Abstract
Secretory cells in submucosal glands (SMGs) secrete antibacterial proteins and mucin glycoproteins into the apical lumen of the respiratory tract, and these are critical for innate immune mucosal integrity. Glandular hyperplasia is manifested in diseases with obstructive respiratory pathologies associated with mucous hypersecretion, and is predominant in the sinus mucosa of patients with chronic rhinosinusitis (CRS), cystic fibrosis (CF), and clinical symptoms of CRS. To gain insights into the molecular basis of SMG hyperplasia in CRS, gene expression microarray analyses were performed to identify the differences in global and specific gene expression in the sinus mucosa of control, CRS, and CRS/CF patients. A marked up-regulation of 11 glandular-associated genes in CRS and CRS/CF sinus mucosa was evident. The RNA and protein expressions of the four most highly up-regulated genes (DSG3, KRT14, PTHLH, and OTX2) were evaluated. An increased expression of DSG3, KRT14, and PTHLH was demonstrated at the mRNA and protein levels in both CRS and CRS/CF sinus mucosa, whereas the increased expression of OTX2 was evident only for CRS/CF sinus mucosa, implicating OTX2 as a CF-specific gene. Immunofluorescence analysis localized DSG3, PTHLH, and OTX2 to serous cells, and KRT14 to myoepithelial cells, in SMGs. Because glandular hyperplasia is a central histologic feature of CRS, the identification of overexpressed glandular genes in the sinus mucosa lays the groundwork for future studies of glandular hyperplasia, and may ultimately lead to the development of novel treatments for mucous hypersecretion in patients with CRS.
Keywords: chronic rhinosinusitis, cystic fibrosis, submucosal glands, hyperplasia, microarrays
Clinical Relevance
To gain insights into the molecular basis of submucosal gland hyperplasia/hypertrophy in chronic rhinosinusitis, we performed gene expression microarray analyses and identified differences in global and specific gene expression in the sinus mucosa of control, chronic rhinosinusitis (CRS), and CRS/cystic fibrosis (CF) patients, focusing especially on genes related to glandular development. Because glandular hyperplasia is a central histologic feature of CRS, identifying overexpressed glandular genes in the sinus mucosa lays the groundwork for future studies on glandular hyperplasia, and may ultimately lead to the development of novel treatments for mucous hypersecretion in patients with CRS. Submucosal gland hyperplasia in the sinus mucosa is a characteristic of patients with CRS. This study delineated genes expressed in sinus mucosa, and determined the cellular localization of glandular-associated gene products that are markedly differentially expressed in CRS or CRS/CF arrays. These findings, in the context of future studies on glandular hyperplasia, may help lead to the development of novel treatments for mucous hypersecretion in patients with CRS.
The mammalian upper and lower respiratory tracts are covered by a mucosal layer that provides a physiologic barrier and a dynamic interface to protect the underlying epithelium against inhaled pathogens and environmental toxins. Secretory cells, for example, goblet cells in the surface epithelium and mucous and serous cells in the submucosal glands (SMGs), are major contributors to respiratory tract mucus. They secrete mucin glycoproteins and antibacterial proteins, which are key components in the mucosal innate immune responses that maintain normal respiratory tract function and homeostasis. Obstructive respiratory pathologies associated with mucous hypersecretion reflect the histologic changes exemplified by goblet-cell hyperplasia, glandular hyperplasia, and hypertrophy. Hyperplasia of goblet cells in the surface epithelium is evident in the lower respiratory tract of patients with asthma (1) or other chronic obstructive pulmonary diseases (2), but is not a characteristic finding in the sinus mucosa of adult (3) or pediatric (4) patients with chronic rhinosinusitis (CRS). Conversely, SMG hyperplasia or hypertrophy is prevalent in the sinus mucosa of adult (3) and pediatric (5) patients with CRS, and in the lower respiratory tracts of patients with chronic obstructive pulmonary diseases (6). Patients with cystic fibrosis (CF) and clinical symptoms of CRS (designated here as CRS/CF) also exhibit SMG hyperplasia/hypertrophy in their sinus mucosa (7).
Submucosal glands are invaginations of the surface epithelium in the cartilaginous airways, and comprise a series of ducts with interconnecting serous and mucous tubules that terminate in acini. They are present at birth in the trachea and bronchi of the lower respiratory tract and in the oronasal passages of the upper respiratory tract, reflecting the initiation of glandular development during embryogenesis (8, 9). The molecular basis of glandular morphogenesis during development was studied in murine (10) and ferret (11) tracheas. The lymphoid enhancer binding factor 1 (Lef1) and the canonical Wnt/wingless signaling pathway were shown to be involved in the development and formation of the initial buds that develop into SMGs in ferrets (12, 13) and mice (14), whereas the bone morphogenetic protein Bmp4 appears to control the development and homeostasis of murine SMGs in the larynx and proximal trachea (15). Studies on the molecular basis of glandular development in the sinus mucosa have not been reported, to the best of our knowledge.
Genes and mechanisms activated during development may likewise be activated during the de novo gland development that results in SMG hyperplasia in the sinus mucosa of patients with CRS. Alternatively, because CRS is considered an inflammatory disease, mediators activated during the response of the sinus mucosa to infection and inflammation may induce SMGs, much as the Th2 cytokine IL-13 mediates goblet cell metaplasia in murine models of allergic asthma (16, 17). The genes and pathways that lead to SMG hyperplasia in respiratory mucosa are unknown. To gain insights into SMG hyperplasia in the upper respiratory tract, gene expression microarray analyses were performed to identify differences in global and specific gene expressions in the sinus mucosa of control and CRS patients. Our initial study identified and validated specific inflammatory and innate immune mediator genes that are up-regulated in CRS and control sinus mucosa (18). Here, we identify glandular gene products differentially expressed in the sinus mucosa of control, CRS, and CRS/CF patients, and we evaluate their cellular localization in SMGs.
