The Epstein-Barr virus (EBV)+ T-cell lymphoproliferative disease (T-LPD) of childhood was newly incorporated into the 2008 World Health Organization classification of lymphoid neoplasms. Two types of EBV-associated T-cell malignancies are included in this category: systemic EBV+ T-LPD of childhood and hydroa vacciniforme (HV)-like lymphoma.1 HV-like lymphoma is a peripheral T-cell lymphoma manifested by dermal lesions in mostly sun-exposed areas. Lesions are papulovesicular with ulceration, crusting, and edematous changes that leave residual scars. Patients have a backdrop of chronic active EBV infection that usually affects T lymphocytes.2 Refractory to chemotherapy, HV-like lymphoma is a smoldering yet fatal disease.3 Allogeneic hematopoietic stem cell transplantation (alloSCT) offers the only established curative option.4 Recent developments in EBV-specific cytotoxic T-lymphocyte (CTL) immunotherapeutics may offer an exciting novel treatment approach for this high-risk group of patients. EBV-CTL immunotherapy has demonstrated efficacy in preventing the onset of type III latency EBV+ LPD when given as prophylaxis to >100 alloSCT recipients and has induced sustained complete remissions (CR) in 11 of 13 patients with EBV+ B-cell post-transplant LPD.5
We report on an Ecuadorian male who presented at age 11 with a 3-year history of recurrent papulovesicular rash on sun-exposed skin that would spontaneously heal leaving pitting scars. At the time of presentation, these lesions were persistent, widespread, and associated with systemic symptoms. Skin biopsy revealed spongiosis, necrosis and a deep atypical CD3+/CD8+/EBER+ cytotoxic T-cell infiltrate. PCR analysis demonstrated monoclonal T-cell receptor (TCR)-gamma gene rearrangement in the skin biopsy, peripheral blood, and bone marrow. EBV PCR and serology testing were strongly positive while liver enzyme and lactate dehydrogenase levels were elevated. CT scan was originally normal, however PET scan revealed cervical adenopathy. Bone marrow and cerebrospinal fluid studies did not show overt morphologic evidence of disease. Based on these findings, a diagnosis of systemic EBV+ HV-like T-cell lymphoma was established.
His disease was refractory to multiple chemotherapy regimens. It persisted despite receiving 2 cycles of CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone), followed by 4 cycles of hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), followed by high-dose methotrexate. The skin lesions regressed transiently with each regimen, however they invariably recurred and the EBV PCR and TCR rearrangement studies remained persistently positive. Refractory to chemotherapy, the patient went on to receive reduced-intensity conditioning followed by alloSCT from a 5/6 HLA-matched related donor who was EBV-positive by serology.
Pre-alloSCT evaluation found the patient with active disease – persistent skin lesions with positive EBV PCR and TCR rearrangement studies. The patient underwent conditioning with the reduced-intensity regimen reported by Corradini et al. combining thiotepa, cyclophosphamide, and fludarabine for recurrent/refractory peripheral T-cell lymphomas.6 Immunosuppression post-alloSCT consisted of mycophenolate mofetil (through day +30) and tacrolimus (taper starting day +60). Neutrophil engraftment was achieved on day +14 with full donor chimerism confirmed on day +26. EBV PCR was 1,239 copies/mL of plasma immediately prior to alloSCT, then subsequently undetectable on day +30 and has remained undetectable since (Figure 1).
Figure 1.
