Abstract
Aims
To study the distribution and clearance of polyethylene glycol (PEG)-ylated single-walled carbon nanotube (SWCNTs) as drug delivery vehicles for the anticancer drug cisplatin in mice.
Materials & methods
PEG layers were attached to SWCNTs and dispersed in aqueous media and characterized using dynamic light scattering, scanning transmission electron microscopy and Raman spectroscopy. Cytotoxicity was assessed in vitro using Annexin-V assay, and the distribution and clearance pathways in mice were studied by histological staining and Raman spectroscopy. Efficacy of PEG-SWCNT–cisplatin for tumor growth inhibition was studied in mice.
Results & discussion
PEG-SWCNTs were efficiently dispersed in aqueous media compared with controls, and did not induce apoptosis in vitro. Hematoxylin and eosin staining, and Raman bands for SWCNTs in tissues from several vital organs from mice injected intravenously with nanotube bioconjugates revealed that control SWCNTs were lodged in lung tissue as large aggregates compared with the PEG-SWCNTs, which showed little or no accumulation. Characteristic SWCNT Raman bands in feces revealed the presence of bilary or renal excretion routes. Attachment of cisplatin on bioconjugates was visualized with Z-contrast scanning transmission electron microscopy. PEG-SWCNT–cisplatin with the attached targeting ligand EGF successfully inhibited growth of head and neck tumor xenografts in mice.
Conclusions
PEG-SWCNTs, as opposed to control SWCNTs, form more highly dispersed delivery vehicles that, when loaded with both cisplatin and EGF, inhibit growth of squamous cell tumors.
Keywords: biodistribution, cancer, carbon nanotube, cisplatin, clearance, drug delivery, dynamic light scattering, growth factor, H & E staining, polyethylene glycol, Raman spectroscopy, STEM
Functionalized single-wall carbon nanotubes (SWCNTs) [1] hold great promise for targeted cancer drug delivery [2]. These nanoparticles are tubular, rolled sheets of graphitic carbon (graphene) with approximately 1 nm diameters [3]. They can be oxidatively shortened to nm-scale lengths and easily outfitted with chemical functionalities to attach biomolecules such as proteins, DNA and RNAi [4–8], thus presenting new opportunities for drug delivery and in vivo imaging [9–11]. For these applications to be translated to clinical reality, challenges presented by issues such as cytotoxicity and biodistribution of SWCNTs in vivo need to be addressed [12–14].
Investigations to date suggest that µm length pristine or nondispersible carbon nanotubes (CNTs) can cause toxicity resulting from metal impurities, aggregation [15], dosage levels and route of administration [16]. Purified SWCNTs implanted via intratracheal instillation in mice resulted in epithelioid granulomas and in some cases interstitial inflammation in a 90-day study [17]. Undoped multiwalled CNTs (MWCNTs) were found to induce severe granulomatous inflammatory responses compared with nitrogen-doped MWCNTs when administered intratracheally in mice [18]. Pristine MWCNTs inhaled by mice for 6 h showed deposition throughout the lungs [19]. Nanotubes were found in lungs and within macrophages, close to the sensitive mesothelial tissue, with evidence of scarring or fibrosis at 2, 6 and 14 weeks postexposure. Furthermore, inhaled MWCNTs suppress immune response in mice [20]. Toxicological data suggest that after intraperitoneal administration of pristine nonfunctionalized CNTs, they behave similarly to asbestos fibers, clogging fine arterial networks and capillaries in the lungs and inducing respiratory irritation, and can result in mesothelioma [21]. However, intraperitoneal injection of nonfunctionalized CNTs directly onto the mesothelium gave data inconclusive to support toxicity.
On the other hand, studies on shortened (e.g., <5 µm), highly dispersed CNTs suggest low toxicity in animals [16]. For example, intravenously injected functionalized SWCNTs with PEG noncovalently in a small group of mice revealed no evidence of toxicity over 4 months [22]. This study suggested that surface modification of SWCNTs to increase dispersibility in water provides nanotubes with less cytotoxicity. Recent updates suggest that appropriately functionalized SWCNTs can potentially provide low toxicity for drug delivery systems (DDS) [23].
In general, increased dispersibility or solubility of any nano DDS improves performance and lowers toxicity [24–26]. Block copolymers [27], such as poly(l-amino acid), poly(ester), and Pluronics, have been used in DDS with doxorubicin, methotrexate, cisplatin and paclitaxel [28–31]. Several DDS have been made using solubilized or dispersed CNTs. Surfactants, such as sodium dodecylsulfate or dodecylbenzene sulphonate, have been used to improve dispersibility [32,33]. CNTs were also wrapped with biomolecules, such as DNA and flavin mononucleotide, which assisted in aqueous dispersion of the nanotubes [34,35]. However, the most popular approach for dispersing the CNTs has been the use of water-soluble polymers [36,37].
Polyethylene glycol (PEG) is a polymer that addresses both increased dispersibility and lowers cytotoxicity [38]. PEG coating of nanomaterials can help in reducing toxicity [39] and, can avoid opsonization (a process by which a pathogen is marked for ingestion and destruction by a phagocyte), making nanoscale DDS less visible to phagocytic cells [40–42]. In addition, nanoparticles and nanotubes with covalently attached PEG had longer blood circulation times than nanomaterials with only surface-adsorbed PEG [43].
Liu et al. demonstrated that functionalizing SWCNTs with branched PEG chains resulted in longer circulation time, less toxicity and more effective clearance in mice [44]. PEGylation of SWCNTs not only makes the hydrophobic nanotubes more soluble, but it also increases their hydrodynamic size and decreases immunogenicity [45]. What remains less clear is whether such a configuration affects the surface-binding capacity of SWCNTs for chemotherapeutic drugs.
For nanotube DDS to be translated to cancer therapy, low toxicity to normal tissues combined with high potency against cancer is needed [46]. We previously demonstrated that oxidized, shortened SWCNTs conjugated with anticancer drug cisplatin [47] and ligand EGF to target EGF receptor killed squamous cancer cells in vitro and limited tumor growth in mice [48]. In this article, we investigate the influence of attached PEG on the performance of similar cisplatin DDSs. Specifically, we report investigations of in vivo distribution, clearance of PEG-SWCNTs and efficacy for targeted tumor therapy in xenograft mouse models. Results suggest that PEG-SWCNTs form more highly dispersed DDS than SWCNTs without PEG, accumulate to a much smaller extent in vital organs of mice compared with non-PEG-SWCNTs, and improved the growth inhibition of squamous cell tumors when loaded with cisplatin and targeted with EGF.
Experimental methods
Preparation of PEGylated nanotubes
The conjugation was achieved by first carboxylating the surface of the nanotubes, providing functionality to covalently bind molecules to the nanotube surface. This process involved ultrasonic dispersion of the high-pressure carbon monoxide conversion SWCNTs (Carbon Nanotechnologies Inc., Houston, TX, USA) in a solution of HNO3–H2SO4 (at a 3:1 ratio, respectively) for 4 h at 70°C. They were subsequently filtered, washed with ultrapure water and dried. The SWCNTs with diameters of 0.8–1.4 nm and 40–400 nm in length were then stored at room temperature until further use. Next, SWCNTs (5 mg oxidized and activated with 1-[3-(dimethylamino) propyl]-3-ethylcarbodiimide hydrochloride [EDC]/N-hydroxysuccinimide [NHS]) were sonicated with 1 ml of 2 mg ml−1 amino-PEG (molecular weight [MW] 5000 Da; Nanocs Inc., New York, NY, USA) in phosphate buffer saline (PBS), for approximately 45 min at room temperature. The resulting PEG-dispersed SWCNTs were centrifuged at 10,000 rpm for 10 min, the resulting supernatant transferred to a clean Eppendorf tube and precipitated SWCNTs discarded. The dispersion was then stored at room temperature for stability monitoring. SWCNT–drug conjugates for tumor targeting were prepared using the PEG-SWCNT dispersion prepared above. The PEG-SWCNTs were resuspended in PBS at a final concentration of 0.5 mg ml−1. They were then incubated with 2 mg ml−1 of EDC/NHS (Sigma-Aldrich, St Louis, MO, USA) for 1 min at room temperature to activate the carboxyl groups on the nanotubes. Subsequently, human EGF (Sigma-Aldrich), along with cisplatin, were added to the nanotube solution and reacted for 1 h at 37°C on a shaker. Finally, the samples were then centrifuged at 1300 rpm for 20 min and the resulting complexes were resuspended in 100 µl Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen Carlsbad, CA, USA). The cisplatin molecules, taken from stock solution, were bound in a similar fashion [49]. The amount of cisplatin was measured by difference in ultraviolet absorbance of adsorbate solutions before and after attachment to SWCNTs. The final stock concentration of SWCNT–cisplatin–EGF was 0.5 mg/ml−1, with approximately 1.33 µM of cisplatin per ml of solution. Approximately 10 cisplatin molecules per 100 nm length of CNTs was estimated. Control SWCNTs were prepared using the same procedure but without EGF.