Materials and Methods
Specimens of Sinus Mucosa
Sinus tissues from patients who underwent craniofacial or neurosurgical procedures for pathologies other than CRS served as control samples. Sinus tissue was obtained from patients with CRS who underwent functional endoscopic sinus surgery at Children's National Medical Center (CNMC, Washington, DC) for CRS refractory to medical management. Exclusion criteria for the control population and the criteria for diagnosing CRS were described previously (18). A diagnosis of CRS in the CRS/CF cohort was based on a combination of clinical presentation, endoscopic nasal examinations, and computerized tomography imaging of the paranasal sinuses. All patients with CRS/CF underwent preoperative evaluations by the Pulmonary Division at CNMC. Patients with ciliary dyskinesias or craniofacial abnormalities were excluded. Clinical data from control and CRS patients were recorded as described previously (4). All patients were entered consecutively into the study after we had obtained appropriate surgical and research consents (and assents, when applicable). This study was reviewed and approved by the Institutional Review Board of the CNMC.
Mucosa from the paranasal sinuses that typically included maxillary and ethmoid tissues was collected from CRS and control patients, as described previously (18). When clinically appropriate, mucosa from the frontal and sphenoid sinuses was also collected. No patients with CRS exhibited nasal polyps, whereas two patients with CRS/CF manifested polyps and polypoid changes in their sinonasal mucosa. The nasal polyps were excised from the mucosa, and were not used in these experiments. Specimens were immediately frozen in liquid nitrogen for RNA or protein isolation, or fixed with 10% neutral-buffered formalin (pH 6.8–7.2; Richard-Allan Scientific, Kalamazoo, MI) for paraffin-embedding and subsequent microscopy.
Gene Expression Profiling and Microarray Data Analyses
Sample preparation, expression profiling, and microarray analyses of sinus mucosa samples were performed at the Microarray Core of CNMC, as previously described (18). All data were analyzed using Microarray Analysis Software, version 5.0 (MAS 5.0 algorithm; Affymetrix, Fremont, CA), along with dChip and PLIER algorithms. We only used probe sets that were statistically significant according to one-way ANOVA and a P value “cutoff” of less than 0.05 in all three algorithms. The dataset from each algorithm was loaded into the data-mining program Gene Spring GX, version 7.3.1 (Silicon Genetics, Redwood City, CA), and the results were visualized using the gene tree cluster feature of the program, which rearranges the order of the probe sets and groups them based on the similarity (Pearson's correlation) of their expression dynamics (19). Hierarchical clustering analyses of all three algorithm datasets were performed using the Hierarchical Clustering Explorer 3.5 program (20). Similarity and distance measures were assessed according to the Pearson correlation coefficient. Functional clustering analyses of the identified genes were performed via Gene Notes software (http://combio.cs.brandeis.edu/GeneNotes/index.htm), which identifies the gene ontology (GO) biological processes significantly enriched with the differentially expressed genes, with Bonferroni correction (21).
Quantitative RT-PCR Analysis
Please see the online supplement for details.
Western Blot Analysis
Please see the online supplement for details.
Immunohistochemical Staining
Please see the online supplement for details.
Cellular Localization by Multichannel Fluorescence Microscopy
Please see the online supplement for details.
Results
Specimens and Patient Demographics
In total, 51 sinus mucosa specimens from three patient cohorts (control, CRS, and CRS/CF) were used for genome-wide microarray, quantitative RT-PCR (qRT-PCR), Western blot, and immunohistochemical/immunofluorescent analyses. Samples were obtained from 17 control patients (8 females) aged 121–222 months, with a mean age of 197 months and a median age of 206 months; from 19 CRS patients (six females) aged 30–214 months, with a mean age of 111 months and a median age of 93 months; and from 15 CRS/CF patients (eight females) ranging in age from 66–355 months, with a mean age of 169 months and a median age of 162 months. Clinical information is provided in the online supplement and in Materials and Methods.
Expression Array Analyses of Sinus Mucosa from Control, CRS, and CRS/CF Patients
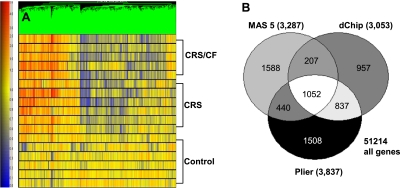
To evaluate the differential expression of genes in the sinus mucosa between control and CRS patients and/or CRS/CF patients, a genome-wide microarray analysis of sinus mucosa from 17 individuals (six patients with CRS, five patients with CRS/CF, and six control subjects) was performed using Affymetrix Human Genome U133_2.0 Arrays. To obtain the most accurate dataset of differentially expressed genes in the three cohorts, the data for each array were subsequently analyzed separately by the MAS 5.0, dChip, and PLIER algorithms. In total, 3,287 probe sets were obtained from MAS 5.0, 3,053 from dChip, and 3,837 from PLIER. The significant probe sets were analyzed by hierarchical clustering in conjunction with the use of an overall F-measure score (which can range from 0–1), with a higher F-measure score reflecting better clustering results in biological samples (20). The results showed that the PLIER algorithm, at F = 0.681, yielded the highest F-measure score and the most consistent grouping of samples in the three cohorts. Thus, the PLIER software program was used for the data presentation of each probe set. The 3,837 probe sets from PLIER were loaded into GeneSpring. The differential expression for each of the 17 patient samples is depicted as a gene tree (Figure 1A). Results showed similarities in expression patterns among patients in each cohort, and a clear-cut stratification of genes in the two CRS cohorts versus the control cohort.
Figure 1.
