Biochem Soc Trans 2009;37:814–818 [DOI] [PubMed] [Google Scholar]
- 12.Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2008;295:L1–L15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol 2008;294:L612–L631 [DOI] [PubMed] [Google Scholar]
- 14.Landau LI. Tobacco smoke exposure and tracking of lung function into adult life. Paediatr Respir Rev 2008;9:39–43 [DOI] [PubMed] [Google Scholar]
- 15.Sandford AJ, Silverman EK. Chronic obstructive pulmonary disease. 1: susceptibility factors for COPD the genotype-environment interaction. Thorax 2002;57:736–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunninghake GM, Cho MH, Tesfaigzi Y, Soto-Quiros ME, Avila L, Lasky-Su J, Stidley C, Melen E, Soderhall C, Hallberg J, et al. MMP12, lung function, and COPD in high-risk populations. N Engl J Med 2009;361:2599–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bush A. COPD: a pediatric disease. COPD 2008;5:53–67 [DOI] [PubMed] [Google Scholar]
- 18.Ewart S, Levitt R, Mitzner W. Respiratory system mechanics in mice measured by end-inflation occlusion. J Appl Physiol 1995;79:560–566 [DOI] [PubMed] [Google Scholar]
- 19.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK. Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 2002;297:843–845 [DOI] [PubMed] [Google Scholar]
- 20.Soutiere SE, Mitzner W. On defining total lung capacity in the mouse. J Appl Physiol 2004;96:1658–1664 [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 22.Halbower AC, Mason RJ, Abman SH, Tuder RM. Agarose infiltration improves morphology of cryostat sections of lung. Lab Invest 1994;71:149–153 [PubMed] [Google Scholar]
- 23.Ohki Y, Mayuzumi H, Tokuyama K, Yoshizawa Y, Arakawa H, Mochizuki H, Morikawa A. Hepatocyte growth factor treatment improves alveolarization in a newborn murine model of bronchopulmonary dysplasia. Neonatology 2009;95:332–338 [DOI] [PubMed] [Google Scholar]
- 24.Meuronen A, Majuri ML, Alenius H, Mantyla T, Wolff H, Piirila P, Laitinen A. Decreased cytokine and chemokine mRNA expression in bronchoalveolar lavage in asymptomatic smoking subjects. Respiration 2008;75:450–458 [DOI] [PubMed] [Google Scholar]
- 25.Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc 2009;6:570–572 [DOI] [PubMed] [Google Scholar]
- 26.Davidson BA, Knight PR, Wang Z, Chess PR, Holm BA, Russo TA, Hutson A, Notter RH. Surfactant alterations in acute inflammatory lung injury from aspiration of acid and gastric particulates. Am J Physiol Lung Cell Mol Physiol 2005;288:L699–L708 [DOI] [PubMed] [Google Scholar]
- 27.Hickman-Davis JM, Wang Z, Fierro-Perez GA, Chess PR, Page GP, Matalon S, Notter RH. Surfactant dysfunction in SP-A−/− and iNOS−/− mice with mycoplasma infection. Am J Respir Cell Mol Biol 2007;36:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 2007;87:1047–1082 [DOI] [PubMed] [Google Scholar]
- 29.Vecchio D, Arezzini B, Pecorelli A, Valacchi G, Martorana PA, Gardi C. Reactivity of mouse alveolar macrophages to cigarette smoke is strain dependent. Am J Physiol Lung Cell Mol Physiol 2010: [DOI] [PubMed] [Google Scholar]
- 30.Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK, Redonnet MR, Seweryniak KE, Sime PJ, Phipps RP. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol 2005;289:L322–L328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson CS, Coote K, Webster R, Johnston H, Atherton HC, Nicholls A, Giddings J, Sugar R, Jackson A, Press NJ, et al. Characterization of cigarette smoke-induced inflammatory and mucus hypersecretory changes in rat lung and the role of CXCR2 ligands in mediating this effect. Am J Physiol Lung Cell Mol Physiol 2005;288:L514–L522 [DOI] [PubMed] [Google Scholar]
- 32.Nagarkar DR, Wang Q, Shim J, Zhao Y, Tsai WC, Lukacs NW, Sajjan U, Hershenson MB. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J Immunol 2009;183:6698–6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbold W, Maus R, Hahn I, Ding N, Srivastava M, Christman JW, Mack M, Reutershan J, Briles DE, Paton JC, et al. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun ( In press ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jobe AH. Blood cytokines and BPD. J Pediatr 2009;154:A2. [DOI] [PubMed] [Google Scholar]
- 35.Bishai JM, Mitzner W. Effect of severe calorie restriction on the lung in two strains of mice. Am J Physiol Lung Cell Mol Physiol 2008;295:L356–L362 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.