Scanning transmission electron microscopy
For electron microscopy, PEG-SWCNTs bioconjugates with and without cisplatin were dispersed in ethanol. Next, 3-µl droplets of this dispersion were placed onto gold lacey-carbon support grids (EMS, Hatfield, PA, USA). Scanning transmission electron microscopy (STEM) images were recorded using an FEI™ Tecnai TF30 electron microscope (FEI, Hillsboro, OR, USA) with a field emission source and acceleration voltage of 300 kV. Z-contrast dark-field STEM images were obtained using a Fischione annular dark-field STEM detector (Fischione, Export, PA, USA). Images were acquired with a probe diameter of approximately 0.3–0.4 nm full-width at half maximum and pixel size 0.027 nm, and postprocessed with a low-pass Gaussian filter of 0.13 nm full-width at half maximum.
Annexin-V assay
HN12 cells, a cell line established from a metastatic head and neck squamous carcinoma [50] were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum, at 37°C in 95% air/5% CO2. Cells grown to 60–70% confluency were treated with either SWCNTs or PEG-SWCNTs to a final concentration of 0.5 mg ml−1. Following 24 h exposure to the nanotubes, cells were trypsinized, harvested and resuspended in 200 µl of 1X binding buffer to a final concentration of 5 × 103 cells µl−1. Next, 5 µl of Annexin V (ApoAlert Annexin V-FITC Apoptosis kit, Clontech, Mountain View, CA, USA) and 10 µl of propidium iodide were added, followed by 15 min incubation in the dark. Cells were subsequently analyzed by flow cytometry (fluorescence-activated cell sorter, Becton Dickinson Mountain View, CA, USA) using a single laser excitation at 488 nm and dual filter set for FITC and rhodamine. γ-irradiation (30 Gy units) from 137Cs-g source (γ-cell 1000, Kanata, ON, Canada) was used as a positive control.
Biodistribution of SWCNTs in vivo
All animal studies were performed according to NIH-approved protocols, in compliance with the Guide for the Care and Use of Laboratory Animals. Female 4–6-week-old athymic mice (nu/nu: Harlan Sprague Dawley, Indianapolis, IN, USA) housed in appropriate sterile filter-capped cages, and fed and hydrated ad libitum were used. Mice were injected with 200 µl of 0.5 mg ml−1 of either SWCNT or PEG-SWCNT in DMEM (two mice per group) intravenously and euthanized 24 h later for removal of vital organs (lung, liver, kidney and spleen) and 5-mm cut slices were fixed in Z-fix (Anatech Ltd., Battle Creek, MI, USA) overnight and 70% ethanol, followed by paraffin embedding. To facilitate histopathalogical analysis, 5-µm sections were cut and stained with hematoxylin and eosin (H&E) for microscopic viewing. A subset of organ tissue sections were collected on glass slides for Raman spectral analysis. In parallel, a separate cohort of animals underwent similar treatment and, at every 24 h postinjection period, solid excreted material from each group was collected, homogenized and subsequently processed for Raman spectral analysis. Tumorgenicity studies using HN12 xenografts were essentially as those previously described [48].
Raman spectroscopy
Raman characterization was used for the analysis of the functionalized PEGylated SWCNT samples for specific signature peaks (D and G bands). Raman characterization for detecting nanotubes in tissues and solid excreted material was carried out using characteristic SWCNT Raman signature peaks (G-band). Samples (tissues or solid excreta) were homogenized in 1% sodium dodecyl sulfate and 1% Triton X-100 and sonicated to form homogenous suspensions. Raman spectra were recorded on a Renishaw Ramanscope 2000 spectrometer equipped with a microscope attachment, and laser spot size was focused to 1 µm diameter (×100 objective), and with a power output of 35 mW. The excitation source was an argon ion laser, 514.5 nm.
Dynamic light scattering
Dynamic light scattering was used to study the aggregation behavior of PEG-SWCNT. Dynamic light scattering was performed using a PD2010 light scattering detector from Precision Detectors (Varian, Inc., Palo Alto, CA, USA). Samples of 0.5 mg ml−1 were 10× using final volume of 400 µl.
Results
Conjugation & dispersion of PEG-SWCNTs
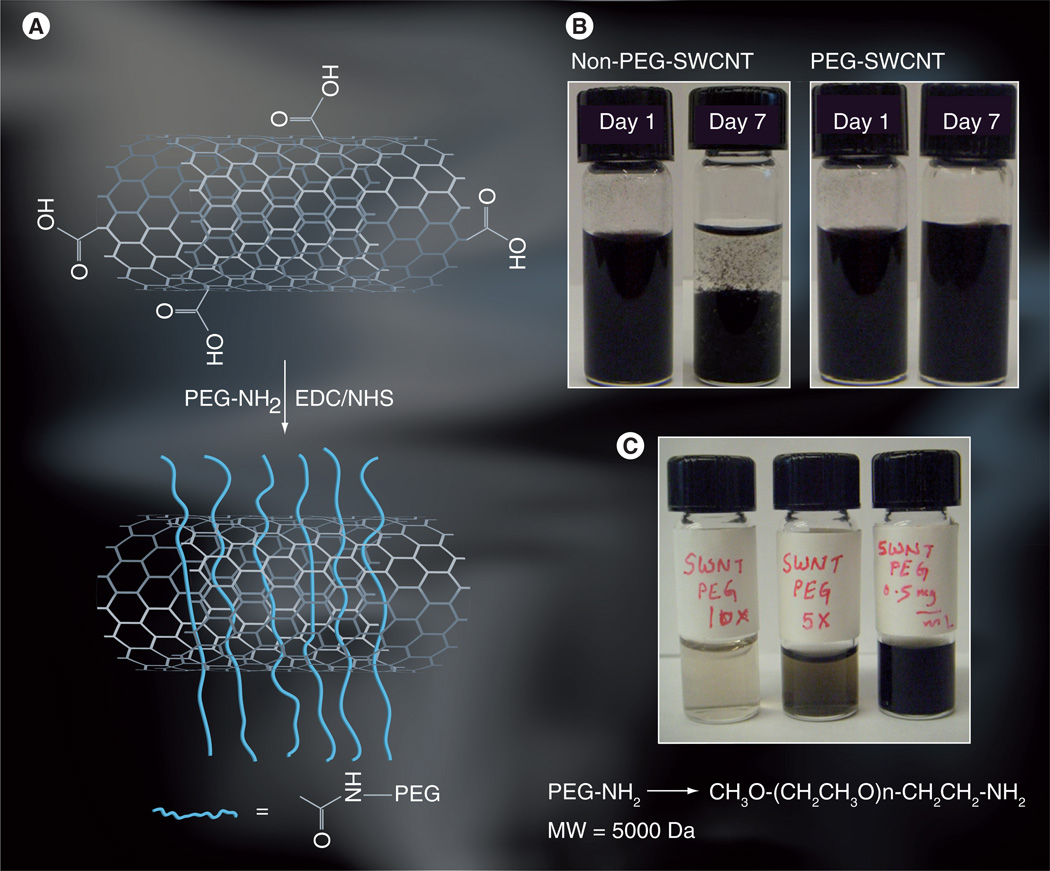
Based on studies reporting that longer and more branched PEG on SWCNTs displayed prolonged blood circulation and lower reticuloendothelial system uptake [51], we chose PEG5000 wrapping of SWCNTs for distribution and clearance in in vivo studies. Amineterminated PEG was reacted with carboxylated SWCNTs that had been treated with amidization promoter EDC/NHS as in Figure 1A. PEG-SWCNTs along with oxidized SWCNTs as control were then dispersed in buffer (PBS). Control SWCNTs were observed initially to disperse readily, but were observed to aggregate within 7 days (Figure 1B, left). By contrast, PEG-SWCNTs remained stable in dispersions for 7 days (Figure 1B, right). This can be better seen in the PEG-SWCNTs diluted tenfold in PBS resulting in clearer dispersions with no aggregates, and these remained clear for 1 week (Figure 1C), and up to 1 month (not shown) both in PBS buffer and serum.