Gene tree derived from PLIER algorithm analyses of 3,837 differentially expressed genes in sinus mucosa from control, chronic rhinosinusitis (CRS), and CRS/cystic fibrosis (CF) patients. (A) The color scale for the gene tree is shown at left. Red and blue represent positive and negative changes in expression, respectively, versus the control condition. Color intensity is proportional to the magnitude of differential expression. Each row represents a separate patient in one of the three cohorts. Each vertical line represents an individual gene. (B) Venn diagrams show the number of statistically significantly altered probe sets generated by the MAS 0.5, dCHIP, and PLIER algorithms in the CRS and/or CRS/CF sinus mucosal specimens. White denotes the common differentially expressed probe sets (1,052) in all three algorithms. P < 0.05.
To better assess the differential expression of CRS, CRS/CF, and control genes in sinus mucosa, the 3,000–3,800 statistically significantly altered probe sets (P < 0.05) in each of the three algorithms were overlapped in a Venn diagram (Figure 1B). The data yielded a list of 1,052 differentially expressed probe sets common to all algorithms that had changed in a statistically significant way in the CRS and/or CRS/CF cohorts. These probe sets were categorized by biological functions in a GO analysis that focused on genes with terms that were overrepresented more frequently than expected by chance, and that proved significant in sinus mucosa after a Bonferroni correction (P < 0.0005) for multiple comparisons. Gene products related to development (GO, 7,275), cell morphogenesis/differentiation (GO, 30,154; GO, 8,283; GO, 9,653; and GO, 902), and inflammatory/immune responses (GO, 6,955; and GO, 6,952) were expressed in sinus mucosa. Inflammatory/immune mediator genes were previously described as significantly up-regulated in the CRS cohort (18). Here, we focused on evaluating the differentiation and developmental gene products relevant to glandular functions (as indicated by mining the Medline database) that were significantly up-regulated in the CRS and CRS/CF cohorts.
Microarray Analysis of Glandular Genes
Glandular development and morphogenesis genes that were up-regulated with statistical significance (P < 0.05) in the CRS and CRS/CF cohorts are listed in Table 1. These genes encoded keratin 14 (KRT14), desmoglein 3 (DSG3), parathyroid hormone-like peptide (PTHLH), transcription factors (OTX2 and TCF19), TNF-associated proteins (TNFAIP2, TNFAIP6, and TNFRSF18), bone morphogenetic-related proteins (BMPR2 and BMP1), and growth factor–related genes (FGFR1). Four of these genes (DSG3, KRT14, PTHLH, and OTX2) were markedly up-regulated (3-fold to 27-fold) in both CRS and CRS/CF sinus mucosa (Table 1) and were selected for further analysis, because their expression and localization in human sinus mucosa were not previously reported.
TABLE 1.
UP-REGULATED GENES (P < 0.05) IN THE SINUS MUCOSA RELATED TO GLANDULAR DEVELOPMENT AND MORPHOGENESIS
| Fold Change |
|||||
| Symbol | Probe Set | Gene Bank Identification Number | Gene Name | CRS | CRS/CF |
| KRT14 | 209351_at | BC002690 | Keratin 14 | 25.33 | 11.94 |
| DSG3 | 235075_at | AI813438 | Desmoglein 3 | 18.88 | 12.39 |
| PTHLH | 210355_at | J03580 | Parathyroid hormone–like hormone | 3.11 | 2.25 |
| OTX2 | 242128_at | BE779765 | Orthodenticle homologue 2 | 3.33 | 7.91 |
| TNFAIP2 | 202510_s_at | NM_006291 | TNF-α–induced protein 2 | 1.99 | 1.78 |
| TNFAIP6 | 206025_s_at | AW188198 | TNF-α–induced protein 6 | 1.86 | 1.52 |
| TNFRSF18 | 224553_s_at | AF117297 | TNF receptor superfamily member 18 | 1.26 | 1.09 |
| TCF19 | 223274_at | BC002493 | Transcription factor 19 (SC1) | 1.55 | 1.33 |
| BMPR2 | 238393_at | AL047534 | Bone morphogenetic protein receptor, Type II | 1.78 | 2.63 |
| BMP1 | 205574_x_at | NM_001199 | Bone morphogenetic protein 1 | 1.76 | 1.30 |
| FGFR3 | 204380_s_at | M58051 | Fibroblast growth factor receptor 3 | 1.68 | 1.58 |
Definition of abbreviations: CF, cystic fibrosis; CRS, chronic rhinosinusitis.
Genes listed in bold were further evaluated in this study.
Evaluation of Glandular Gene mRNA Expression Levels by qRT-PCR
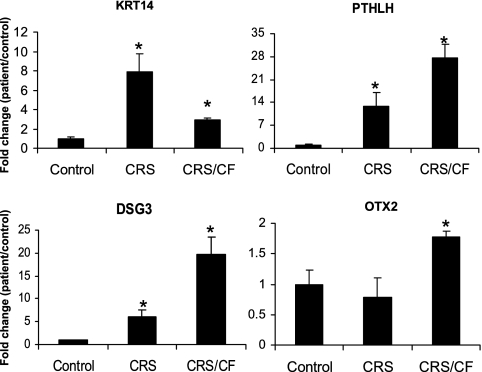
An independent set of tissues (separate from those used in the microarray analyses) from four control, five CRS, and four CRS/CF patients was used to determine the validity of the expression array results. The data indicated that DSG3, KRT14, and PTHLH mRNA were up-regulated in both CRS and CRS/CF sinus mucosa compared with control sinus mucosa, and that the differences were statistically significant (Figure 2). This independent mRNA analysis confirmed the significant increase observed between CRS and CRS/CF patients and controls in the microarray data. However, OTX2, which showed a statistically significant increase in both the CRS and CRS/CF cohorts according to microarray analyses, was only increased in sinus mucosa from CRS/CF patients, according to qRT-PCR analyses in the independent set of sinus mucosa tissues.