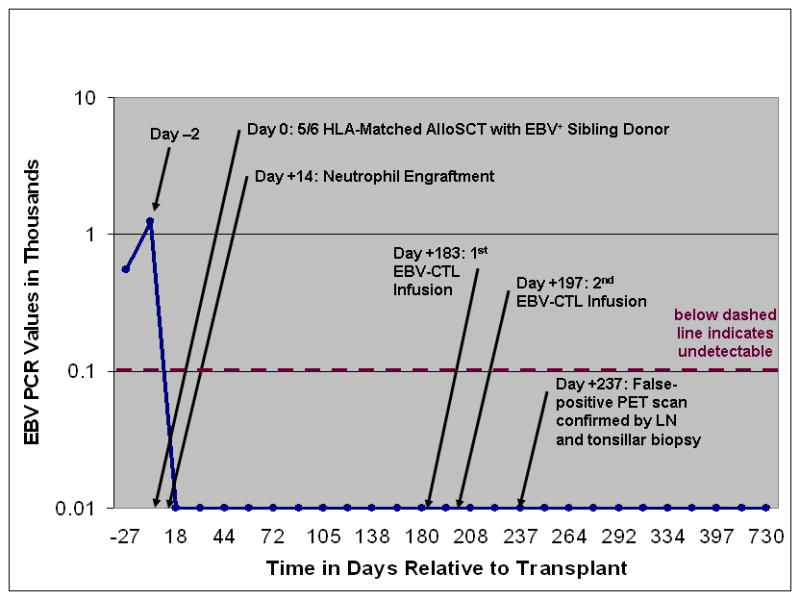
EBV Quantitative RT PCR DNA Copies/mL of Plasma Pre and Post Allogeneic Stem Cell Transplantation
The patient was enrolled on a clinical trial with the Center for Cell and Gene Therapy at Baylor College of Medicine investigating the efficacy of EBV-specific CTL immunotherapy in patients with type II latency EBV+ malignancies. CTLs targeting the iummunosubdominant EBV-associated latent membrane protein-2 (LMP2) antigen were derived from the sibling donor’s peripheral blood mononuclear cells as we have previously described.5 The patient received LMP2-specific CTL infusions on day +183 and day +197 without experiencing greater than grade I toxicities. He did not experience graft-versus-host disease prior to CTL infusions and had a mild eczematous skin rash post-CTL that resolved with topical steroids. He is currently 2 years post-alloSCT and in sustained CR with negative TCR rearrangement studies and an undetectable EBV PCR.
PET/CT was utilized to monitor for disease recurrence post-alloSCT. The patient had unremarkable PET scans prior to alloSCT and at days +60, +93, and +181 showing minimal levels of FDG activity based upon maximum standardized uptake values (SUV). However, FDG-PET after the EBV-CTL infusions was highly abnormal with a steep increase in SUV levels (11 in Waldeyer’s tonsillar ring and 6 in an inguinal lymph node [LN]) on day +237 (Figure 2A). With high suspicion for relapse, inguinal LN biopsy and tonsillectomy were performed. Pathology review of both specimens revealed reactive lymphoid tissue without evidence of lymphoma (Figure 2B). TCR rearrangement studies were polyclonal. These findings suggest that the CTL infusions were associated with lymphoid tissue activation resulting in the false-positive FDG-PET scans. Subsequent scans showed gradual normalization.
Figure 2.
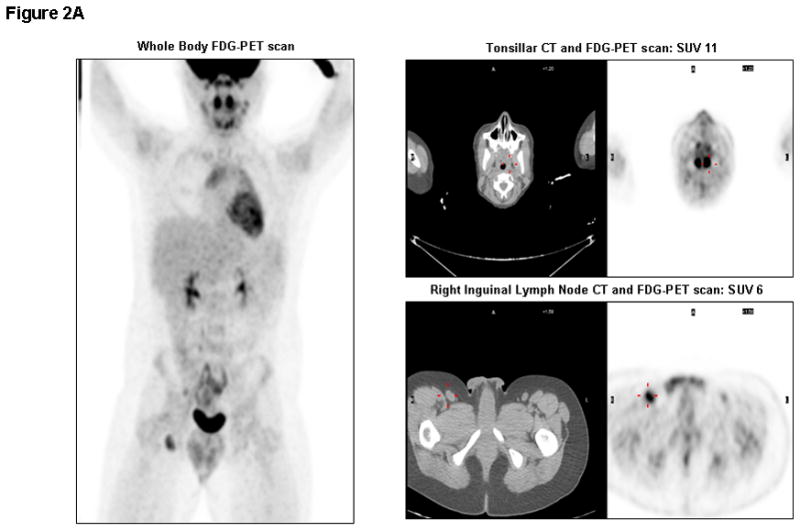
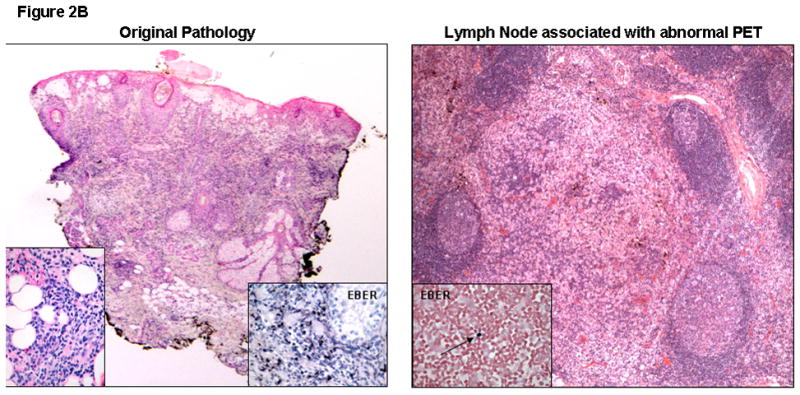
Figure 2A. Whole Body FDG-PET/CT scan on day +237: Significantly elevated metabolic activity in bilateral tonsils (SUV 11) and right inguinal lymph nodes (SUV 6), in addition to cervical (SUV 4.5), mediastinal (SUV 5), and mesenteric (SUV 3.6) lymph nodes. Corresponding CT images of tonsils and inguinal lymph node are shown with abnormal signals marked in red.