Figure 1. PEGylated single-walled carbon nanotube attachment and dispersions.
(A) Oxidized SWCNT attachment by amide linkage to amino-PEG providing very high dispersion. (B) Photographs of PEG-SWCNT dispersion 1 and 7 days after derived, showing the stability of these nanotube dispersions. Precipitation was seen for non-PEG-SWCNT dispersions. (C) Photographs of PEG-SWCNT dispersions in phosphate buffer saline at various dilutions showing stable dispersions are obtained at higher dilutions.
EDC: 1-[3-(dimethylamino) propyl]-3-ethylcarbodiimide hydrochloride; MW: Molecular weight; NHS: N-hydroxysuccinimide; PEG: Polyethylene glycol; SWCNT/SWNT: Single-walled carbon nanotube.
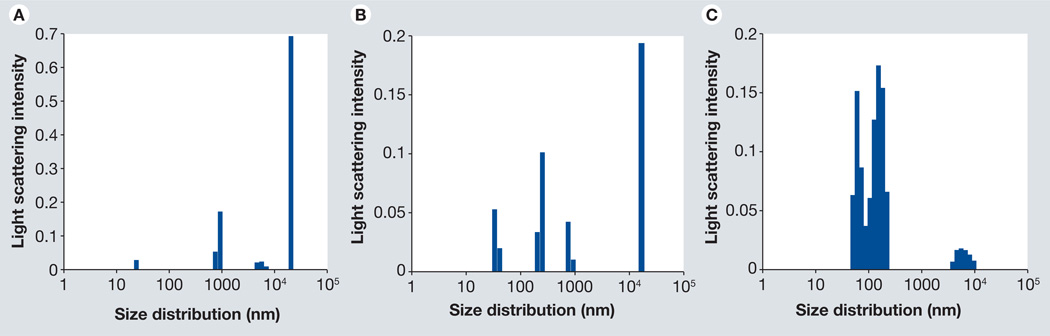
Dynamic light scattering [52,53] showed narrow nominal size distributions of the PEG-SWCNTs (35% aggregates of 63.3 nm, 59% of 153 nm), compared with large aggregates of pristine SWCNTs (22% aggregates of 879 nm, 69% of 20300 nm) and oxidized SWCNTs (13% aggregates of 245 nm, 75% of 19400 nm) (Figure 2). Pristine and oxidized SWCNTs showed much higher degrees of polydispersity and aggregation compared with PEG-SWCNTs, suggesting that PEGylation of the SWCNTs renders them more hydrophilic and more dispersible.
Figure 2. Nominal size distributions of PEGylated single-walled carbon nanotubes dispersed in water from dynamic light scattering.
(A) Pristine single-walled carbon nanotube (SWCNT) show a high degree of aggregation in aqueous media along with severe polydispersity. (B) Oxidized SWCNTs show somewhat less aggregation compared with pristine SWCNTs, but still have considerable polydispersity. (C) Polyethylene glycol-SWCNT dispersion shows one major distribution with a smaller secondary one, suggesting less aggregation compared with pristine and oxidized SWCNTs.
STEM & Raman spectra of PEG-SWCNTs
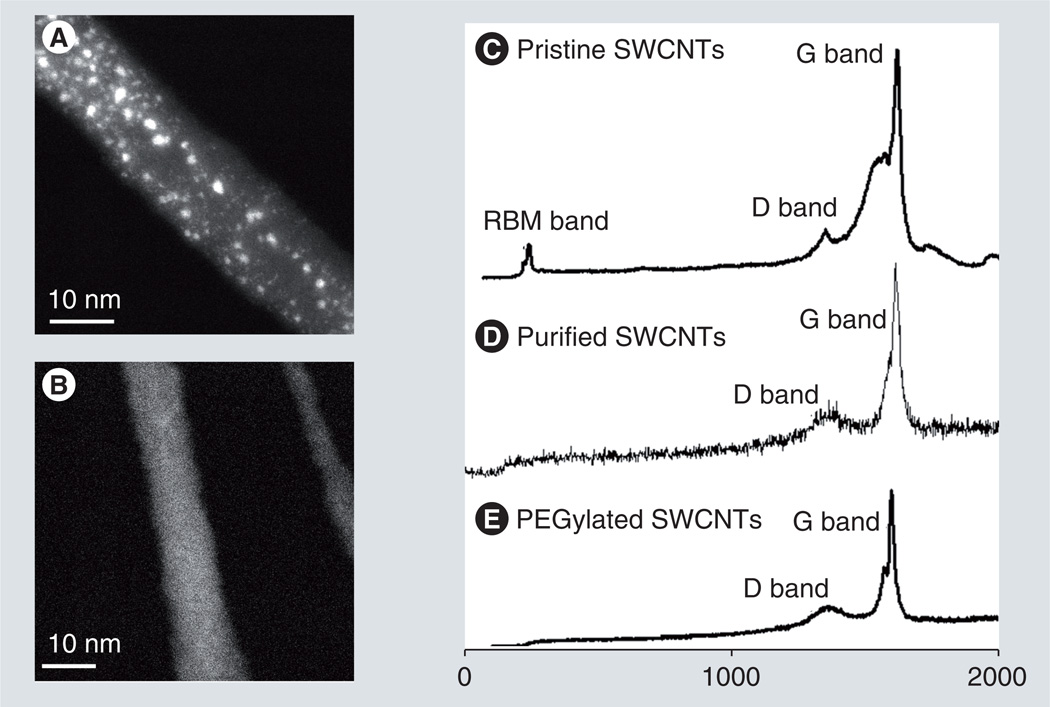
Scanning transmission electron microscopy and Raman spectroscopy were employed to characterize the PEG-SWCNT structures [54–57]. In Z-contrast STEM, image intensity scales with the square of atomic number, and so heavy atoms such as Pt appear bright and with high contrast on a darker background from materials of light atoms such as SWCNTs. STEM imaging of PEG-SWCNT–cisplatin bioconjugates shows SWCNTs packed as a bundle of approximately 10 nm in diameter as well as numerous bright spots of varying sizes dispersed throughout the bundle (Figure 3A). Based on our previous studies of cisplatin loaded onto carboxylated SWCNTs, we expect that the smallest bright spots in Figure 3A correspond to individual atoms of Pt covalently bound to the nanotubes, whereas the larger bright spots derive from clusters of Pt formed by many atoms [56]. STEM images of control PEG-SWCNTs bearing no cisplatin show no bright spots (Figure 3B).
Figure 3. Z-contrast scanning transmission electron microscopy images and Raman spectra of PEGylated single-walled carbon nanotubes.
(A) Scanning transmission electron microscopy image of a PEG-SWCNT bundle conjugated with anticancer drug cisplatin. Bright dots correspond to clusters of Pt atoms (i.e., cisplatin molecules). (B) Scanning transmission electron microscopy image of a PEG-SWCNT bundle alone shows no bright dots. (C–E) Raman spectra showing signature peaks (relative intensities) of (C) pristine SWCNTs, (D) purified or acid-treated SWCNTs, and (E) SWCNTs wrapped with PEG.
PEG: Polyethylene glycol; RBM: Radial breathing mode; SWCNT: Single-walled carbon nanotube.
In Raman spectra of PEG-SWCNTs, the radial breathing mode, defect D band and the tangential mode (G band) signature peaks (Figure 3C–E) were consistent with similar data reported by others [58]. Raman spectra of pristine SWCNTs clearly show the radial breathing mode bands, along with overlapping peaks in the D and G band region (Figure 3C). Oxidatively purified SWCNT and PEG-SWCNT samples give broad D bands at 1290 cm−1 and much better resolved G bands at 1500–1700 cm−1 (Figures 3D & 3E). Figure 3E indicates that the PEG wrapping around the SWCNT surface is not 100% and that there are vacant defect (D band) sites for further conjugation with EGF/drug.