Figure 2.
Quantitative RT-PCR analyses of four up-regulated genes identified in our sinus mucosa microarray study. Data show fold-changes (with standard errors) in transcript levels of tissues from CRS (n = 6) and CRS/CF (n = 5) compared with control samples (n = 6), which are normalized to one. *Statistically significant differences (P < 0.05) between CRS/CF and CRS cohorts and control subjects. KRT14, keratin 14; PTHLH, parathyroid hormone–like hormone; DSG3, desmoglein 3; OTX2, orthodenticle homologue 2.
Differential Expression of Glandular Proteins in CRS Sinus Mucosa
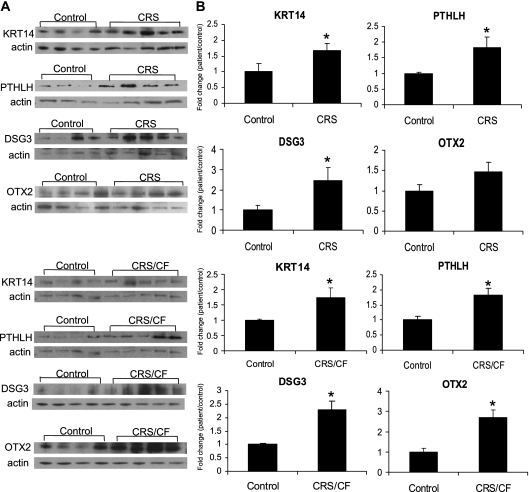
The four glandular gene products of interest were evaluated by Western blot analysis (Figure 3) in sinus tissues from five control, seven CRS, and five CRS/CF patients, to determine whether differential expression also occurred at the protein level. The data showed a statistically significant increase in DSG3, KRT14, and PTHLH protein levels in both CRS and CRS/CF sinus mucosa compared with control sinus mucosa (P < 0.05), in agreement with the qRT-PCR validation data (Figure 2). Likewise, in agreement with the RNA validation studies, OTX2 protein was increased with statistical significance (P < 0.05) only in the sinus mucosa of CRS/CF but not CRS patients.
Figure 3.
Quantification analysis of protein expression of four glandular gene products by Western blot analysis used sinus mucosa tissue from control, CRS, and CRS/CF patients. (A) Representative Western blots depict the protein expression of KRT14, PTHLH, OTX2, and DSG3 in sinus mucosa tissue homogenates from control, CRS, and CRS/CF patients. Actin was immunoblotted as an internal control. (B) Quantitation of band intensity from blots (A) by densitometry. Results are presented as means ± SEM. The relative expression of each protein was set at 100% for control tissues after normalization against actin and protein measurements, respectively. n = 4 for control, n = 4 or 5 for CRS or CRS/CF patients. *P < 0.05, compared with control subjects.
Localization of DSG3, KRT14, PTHLH, and OTX2 in Sinus Mucosa
The localization of the four proteins shown by Western blot analysis (Figure 3) to be expressed in the sinus mucosa was determined. Two distinct microanatomic compartments in the sinus mucosa (the surface epithelium and submucosa) were evaluated using sinus tissues of three CRS, four CRS/CF, and four control patients. KRT14, DSG3, PTHLH, and OTX2 were detected in ciliated and basal cells in the surface epithelium (data not shown). All four proteins were strongly expressed in SMGs, as shown in the online supplement (Figure E1). Among control samples, those with detectable glands were selected for analyses. The expression patterns were similar in CRS, CRS/CF, and control sinus mucosa, which typically contained far fewer SMGs than CRS sinus mucosa.
Localization of DSG3, KRT14, PTHLH, and OTX2 in SMG Cells
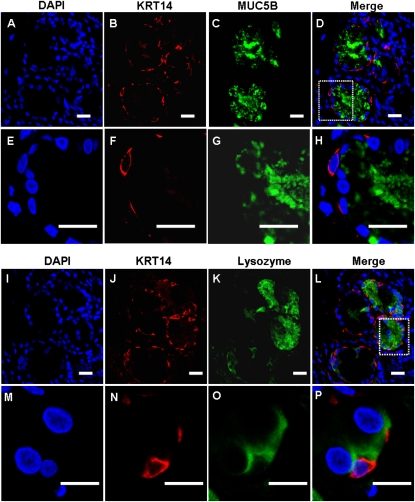
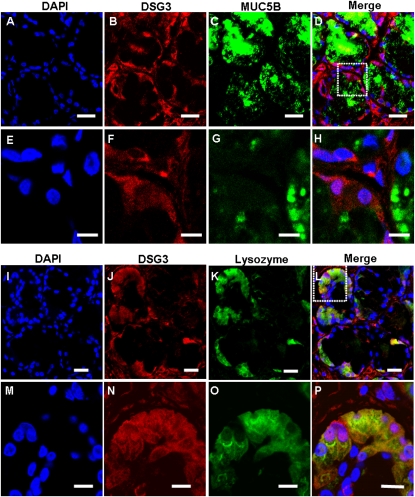
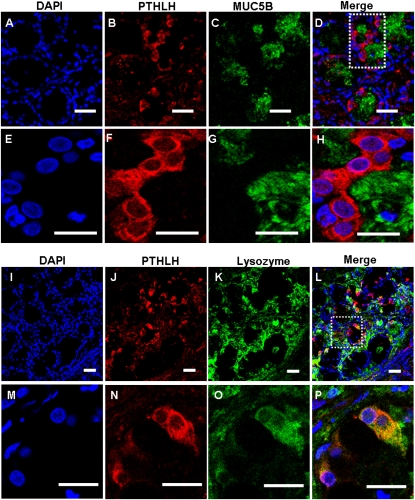
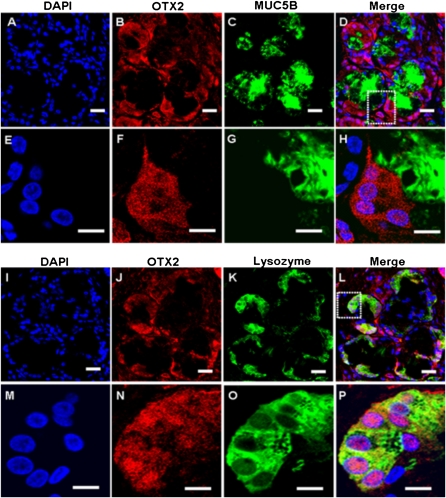
To determine the cellular localization of the four glandular genes of interest in sinus SMGs, double immunofluorescent staining and multichannel fluorescence microscopy were performed. Colocalization studies of each of the four glandular proteins with mucin-5B (MUC5B) or lysozyme, which are markers for mucous or serous cells, respectively (22, 23), were performed (Figures 4–7). Immunofluorescence showed that KRT14 was not expressed in mucous or serous cells (Figure 4). However, KRT14 was expressed in myoepithelial cells, and the colocalization of KRT14 with the myoepithelial cell marker, α–smooth muscle actin (24), was evident (Figure E2). The double-labeling studies also showed that DSG3 (Figure 5), PTHLH (Figure 6), and OTX2 (Figure 7) colocalized with lysozyme but not MUC5B, indicating the restriction of DSG3, PTHLH, and OTX2 to serous cells in sinus SMGs.