Assessment of cytotoxicity in vitro
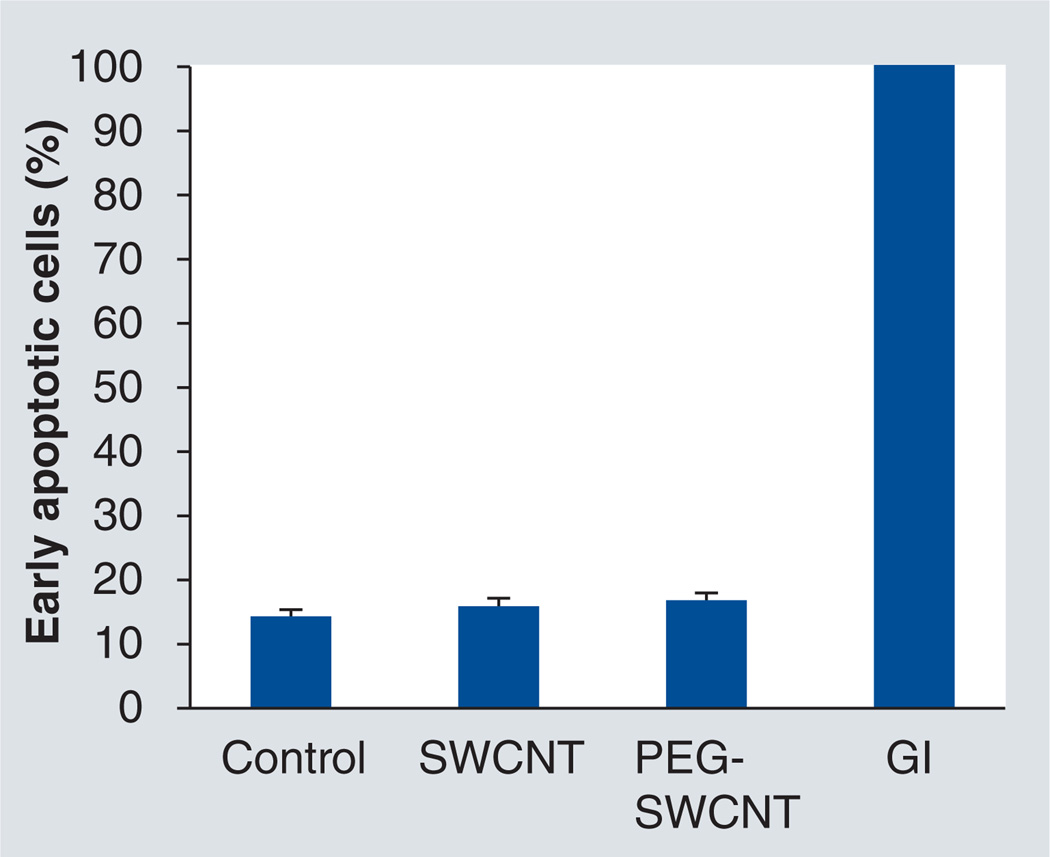
Before assessing PEG-SWCNTs in vivo, we tested them for signs of cytotoxicity on head and neck cancer HN12 cells using the Annexin V assay. As shown in Figure 4, cells treated with 0.5 mg ml−1 of PEG-SWCNTs showed no difference in the cytotoxicity assay when compared with cells alone (negative control), or cells treated with non-PEG-SWCNTs. The in vitro profile for SWCNTs alone was consistent with earlier studies using cell viability 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay where cell proliferation was not hindered [48]. A positive control exposed to γ-irradiation (GI: 30 Gy units) resulted in nearly 100% apoptosis. The data suggest that PEG-SWCNTs do not induce apoptotic responses in vitro in HN12 cells.
Figure 4. Apoptosis assays of HN12 head and neck cancer cells treated with single-walled carbon nanotubes and PEGylated single-walled carbon nanotubes.
Analysis by the Annexin-V assay and subsequent flow cytometry revealed no significant deviation of the percentage of early-stage apoptotic cells among the experimental groups and negative control. Conversely, approximately 100% of the cells irradiated with γ-radiation were apoptotic, serving as a positive control. Concentrations of SWCNT and PEG-SWCNT were 0.5 mg ml−1. The assay was performed 24 h postapplication. Control: Cells only; GI: γ-irradiation control; PEG: Polyethylene glycol; SWCNT: Single-wall carbon nanotube.
Histopathological analysis of vital organ tissues
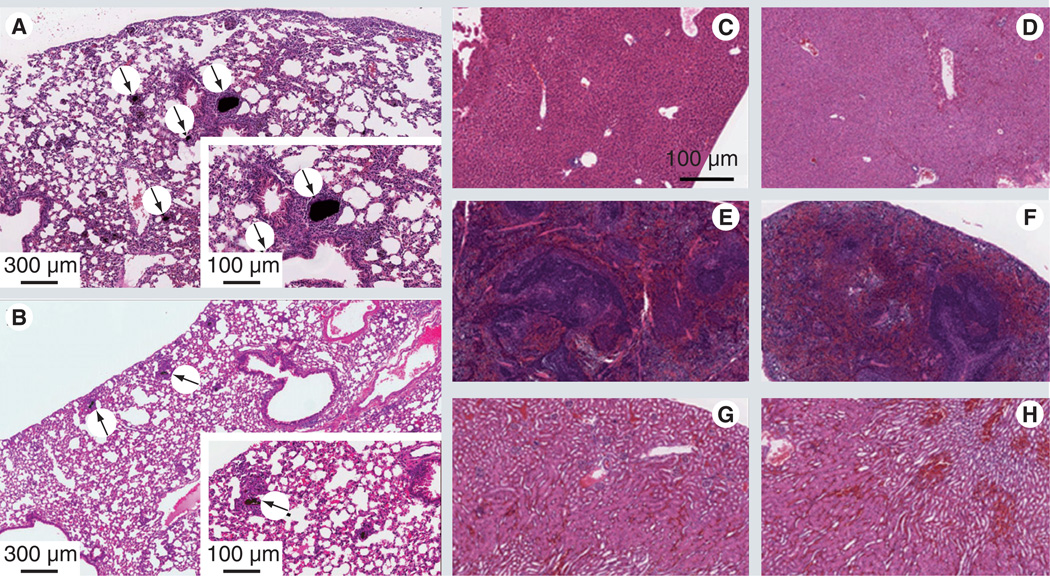
Vital organs from mice injected with PEG-SWCNTs or non-PEG-SWCNTs through the intravenous route, were analyzed by using H&E staining for any signs of acute toxicity and evidence of CNT distribution. Sections 5-µm thick cut from paraffin-embedded tissues were stained with H&E for histopathological assessment. Figures 5A–H show stained images of vital organs for PEGylated and control SWCNTs. Figure 5A shows lung tissue of control mice injected with oxidized SWCNTs. Embedded in the lung parenchyma are concentrations of dark, particulate material likely to be non-PEG-SWCNTs, which at this resolution could be either free and densely packed material or contained in the cytoplasm of macrophages. This accumulation does not appear to be within airways, but rather in the interior of blood vessels that have been clogged by these concretions. Besides macrophages, other inflammatory mononuclear cells are seen to be accumulated surrounding the affected areas. In Figure 5B, lung tissue from mice injected with PEG-SWCNTs shows black granular concretions, but appears to be smaller and fewer in number. These aggregates are also surrounded by mononuclear reactive cells, which are most likely macrophages. Even though the presence of these types of cells signals the presence of a mild tissue reaction, the degree of inflammation was much lower as that seen in the non-PEG-SWCNTs.
Figure 5. Histological analysis of vital organs of mice treated with single-walled carbon nanotubes and PEGylated single-walled carbon nanotubes.