Figure 4.
Immunofluorescent double labeling of KRT14 and mucin-5B (MUC5B) or lysozyme in human sinus submucosal glands (SMGs). (A–H) Double labeling of KRT14 and MUC5B. (I–P) Double labeling of KRT14 and lysozyme. Labeling by the KRT14 antibody was detected with a rhodamine-labeled secondary antibody (red). Nulear was stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Labeling of MUC5B and lysozyme were detected with FITC-labeled secondary antibody (green) in the same section. Double labeling revealed no colocalization of KRT14 and MUC5B or lysozyme in SMG cells. Instead, KRT14 was expressed in myoepithelial cells adjacent to the acini. Scale bars, 20 μm in A–L; 10 μm in M–P. The images in the white dotted box in D and L are magnified and shown in E–H and M–P, respectively.
Figure 5.
Immunofluorescent double labeling of DSG3 and MUC5B or lysozyme in human sinus SMGs. (A–H) Double-labeling of DSG3 and MUC5B. (I–P) Double labeling of DSG3 and lysozyme. Labeling by the DSG3 antibody was detected with a rhodamine-labeled secondary antibody. Labeling of MUC5B and lysozyme were detected with FITC-labeled secondary antibody in the same section. The overlap of DSG3 (red) and MUC5B or lysozyme (green) labeling appeared orange (Merge). Double labeling revealed colocalization of DSG3 and lysozyme in the same SMG cells, but no colocalization of DSG3 and MUC5B. No staining was evident under negative control conditions. Scale bars, 20 μm in A–D and I–L; 10 μm in E–H and M–P. The images in the white dotted box in D and L are magnified and shown in E–H and M–P, respectively.
Figure 6.
Immunofluorescent double labeling of PTHLH and MUC5B or lysozyme in human sinus SMGs. (A–H) Double labeling of PTHLH and MUC5B. (I–P) Double labeling of PTHLH and lysozyme. Labeling by the PTHLH antibody was detected with a rhodamine-labeled secondary antibody. Labeling of MUC5B and lysozyme were detected with FITC-labeled secondary antibody on the same section. The overlap of PTHLH (red) and MUC5B or lysozyme (green) labeling appeared orange (Merge). Double labeling revealed colocalization of PTHLH and lysozyme in the same SMG cells, but no colocalization of PTHLH and MUC5B. Scale bars, 20 μm in A–D and I–L; 10 μm in E–H and M–P. The images in the white dotted box in D and L are magnified and shown in E–H and M–P, respectively.
Figure 7.
Immunofluorescent double labeling of OTX2 and MUC5B or lysozyme in human sinus SMGs. (A–H) Double labeling of KRT14 and MUC5B. (I–P) Double labeling of OTX2 and lysozyme. Labeling by the OTX2 antibody was detected with a rhodamine-labeled secondary antibody. Labeling of MUC5B and lysozyme was detected with FITC-labeled secondary antibody in the same section. The overlap of OTX2 (red) and MUC5B or lysozyme (green) labeling appeared orange (Merge). Double labeling revealed no colocalization of OTX2 and MUC5B, but colocalization of OTX2 and lysozyme in the same SMG cells. Scale bar, 20 μm. The images in the white dotted box in D and L are magnified and shown in E–H and M–P respectively.
Colocalization of KRT14 and DSG3
Previous studies showed that Dsg3 binds to Krt14 in mouse keratinocytes (25), and increases the expression of Krt14 in murine suprabasal epidermis (26). To determine whether DSG3 might interact with KRT14 in SMG cells in sinus mucosa, their cellular localization was investigated using multichannel fluorescence microscopy. Immunofluorescence data indicated the colocalization of KRT14 and DSG3 in a subset of myoepithelial cells (Figure E3).
Discussion
Mucus hypersecretion, a characteristic phenotype of patients with obstructive respiratory pathologies, to some extent reflects the epithelial tissue remodeling subsequent to infection and inflammation (27). In the sinus mucosa, this hypersecretion is manifested as SMG hyperplasia (3, 5) rather than goblet cell hyperplasia (3, 4). Microarray analyses of the sinus mucosa of control and CRS patients, including CRS/CF patients, were performed to identify differences in global and specific gene expression between healthy and remodeled sinus mucosa, and to gain initial insights into the genes implicated in glandular hyperplasia in CRS. Several inflammatory and immune mediator genes significantly up-regulated in CRS versus control sinus mucosa were previously identified and validated (18).