Tissue samples were procured from 6-week-old athymic mice that were injected intravenously with 200 µl of 0.5 mg ml−1 of single-walled carbon nanotube (SWCNT) (A, C, E & G) and polyethylene glycol (PEG)-SWCNT (B, D, F & H) and stained with hematoxylin–eosin. (A) Lung parenchyma showing dark, particulate material, which appears to be free and densely packed, or contained in the cytoplasm of macrophages. The accumulations do not seem to have relations with the airways, but rather appear in the interior of blood vessels that have been clogged by the concretions. Besides the macrophages, other inflammatory mononuclear cells are accumulated surrounding the affected areas. (B) The PEG-SWCNT lungs shows much smaller and fewer concretions similar to those described above. They are also surrounded by mononuclear reactive cells, most likely macrophages. Isolated (segmental) angiocentric chronic inflammatory infiltrates are also present. (C & D) Liver parenchyma with no apparent histological signs of toxicity. (E & F) The kidney has no noticeable histological signs of nanotube toxicity. (G & H) Spleen tissue with no significant histological changes.
By contrast, no significant histological changes are seen in PEG-SWCNT-treated animals compared with controls injected with PBS alone. No dark material was observed in the H&E stained paraffin-embedded cross-sections of tissues from vital organs (Figures 5C–H). The data suggest that PEG-SWCNTs do not accumulate in vital organs when injected intravenously into mice. The short-term histological analysis of tissue samples of animals treated with PEG-SWCNTs did not show any adverse inflammatory response in vivo [59].
Tracking PEG-SWCNTs ex vivo using Raman spectroscopy
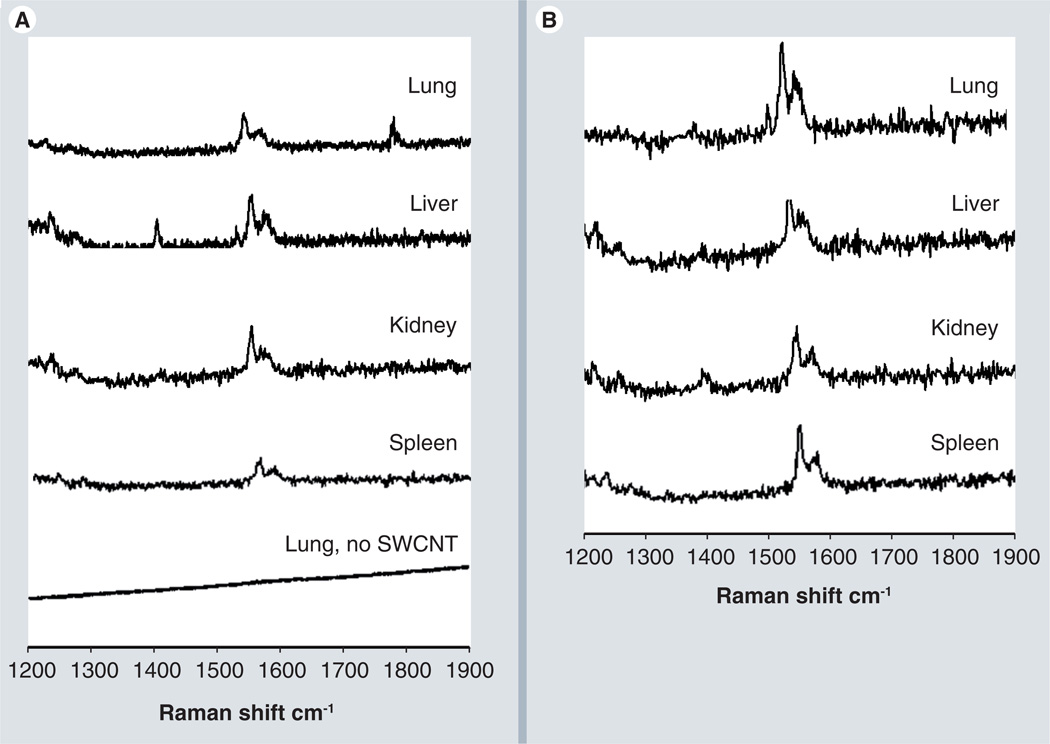
To track the fate of CNTs, Raman spectra were performed on tissues procured from vital organs as indicated above. Tissues from mice injected with PBS did not show the characteristic G-band peak of the CNTs (Figure 6). Instead, featureless spectrum was observed with an elevated background, likely coming from tissue autofluorescence, as in earlier reports [41]. Raman spectroscopy was adequately sensitive to detect the G bands, but with very small intensities (Figure 6).
Figure 6. Raman analysis of tissue from vital organs.
(A) Raman spectra of tissues from vital organs removed from the mice after being treated with polyethylene glycol (PEG)-single-walled carbon nanotubes (SWCNTs). Mice treated with PEG-SWCNTs 24 h post intravenous injection, tissue removed 24 h post intravenous injection for Raman analysis with control lung tissue. The Raman G band for SWCNTs in the 1500–1600 cm−1 region is clearly observed in mice treated with PEG-SWCNTs, while spectral analysis of tissues of mice not treated with SWCNT (bottom trace) showed no indication of the characteristic G band. (B) Raman spectra of tissues of vital mouse organs after being treated with non-PEG-SWCNTs, 24 h post intravenous injection. All spectra are the average of three spots from a given tissue slide.
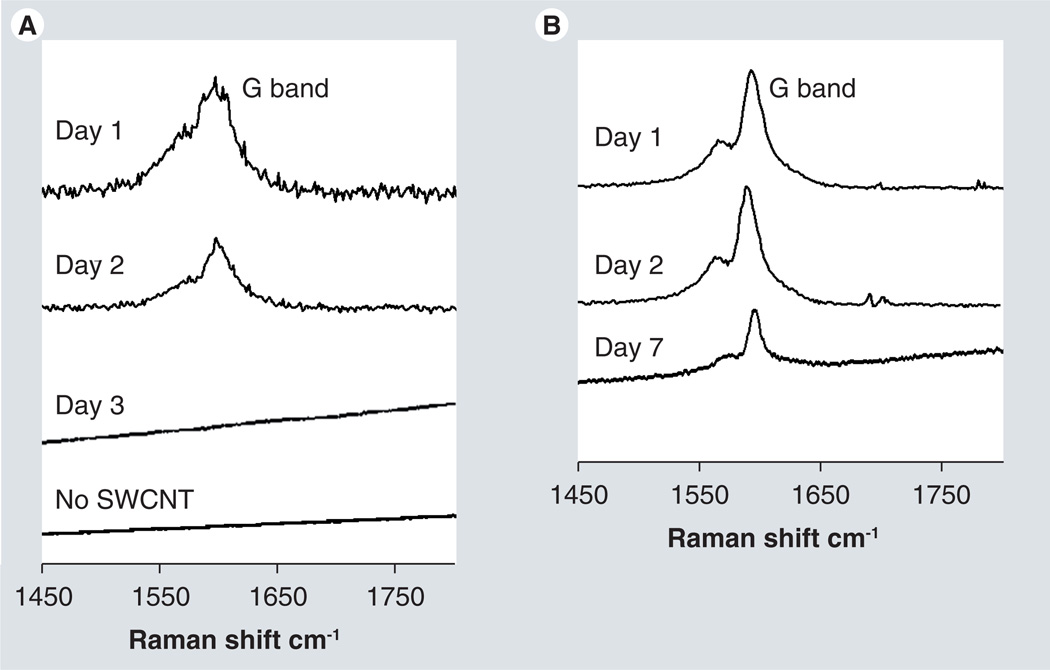
Solid excreted material collected from parallel cohorts of mice was also analyzed by Raman spectroscopy. Samples from mice injected with PBS (Figure 7A) were collected 24-h postinjection, but the excreted material gave no G bands. Raman peaks from mice receiving non-PEG-SWCNTs disappeared after 2 days (Figure 7A), while those for PEGylated SWCNTs were observed for up to 7 days (Figure 7B).
Figure 7. Raman analysis of powdered fecal samples.
(A) Raman spectra of excreted material from mice treated with SWCNTs (days 1–3) or phosphate buffer (no SWCNT). Spectra shown are the average of three spots from the tissue slide. (B) Raman spectra of excreted material from mice treated with polyethylene glycol-SWCNTs treated mice at indicated times (1–7 days). Spectra are the average of three spots from a slide on which the powdered samples were spread.
SWCNT: Single-walled carbon nanotube.
Inhibited growth of HN12 xenografts by PEG-SWCNT–cisplatin–EGF bioconjugate
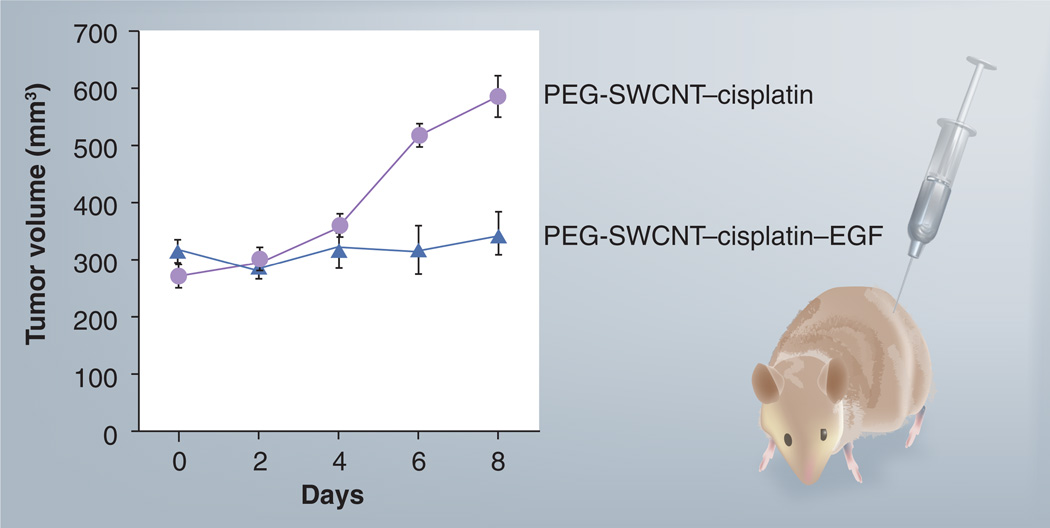
As the tumor microenvironment is known to be acidic (pH 5.7–7.8) [60] and our in vitro dialysis data indicates the release of cisplatin from the conjugates in acid conditions (data not shown), we explored the tumor-targeting efficacy of PEG-SWCNT–cisplatin–EGF equipped with a cancer-directing ligand to study the effect on tumor progression. Athymic nude mice were implanted with a HN12 tumor xenograft subcutaneously. PEG-SWCNTs equipped with the targeting ligand EGF and anticancer drug cisplatin formed highly dispersible and stable delivery vehicles, and when injected intravenously resulted in inhibition of tumor growth more effectively compared with non-PEG-SWCNT (Figure 8). The group treated with a single injection of PEG-SWCNT–cisplatin–EGF showed 6.25% tumor volume growth from days 0 to 8. By contrast, the group treated with the corresponding non-EGF bioconjugates (control) showed 55% tumor volume growth from days 0 to 8. The data suggest that a single dose of PEG-SWCNT–cisplatin–EGF drug conjugate (~1.33 µM of cisplatin), is able to decrease tumor growth significantly. By contrast, in our previous study using the non-PEG-SWCNT–cisplatin–EGF, three injections of the DDS (each with ~1.33 µM of cisplatin) were required to achieve a slightly less effective tumor grown inhibition over 10 days [48].
Figure 8. Inhibition of pre-established HN12 head and neck squamous carcinoma xenografts by PEG-SWCNTs–cisplatin–EGF.
A single injection of the two bioconjugates (PEG-SWCNT–cisplatin with and without EGF 0.5 mg ml−1; approximately 1.33 µM cisplatin concentration) was administered intravenously as shown in the schematic (right) and lesions were measured every 2 days for tumor volume (left). Error bars represent n = 3.
PEG: Polyethylene glycol; SWCNT: Single-walled carbon nanotube.
Discussion
Our previous results demonstrated that non-PEG-SWCNT–cisplatin–EGF can effectively retard cancer progression in xenograft mouse models [48]. Two-photon in-vivo microscopy confirmed that the non-PEG-SWCNT–cisplatin–EGF bioconjugates were tumor specific. While non-PEG-SWCNTs did not show toxicity on cancer cell lines in vitro, these nanotubes administered intravenously to mice were found lodged in the lungs in large aggregates with signs of inflammation.
In this study, our strategy was to conjugate anticancer drugs/growth factors (targeting ligands) onto the surface of the SWCNTs while at the same time making the nanotubes highly aqueous dispersible. However, for covalent conjugation the availability of defect sites on the SWCNT surface is necessary. In order to assess whether defect sites were available on SWCNTs wrapped with PEG, we performed Raman analysis, which essentially gives specific signature peaks (D band, ~1290–1310 cm−1) for CNTs indicating the availability of free defect sites. Raman spectra of the PEGylated SWCNT samples (Figure 3E) gave an indication that open sites for EGF/drug conjugation are available on the SWCNT surface, and that exposed EGF conjugates will recognize its cognate receptor leading to activation and followed by internalization, essentially as described in our previous study [48]. In support, PEG-derived SWCNTs have also been used by other groups for biomedical applications with various biomolecules on the surface of the nanotubes. For example, PEGylated SWCNT tumor targeting using RGD was demonstrated by Liu et al. while covalently linked PEG to SWCNTs have been shown to act as stealth delivery systems in vivo, as reported by Yang et al. and others [61–63].
Results presented in this article show that a coating of PEG on SWCNTs increases dispersibility and decreases adverse reactions (Figure 1). In addition, in our previous studies we have determined relative levels and distribution of conjugated EGF and cisplatin on carboxylated SWCNTs. Here, we estimated, by using differences in absorption spectroscopy and STEM, that there were approximately 36 ± 10 EGF molecules per 100 nm length of SWCNTs, and 1.33 µM of cisplatin per dispersion [48,56]. In our current study, we followed the same model for conjugation, but prior to this, the PEGylated SWCNTs underwent characterization. Raman analysis of the PEGylated SWCNTs showed defect (due to oxidation by acid treatment) D-band peaks on the surface of the nanotubes (Figure 3C–E). This data likely suggests that the PEG wrapping around the SWCNTs is not 100% and contains free conjugation sites. In support, a recent study using thermogravimetric analysis demonstrated that covalent wrapping of PEG around SWCNT surface is approximately 18% of the weight portion [64]. Furthermore, estimating the number of PEG molecules per SWCNT would be problematic, primarily due to the fact that SWCNTs as observed from the STEM images (Figure 3A & 3B), also exists in bundle form. Therefore, in order to obtain and better understand individually separated PEG-SWCNT dispersions, optimization studies of PEGylation around SWCNT surface are currently in progress.
PEGylation, from an empirical standpoint, does not affect the binding capabilities of the SWNCT for cisplatin or the potency of the DDS (Figure 8). Similar cisplatin concentration levels and numbers of molecules per 100 nm of nanotube were achieved with or without PEG attached to the nanotubes. Results in the mouse model suggest that covalent PEGylation may increase circulation time of SWCNTs in vivo (Figure 7) while limiting the tendency of nanotubes to aggregate. Unlike non-PEG-SWCNTs, PEG-SWCNTs did not induce inflammatory responses in the lungs or elsewhere (Figure 5). It must be noted that these were short-term studies with a small set of animals, so that chronic nanotube-induced toxicity or other adverse responses may not have been detected. We also confirmed that both configurations of nanotubes have no adverse cytotoxic effect in vitro (Figure 4). Tumor growth inhibition in mice was at least as effective with PEG-SWCNT–cisplatin–EGF (Figure 8) as for non-PEG-SWCNT–cisplatin–EGF [48]. These results validate PEG as a feasible coating to lower toxicity while concurrently maintaining tumor-inhibiting functionality. It is noteworthy that these essentially formed proof-of-concept studies for our DDS, involving SWCNT–drug–EGF conjugates, and consequently we chose to carry out short duration studies, primarily to optimize the efficient delivery of the conjugates given by intravenous injections. In our initial study, we used a treatment window of three injections over a period of 4 days, whereby a single injection was administered every 2 days (days 0, 2 and 4) [48], and was stopped due to a response being noted after the last injection (day 4). However, this study was terminated due to limitation of the size of the tumor xenografts permissible. Consequently, this model was used for repeat experiments as those described in this current study, with the exception that only a single injection was administered (day 0), thus allowing for the basis of making a side-by-side comparison of the two types of conjugates in vivo. Nonetheless, in our previous and current study, our working hypothesis was based on the premise that once the ligand (on the conjugates) binds to the overexpressing receptors on the target cells, the whole complex is then internalized and the cisplatin released. In this context, low and safe levels of cisplatin (~1.33 µM) would accumulate and concentrate only in cancer cells, and consequently trigger a cytotoxic desired effect, as seen in our in vitro (apoptosis) and in vivo (tumor inhibition) experiments. In this regard, as our in vitro studies had demonstrated that 1.33 µM of cisplatin alone had a minimal cytotoxic effect on the target cells, this essentially guided our in vivo experiments to use conjugates with cisplatin that were with and without EGF, for a two-arm comparison. A caveat though, is that serum levels of cisplatin in human cancer patients receiving this agent as standard-of-care is in the region of 10 µM, and this is often associated with severe toxicity, while our SWCNT–drug–EGF conjugates could potentially avoid these side effects. As a follow-up, we propose long-term studies to better assess the biological response of tumors in mice given the respective conjugates, including the fate of cisplatin in the target cells.