The present study focused on glandular-associated genes, to identify, validate, and characterize the cellular localization of four gene products (DSG3, KRT14, PTHLH, and OTX2) that exhibited significantly increased mRNA expression in the sinus mucosa of both CRS and CRS/CF patients relative to control patients. The increased expression of DSG3, KRT14, and PTHLH mRNA and protein was validated both in CRS and CRS/CF sinus mucosa, indicating that they may comprise glandular-associated genes up-regulated in CRS and CRS/CF sinus mucosa in response to the chronic inflammation inherent in the sinus mucosa of all patients with CRS. In contrast, the increased mRNA and protein expression of glandular transcription factor OTX2 was validated only in CRS/CF sinus mucosa, suggesting that OTX2 may be a CF-specific gene that contributes to SMG hyperplasia in CF respiratory mucosa.
We reasoned that if these four gene products were relevant to glandular hyperplasia in CRS or CRS/CF patients, they would be well-expressed in glandular cells and perhaps detectable in the surface epithelium, which is thought to be the source of epithelial stem and progenitor cells, at least in the trachea (10). Each of the four proteins was detected in basal or ciliated cells in the surface epithelium, and each was highly expressed in SMGs, with the expression of DSG3, PTHLH, and OTX2 observed in serous but not mucous cells, and KRT14 observed in myoepithelial cells adjacent to glandular acini. Serous cells are thought to be stem cells for the renewal of respiratory tract epithelium (28, 29). The expression of DSG3, PTHLH, and OTX2 in serous cells (but not mucous cells) raises the possibility that progenitor cells in the surface epithelium can differentiate into serous cells that then function as the “leading edge” of gland tubules during invagination of the epithelium. Although neither serous nor stem cells in the human sinus mucosal epithelium have yet been identified, progenitor cells that are serous cell precursors may proliferate after inflammation, which could lead to SMG hyperplasia.
The genes that participate in the morphogenesis and differentiation of SMGs in respiratory tract mucosa are markedly understudied. However, the four proteins that are markedly up-regulated in sinus mucosa (DSG3, KRT14, PTHLH, and OTX2) were implicated in glandular formation in other epithelial systems. In the mammary gland, desmosomal adhesion is important during epithelial morphogenesis. Blocking of the cell adhesion recognition sites in desmosomal cadherins inhibits alveolar morphogenesis by epithelial cells from the mammary lumen, and disrupts the positional sorting of luminal and myoepithelial cells in aggregates formed by the reassociation of isolated cells (30). The desmosomal cadherin superfamily provides mechanical strength to epithelial tissues by forming stable intercellular contacts anchored to KRT intermediate filaments. DSG3, a member of the desmosomal superfamily, is implicated in the intercellular attachment of skin and mucous membranes (25). Dsg3 anchors to Krt14 in murine keratinocytes (25), and the expression of human DSG3 in transgenic mice increases the expression of murine Krt14 in the suprabasal epidermis (26).
KRT14 is a member of the Type I keratin family that forms the cytoskeleton of keratinocytes and other epithelial cells. Specific keratins are considered markers for hair-follicle stem cells (31) and rabbit cornea stem cells (32). In the salivary glands, KRT14 is expressed in myoepithelial cells, which were proposed to function as luminal cell progenitors (33). In murine tracheae, distinct populations of cells in glandular ducts near the cartilage-intercartilage junction that express high concentrations of Krt14, Krt18, and Krt5 after epithelial damage are considered stem cells (10). Krt14/Krt5 is considered a marker of murine lung epithelial stem cells (34, 35). In human sinus mucosa, we observed the colocalization of KRT14 with KRT5 in SMG ductal cells (data not shown), but also observed that KRT14 was expressed in myoepithelial cells adjacent to glandular acini, as well as in basal cells in the surface epithelium. Although DSG3 was expressed in serous cells and KRT14 was expressed in myoepithelial cells, the colocalization of KRT14 and DSG3 in a subset of myoepithelial cells was evident (Figure E3). Taken together, the information suggests that the increased concentrations of DSG3 observed in CRS sinus mucosa may contribute to the marked up-regulation of KRT14 in CRS sinus mucosa, and may subsequently elicit stem cell self-renewal, leading to glandular hyperplasia. Further study of the interaction between DSG3 and KRT14 in sinus mucosa glandular cells could serve as a future direction for experiments into the pathogenesis of glandular hyperplasia in CRS sinus mucosa.
A potential role in glandular development or hyperplasia is less clear for the other two glandular genes of interest (PTHLH and OTX2) that are up-regulated in CRS or CRS/CF sinus mucosa and expressed in serous SMG cells. PTHLH is expressed in the myoepithelial cells of sweat and parotid glands (36), and participates in the regulation of epithelial–mesenchymal interactions during embryonic mammary development and adolescent ductal morphogenesis (37). OTX2, a homeodomain-containing transcription factor well-expressed in the Xenopus cement gland, a mucus-secreting neural organ, has an essential role in activating the ectopic formation of the cement gland (38). The increased expression of OTX2 was validated only in CRS/CF and not CRS sinus mucosa, suggesting that OTX2 may be a CF-specific gene that affects the ontogeny of SMG hyperplasia at least in the upper respiratory tract mucosa of patients with CF.
The expression of three members (TNFAIP2, TNFAIP6, and TNFRSF18) of the TNF-associated protein family was indicated by microarray analyses to be modestly but significantly up-regulated in CRS sinus mucosa. This finding may indicate a possible role for the TNF superfamily in glandular formation in the respiratory tract, if the increased expression of these gene products were to be validated. Interestingly, mutations in specific members of the TNF superfamily (EDA, EDAR, and EDARADD) were shown to result in a lack of mucus-producing glands in the nose, larynx, and bronchi of patients with ectodermal dysplasia (39). However, these genes were not significantly up-regulated in patients with CRS or CRS/CF in our array datasets.