Raman analysis of solid excreted material from mice treated with PEG-SWCNTs showed a slow but persistent decrease in the SWCNT Raman G band (Figure 7), suggesting that clearance of SWCNTs was occurring in the mice. Furthermore, non-PEG-SWCNTs either got excreted rapidly (1–2 days), or became lodged in various vital organs of the mice. The SWCNT Raman analysis of fecal material gives an indication of a biliary excretion pathway for the CNTs, while the observation of SWCNT Raman bands in the kidney also suggests that CNTs are likely excreted via the renal pathway.
Conclusion & future perspective
Distribution and clearance data for PEG-SWCNTs in mice demonstrate the effectiveness and lower toxicity of highly aqueous dispersible PEG-SWCNTs for drug delivery. Raman analysis of vital organs and fecal material suggest a biliary/renal pathway of excretion. In addition to facilitating slower excretion of SWCNTs in vivo, PEG does not interfere with drug delivery properties. On the contrary, PEG appears to enhance DDS performance. These observations lay the groundwork for further toxicology and drug delivery studies for PEG-SWCNT systems. Assessing the pharmacokinetics of CNTs remains an unmet challenge that mandates long-term studies and sufficient controls. However, results in this article give further credence to the probability of future, more effective DDS made with SWCNTs.
Executive summary.
-
•
Polyethylene glycol (PEG)-ylation of single-walled carbon nanotubes (SWCNTs) renders them more hydrophilic and makes them more aqueous dispersible compared with simple oxidized SWCNTs.
-
•
Raman analysis points towards either biliary or renal clearance pathways.
-
•
PEG-SWCNTs can be loaded with anticancer drugs along with an appropriate ligand to form a targeted therapeutic combination, which successfully inhibits tumor progression in mice.
Acknowledgments
This research was supported by the Intramural Programs of the National Institute of Dental and Craniofacial Research and the National Institute of Biomedical Imaging and Bioengineering, NIH, and in part by PHS grant ES013557 from NIEHS/NIH to JFR, University of Connecticut.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Hirsch A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002;41:1853–1859. doi: 10.1002/1521-3773(20020603)41:11<1853::aid-anie1853>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2. Bianco A, Kostarelos K, Prato M. Opportunities and challenges of carbon-based nanomaterials for cancer therapy. Expert Opin. Drug Deliv. 2008;5:331–342. doi: 10.1517/17425247.5.3.331. •• Describes the biomedical applications of a variety of carbon nanomaterials studied, especially nanotubes. Discusses the potential benefits and risks of carbon nanomaterials towards clinical application.
- 3.Dillon AC, Yudasaka M, Dresselhaus MS. Employing Raman spectroscopy to qualitatively evaluate the purity of carbon single-wall nanotube materials. J. Nanosci. Nanotechnol. 2004;4:691–703. doi: 10.1166/jnn.2004.116. [DOI] [PubMed] [Google Scholar]
- 4.Kam S, Dai H. Carbon nanotubes as intracellular protein transporters. generality and biological functionality. J. Am. Chem. Soc. 2005;127:6021–6026. doi: 10.1021/ja050062v. [DOI] [PubMed] [Google Scholar]
- 5.Yang R, Yang X, Zhang Z, et al. Single-walled carbon nanotubes-mediated in vivo and in vitro delivery of siRNA into antigen-presenting cells. Gene Therapy. 2006;13:1714–1723. doi: 10.1038/sj.gt.3302808. [DOI] [PubMed] [Google Scholar]
- 6.Singh R, Pantarotto P, McCarthy D, et al. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J. Am. Chem. Soc. 2005;127(12):4388–4396. doi: 10.1021/ja0441561. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Winters M, Holodniy M, Dai H. siRNA delivery into human T cells and primary cells with carbon-nanotube transporters. Angew. Chem. Int. Ed. 2007;46:2023–2027. doi: 10.1002/anie.200604295. [DOI] [PubMed] [Google Scholar]
- 8.Kam NWS, Liu Z, Dai HJ. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. Am. Chem. Soc. 2005;127:6021–6026. doi: 10.1021/ja053962k. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Chen K, Davis C, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat. Nanotechnol. 2009;4:627–633. doi: 10.1038/nnano.2009.241. •• Provides the most recent advances in carbon nanotube-based imaging and therapeutics. Highlights the current trends and what needs to be addressed next towards carbon nanotubes as delivery vectors.
- 11.Kim DK, Dobson J. Nanomedicine for targeted drug delivery. J. Mater. Chem. 2009;19:6294–6307. [Google Scholar]
- 12.Wang H, Chen XY. Applications for site-directed molecular imaging agents coupled with drug delivery potential. Expert Opin. Drug Deliv. 2009;6:745–768. doi: 10.1517/17425240902889751. [DOI] [PubMed] [Google Scholar]
- 13.Pietronave S, Iafisco M, Locarno D, Rimondini L, Prat M. Functionalized nanomaterials for diagnosis and therapy of cancer. J. Appl. Biomater. Biomech. 2009;7:77–89. [PubMed] [Google Scholar]
- 14.Lison D, Muller J. Lung and systemic responses to carbon nanotubes (CNT) in mice. Toxicol. Sci. 2008;101:179–180. doi: 10.1093/toxsci/kfm249. [DOI] [PubMed] [Google Scholar]
- 15.Quintana M, Prato M. Supramolecular aggregation of functionalized carbon nanotubes. Chem. Commun. 2009;40:6005–6007. doi: 10.1039/b915126e. [DOI] [PubMed] [Google Scholar]
- 16.Kostarelos K. The long and short of carbon nanotube toxicity. Nat. Biotechnol. 2008;26:774–776. doi: 10.1038/nbt0708-774. [DOI] [PubMed] [Google Scholar]
- 17.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 18.Carrero-Sanchez JC, Elías AL, Mancilla R, et al. Biocompatibility and toxicological studies of carbon nanotubes doped with nitrogen. Nano Lett. 2006;6:1609. doi: 10.1021/nl060548p. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson K, Poland C. Nanotoxicology: new insights into nanotubes. Nat. Nanotechnol. 2009;4:708–710. doi: 10.1038/nnano.2009.327. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat. Nanotechnol. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poland C, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 22.Schipper ML, Nakayama-Ratchford N, Davis CR. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 23. Simeonova P. Update on carbon nanotube toxicity. Nanomedicine. 2009;4:373–375. doi: 10.2217/nnm.09.25. • This short update highlights the main toxicity issues involving carbon nanotubes.
- 24.Veronese F, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov. Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 25.Jiang W, Mardyani S, Fischer H, Chan WCW. Design and characterization of lysine cross-linked mercapto-acid biocompatible quantum dots. Chem. Mater. 2006;4:872–878. [Google Scholar]
- 26.Mulder WJM, Koole R, Brandwijk RJ, et al. Surfactant-assisted synthesis of water-soluble and biocompatible semiconductor quantum dot micelles. Nano Lett. 2006;6:1–6. doi: 10.1021/nl050017l. [DOI] [PubMed] [Google Scholar]
- 27.Adams M, Lavasanifar A, Kwon G. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003;92:1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- 28.Kwon GS, Suwa S, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Enhanced tumor accumulation and prolonged circulation times of micelle-forming poly(ethylene oxide-aspartate) block copolymer–adriamycin conjugates. J. Control. Release. 1994;29:17–23. [Google Scholar]
- 29.Li Y, Kwon GS. Methotrexate esters of poly(ethylene oxide)-block-poly(2-hydroxyethyl-l-aspartamide). I. Effects of the level of methotrexate conjugation on the stability of micelles and on drug release. Pharm. Res. 2000;17:607–611. doi: 10.1023/a:1007529218802. [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama N, Kataoka K. Preparation and characterization of size-controlled polymeric micelle containing cis-dichlorodiammineplatinum (II) in the core. J. Control. Release. 2001;74:83–94. doi: 10.1016/s0168-3659(01)00314-5. [DOI] [PubMed] [Google Scholar]
- 31.Burt HM, Zhang X, Toleikis P, Embree L, Hunter WL. Development of copolymers of poly(d,l-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf. B Biointerfaces. 1999;16:161–171. [Google Scholar]
- 32.Nish A, Nicholas J. Temperature induced restoration of fluorescence from oxidised single-walled carbon nanotubes in aqueous sodium dodecylsulfate solution. Phys. Chem. Chem. Phys. 2006;8:3547–3551. doi: 10.1039/b604045d. [DOI] [PubMed] [Google Scholar]
- 33.Matarredona O, Rhoads H, Zhongrui L, Harwell J, Balzano L, Resasco D. Dispersion of single-walled carbon nanotubes in aqueous solutions of the anionic surfactant NaDDBS. J. Phys. Chem. B. 2003;107:13357–13367. [Google Scholar]
- 34.Zheng M, Jagota A, Semke E, et al. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003;2:338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 35.Ju S, Doll J, Sharma I, Papadimitrakopoulos F. Selection of carbon nanotubes with specific chiralities using helical assemblies of flavin mononucleotide. Nat. Nanotechnol. 2008;3:356–362. doi: 10.1038/nnano.2008.148. [DOI] [PubMed] [Google Scholar]
- 36.Yang M, Koutsos V, Zaiser M. Interactions between polymers and carbon nanotubes: a molecular dynamics study. J. Phys. Chem. B. 2005;109:10009–10014. doi: 10.1021/jp0442403. [DOI] [PubMed] [Google Scholar]
- 37.Nish A, Hwang J, Doig J, Nicholas R. Highly selective dispersion of single-walled carbon nanotubes using aromatic polymers. Nat. Nanotechnol. 2007;2:640–646. doi: 10.1038/nnano.2007.290. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Sun X, Ratchford N, Dai H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 2007;1:50–56. doi: 10.1021/nn700040t. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Wu D, Zhang W, et al. Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew. Chem. Int. Ed. 2005;44:4782–4785. doi: 10.1002/anie.200500042. [DOI] [PubMed] [Google Scholar]
- 40.Owens D, Peppas N. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J.Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Yang S, Fernando K, Liu J, et al. Covalently PEGylated carbon nanotubes with stealth character in vivo. Small. 2008;4:940–944. doi: 10.1002/smll.200700714. [DOI] [PubMed] [Google Scholar]
- 42.Ryan SM, Mantovani G, Wang X, Haddleton DM, Brayden DJ. Advances in PEGylation of important biotech molecules: delivery aspects. Expert Opin. Drug Deliv. 2008;5:371–383. doi: 10.1517/17425247.5.4.371. [DOI] [PubMed] [Google Scholar]
- 43.Wang A, Gu F, Zhang L, et al. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin. Biol. Ther. 2008;8:1063–1070. doi: 10.1517/14712598.8.8.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl Acad. Sci. USA. 2008;105:1410–1415. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeineldin R, Al-Haik M, Hudson L. Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells. Nano Lett. 2009;9:751–757. doi: 10.1021/nl8033174. [DOI] [PubMed] [Google Scholar]
- 46.Podesta J, Al-Jamal K, Herrero M, et al. Antitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic siRNA silencing in a human lung xenograft model. Small. 2009;5:1176–1185. doi: 10.1002/smll.200801572. [DOI] [PubMed] [Google Scholar]
- 47.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 48. Bhirde A, Patel V, Gavard J, et al. Targeting killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACSNano. 2009;3:307–316. doi: 10.1021/nn800551s. • Describes the site-specific targeting capabilities of carbon nanotubes for chemotherapeutic drug targeting both in vitro and in vivo.
- 49.Gabano E, Ravera M, Cassino C, Bonetti S, Palmisano G, Osella D. Stepwise assembly of platinum–folic acid conjugates. Inorganica Chimica Acta. 2008;361:1447–1455. [Google Scholar]
- 50.Cardinali M, Pietraszkiewicz H, Ensley J, Robbins K. Tyrosine phosphorylation as a marker for aberrantly regulated growth-promoting pathways in cell lines derived from head and neck malignancies. Int. J. Cancer. 1995;61:98–103. doi: 10.1002/ijc.2910610117. [DOI] [PubMed] [Google Scholar]
- 51.Prencipe G, Tabakman S, Welsher K, et al. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J. Am. Chem. Soc. 2009;131:4783–4787. doi: 10.1021/ja809086q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J, Kim J, An K, et al. Electrophoretic and dynamic light scattering in evaluating dispersion and size distribution of single-walled carbon nanotubes. J. Nanosci. Nanotech. 2005;5:1045–1049. doi: 10.1166/jnn.2005.160. [DOI] [PubMed] [Google Scholar]
- 53.Isobe H, Tanaka T, Maeda R, et al. Preparation, purification, characterization, and cytotoxicity assessment of water-soluble, transition-metal-free carbon nanotube aggregates. Angew. Chem. Int. Ed. 2006;45:6676–6680. doi: 10.1002/anie.200601718. [DOI] [PubMed] [Google Scholar]
- 54.Hong S, Tobias G, Ballesteros B, et al. Atomic scale detection of organic molecules coupled to single-wall carbon nanotubes. J. Am. Chem. Soc. 2007;129:10966–10967. doi: 10.1021/ja069080i. [DOI] [PubMed] [Google Scholar]
- 55.Porter A, Gass M, Bendall J, et al. Uptake of noncytotoxic acid-treated single-walled carbon nanotubes into the cytoplasm of human macrophage cells. ACS Nano. 2009;3:1485–1492. doi: 10.1021/nn900416z. [DOI] [PubMed] [Google Scholar]
- 56. Bhirde A, Sousa A, Patel V, et al. Imaging the distribution of individual platinum-based anticancer drug molecules attached to single-wall carbon nanotubes. Nanomedicine. 2009;4:763–772. doi: 10.2217/nnm.09.56. • Describes the distribution of chemotherapeutic drug cisplatin onto single-walled carbon nanotubes using electron microscopy.
- 57.Liu Z, Cai W, He L, et al. In vivo biodistribution and highly efficient tumor targeting of carbon manotubes in mice. Nat. Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 58.Graupner R. Raman spectroscopy of covalently functionalized single-wall carbon nanotubes. J. Raman Spectrosc. 2007;38:673–683. [Google Scholar]
- 59.Schipper M, Ratchford N, Davis C, et al. A Pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 60.Shen Y, Tang H, Radosz M, Van Kirk E, Murdoch WJ. pH-responsive nanoparticles for cancer drug delivery. Methods Mol. Biol. 2008;437:183–216. doi: 10.1007/978-1-59745-210-6_10. [DOI] [PubMed] [Google Scholar]
- 61.Liu Z, Cai W, He L, et al. In vivo biodistribution and highly efficient tumor targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 62.Zeineldin R, Al-Haik M, Hudson L. Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells. Nano Lett. 2009;9:751–757. doi: 10.1021/nl8033174. [DOI] [PubMed] [Google Scholar]
- 63.Yang S, Fernando K, Liu J, et al. Covalently PEGylated carbon nanotubes with stealth character in vivo. Small. 2008;4:940–944. doi: 10.1002/smll.200700714. [DOI] [PubMed] [Google Scholar]
- 64.Jung H, Ko K, Jung T. Aggregation behavior of chemically attached poly(ethylene glycol) to single-walled carbon nanotubes (SWNTs) ropes. Mater. Sci. Eng. C. 2004;24:117–121. [Google Scholar]