The modest but significantly increased expression of growth factors (BMP1, BMPR2, and FGFR3) was also evident in our CRS sinus mucosa microarrays, suggesting that specific members of the BMP and fibroblast growth factor (FGF) families play a role in the differentiation of glands that results in SMG hyperplasia in CRS. Various FGFs, growth factor receptors, BMPs, and transcription factors are known to mediate interactions between mesenchymal and epithelial components during embryonic glandular development (40). For example, Bmp7 and Pax6 play important roles during the embryonic SMG branching morphogenesis of murine salivary glands (41), whereas Bmp4 (42) and Fgf10 (43) mediate interactions between mesenchymal and epithelial components that are important for the initial budding and subsequent branching morphogenesis of the epithelial placodes in mice.
The pathways leading to the cellular differentiation that results in gland formation in the upper or lower respiratory mucosa are not well understood. The canonical Wnt signaling pathway and Lef1 play a role during embryogenesis in the development and formation of the initial buds that develop into tracheal SMGs in ferrets (12, 13). These genes and pathways may be transiently activated during the differentiation of respiratory mucosa that leads to de novo glandular development and hyperplasia in respiratory tract diseases, but may not be up-regulated in chronic conditions. Along this line, Lef1 mRNA was not differentially regulated in CRS sinus mucosa, suggesting either that the Wnt pathway is not active in chronic conditions, or that an alternate differentiation pathway is initiated by chronic inflammation in patients with CRS. Investigations of the processes that lead to the initiation and development of glands in the sinus mucosa and subsequent glandular hyperplasia will likely be facilitated by the recent development of an in vitro three-dimensional model for the differentiation of glandular acini from respiratory tract epithelial cells (44).
Supplementary Material
Acknowledgments
The authors thank the Cellular Imaging Core in the Center for Neuroscience Research (CNMC, Washington, DC) for training in, and the use of, confocal microscopes. The authors also thank Dr. Eric Hoffman and the Microarray Core at the Center for Genetic Medicine Research, CNMC, for providing microarray and bioinformatics support, and Drs. Diego Preciado, Robert Freishtat, and Alan Watson for reviewing the manuscript before submission.
Footnotes
This study was supported by a Cystic Fibrosis Foundation Pilot/Feasibility grant (M.T.P.), and by National Institutes of Health grants GCRC 5-MO1-RR-020359-02 from the General Clinical Research Center, NCMRR 5R24HD050846–02 from the National Center for Medical Rehabilitation Research, and R21 AI083995-01 (M.T.P. and M.C.R.). Confocal microscopy imaging was supported by Core Grant 1P30HD40677 from the National Institute of Child Health and Human Development (NICHD) to the Children's Mental Retardation and Developmental Disabilities Research Center.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0133OC on December 22, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001;163:517–523 [DOI] [PubMed] [Google Scholar]
- 2.Rogers DF. Mucus hypersecretion in chronic obstructive pulmonary diseases. Novartis Found Symp 2001;234:65–77 [DOI] [PubMed] [Google Scholar]
- 3.Tos M, Mogensen C. Mucus production in chronic maxillary sinusitis: a quantitative histopathological study. Acta Otolaryngol 1984;97:151–159 [DOI] [PubMed] [Google Scholar]
- 4.Pena MT, Aujla PK, Patel KM, Zalzal GH, Rose MC. Immunohistochemical analyses of MUC5AC mucin expression in sinus mucosa of children with sinusitis and controls. Ann Otol Rhinol Laryngol 2005;114:958–965 [DOI] [PubMed] [Google Scholar]
- 5.Pena MT, Aujla PK, Zudaire E, Watson AM, Zalzal GH, Rose MC. Localization and expression of MUC5B and MUC7 mucins in pediatric sinus mucosa. Ann Otol Rhinol Laryngol 2007;116:389–397 [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2009;4:435–459 [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Amorn M, Aujla PK, Rice S, Mimms R, Watson AM, Peters-Hall JR, Rose MC, Peña MT. Histology and mucin immunohistochemistry of cystic fibrosis sinus mucosa. Archives Otolaryngology ( In press ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurlbeck WM, Benjamin B, Reid L. Development and distribution of mucous glands in the foetal human trachea. Br J Dis Chest 1961;55:54–64 [DOI] [PubMed] [Google Scholar]
- 9.Bucher U, Reid L. Development of the mucus-secreting elements in human lung. Thorax 1961;16:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 2001;24:662–670 [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Driskell RR, Engelhardt JF. Airway glandular development and stem cells. Curr Top Dev Biol 2004;64:33–56 [DOI] [PubMed] [Google Scholar]
- 12.Driskell RR, Goodheart M, Neff T, Liu X, Luo M, Moothart C, Sigmund CD, Hosokawa R, Chai Y, Engelhardt JF. Wnt3a regulates Lef-1 expression during airway submucosal gland morphogenesis. Dev Biol 2007;305:90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan D, Yue Y, Zhou W, Labed B, Ritchie TC, Grosschedl R, Engelhardt JF. Submucosal gland development in the airway is controlled by lymphoid enhancer binding factor 1 (LEF1). Development 1999;126:4441–4453 [DOI] [PubMed] [Google Scholar]
- 14.Driskell RR, Liu X, Luo M, Filali M, Zhou W, Abbott D, Cheng N, Moothart C, Sigmund CD, Engelhardt JF. Wnt-responsive element controls Lef-1 promoter expression during submucosal gland morphogenesis. Am J Physiol Lung Cell Mol Physiol 2004;287:L752–L763 [DOI] [PubMed] [Google Scholar]
- 15.Rawlins EL, Hogan BL. Intercellular growth factor signaling and the development of mouse tracheal submucosal glands. Dev Dyn 2005;233:1378–1387 [DOI] [PubMed] [Google Scholar]
- 16.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261 [DOI] [PubMed] [Google Scholar]
- 17.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Ghimbovschi S, Aujla PK, Patel KC, Rose MC, Pena MT. Expression profiling of inflammatory mediators in pediatric sinus mucosa. Arch Otolaryngol Head Neck Surg 2009;135:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almon RR, DuBois DC, Yao Z, Hoffman EP, Ghimbovschi S, Jusko WJ. Microarray analysis of the temporal response of skeletal muscle to methylprednisolone: comparative analysis of two dosing regimens. Physiol Genomics 2007;30:282–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo J, Gordish-Dressman H, Hoffman EP. An interactive power analysis tool for microarray hypothesis testing and generation. Bioinformatics 2006;22:808–814 [DOI] [PubMed] [Google Scholar]
- 21.Hong P, Wong WH. GeneNotes: a novel information management software for biologists. BMC Bioinformatics 2005;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma P, Dudus L, Nielsen PA, Clausen H, Yankaskas JR, Hollingsworth MA, Engelhardt JF. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am J Respir Cell Mol Biol 1998;19:30–37 [DOI] [PubMed] [Google Scholar]
- 23.Basbaum C, Finkbeiner W. Mucus-producing cells of the airways. : Massaro D, editor Lung cell biology. New York: Marcel Dekker; 1989. pp. 37–80 [Google Scholar]
- 24.Gugliotta P, Sapino A, Macri L, Skalli O, Gabbiani G, Bussolati G. Specific demonstration of myoepithelial cells by anti–alpha smooth muscle actin antibody. J Histochem Cytochem 1988;36:659–663 [DOI] [PubMed] [Google Scholar]
- 25.Caldelari R, de Bruin A, Baumann D, Suter MM, Bierkamp C, Balmer V, Müller E. A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgaris. J Cell Biol 2001;153:823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merritt AJ, Berika MY, Zhai W, Kirk SE, Ji B, Hardman MJ, Garrod DR. Suprabasal desmoglein 3 expression in the epidermis of transgenic mice results in hyperproliferation and abnormal differentiation. Mol Cell Biol 2002;22:5846–5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278 [DOI] [PubMed] [Google Scholar]
- 28.Basbaum CB, Jany B, Finkbeiner WE. The serous cell. Annu Rev Physiol 1990;52:97–113 [DOI] [PubMed] [Google Scholar]
- 29.Evans MJ, Shami SG, Cabral-Anderson LJ, Dekker NP. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol 1986;123:126–133 [PMC free article] [PubMed] [Google Scholar]
- 30.Runswick SK, O'Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol 2001;3:823–830 [DOI] [PubMed] [Google Scholar]
- 31.Coulombe PA, Kopan R, Fuchs E. Expression of keratin K14 in the epidermis and hair follicle: insights into complex programs of differentiation. J Cell Biol 1989;109:2295–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schermer AS, Glavin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 1986;103:49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa Y, Toyosawa S, Ishida T, Ijuhin N. Keratin 14 immunoreactive cells in pleomorphic adenomas and adenoid cystic carcinomas of salivary glands. Virchows Arch 2000;437:58–68 [DOI] [PubMed] [Google Scholar]
- 34.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res 2001;27:401–415 [DOI] [PubMed] [Google Scholar]
- 35.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med 1998;157:2000–2006 [DOI] [PubMed] [Google Scholar]
- 36.Zabel M, Murawski M, Surdyk-Zasada J, Salwa-Zurawska W, Radziemski A. Immunocytochemical localisation of PTHrP (parathormone-related peptide) in myoepithelial cells of human sweat and parotid glands. Folia Histochem Cytobiol 1999;37:167–172 [PubMed] [Google Scholar]
- 37.Dunbar ME, Wysolmerski JJ. Parathyroid hormone–related protein: a developmental regulatory molecule necessary for mammary gland development. J Mammary Gland Biol Neoplasia 1999;4:21–34 [DOI] [PubMed] [Google Scholar]
- 38.Gammill LS, Sive H. Identification of OTX2 target genes and restrictions in ectodermal competence during Xenopus cement gland formation. Development 1997;124:471–481 [DOI] [PubMed] [Google Scholar]
- 39.Smythe WR, Bridges ND, Gaynor JW, Nicolson S, Clark BJ, Spray TL. Bilateral sequential lung transplant for ectodermal dysplasia. Ann Thorac Surg 2000;70:654–656 [DOI] [PubMed] [Google Scholar]
- 40.Hogan BL. Morphogenesis. Cell 1999;96:225–233 [DOI] [PubMed] [Google Scholar]
- 41.Jaskoll T, Zhou YM, Chai Y, Makarenkova HP, Collinson JM, West JD, Hajihosseini MK, Lee J, Melnick M. Embryonic submandibular gland morphogenesis: stage-specific protein localization of FGFs, BMPs, Pax6 and Pax9 in normal mice and abnormal SMG phenotypes in FgfR2-IIIc(+/Delta), BMP7(−/−) and Pax6(−/−) mice. Cells Tissues Organs 2002;170:83–98 [DOI] [PubMed] [Google Scholar]
- 42.Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J 2000;19:6664–6674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makarenkova HP, Ito M, Govindarajan V, Faber SC, Sun L, McMahon G, Overbeek PA, Lang RA. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development 2000;127:2563–2572 [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Peters-Hall J, Bose S, Pena MT, Rose MC. Human bronchial epithelial cells differentiate to 3D glandular acini on basement membrane matrix. Am J Respir Cell Mol Biol 2010; Aug 19. [Epub ahead of print] PMID: 20724555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.