Figure 2B. Skin Biopsy at Original Presentation: Histopathological examination of a lesion on the face showed acute spongiosis with prominent vesiculation and necrosis, as well as a dense superficial and deep infiltrate with adnexal involvement and extension into the subcutaneous fat. Left inset demonstrates an infiltrate composed of large, atypical angulated lymphocytes. Right inset reveals diffusely EBV positive lymphocytes on EBERish stain (hematoxylin and eosin, 20X; insets, original magnification 400X).
LN Biopsy post-CTL Infusion (Day+243): Histopathological examination of the inguinal lymph node showed preserved architecture and reactive changes, in the form of nodular paracortical hyperplasia (dermatopathic changes), follicular hyperplasia and sinus histiocytosis. A rare EBV positive small lymphocyte (inset, arrow) was seen (hematoxylin and eosin, 40X; inset, EBERish for EBV, original magnification 400X). EBERish: in situ hybridization for EBV encoded small RNAs.
EBV+ HV-like T-cell lymphoma is a notoriously refractory disease with poor outcomes. This is the first report of sequential matched EBV+ sibling alloSCT followed by consolidation therapy with donor-derived EBV-specific CTL immunotherapy for a patient with an EBV+ T-cell malignancy. The life saving role of EBV+ alloSCT in the setting of this rare chemotherapy resistant disease validates the critical importance in this adaptive immunotherapeutic approach to cure. We demonstrate that combination of reduced-intensity conditioning followed by EBV+ alloSCT with subsequent EBV-CTL infusion is feasible and well tolerated. While it remains unknown whether the patient would have experienced a sustained CR with EBV+ alloSCT alone, the strong evidence for the efficacy of EBV-CTL therapy in patients with type III latency EBV+ B-cell LPD offers compelling rationale for optimizing treatment options with this consolidated approach in type II latency EBV+ T-cell lymphomas. Furthermore, we report on the occurrence of false-positive FDG-PET after infusion of LMP2-CTLs with subsequent self-resolution. PET scans have been reported to appear abnormal secondary to LN activation.7 It is plausible that our patient experienced significant lymphoid activation after infusion of LMP2-CTLs resulting in the abnormal PET images. The encouraging outcomes for this patient with an EBV+ T-cell malignancy, a disease that characteristically carries a dismal prognosis, offers hope that this immunotherapeutic approach may offer improved chances for long-term survival in this group of high-risk patients. The Childhood, Adolescent, Young Adult Lymphoma Cell Therapy Consortium is a new initiative aiming to provide targeted cell therapy for patients with EBV+ lymphoma and evaluate the efficacy of these novel cellular immunotherapies on a larger scale.
References
- 1.Lim MS, De Leval L, Quintanilla-Martinez L. Commentary on the 2008 WHO classification of mature T- and NK-cell neoplasms. J Hematopathol. 2009;2:65–73. doi: 10.1007/s12308-009-0034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol. 2009;20(9):1472–82. doi: 10.1093/annonc/mdp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrionuevo C, Anderson VM, Zevallos-Giampietri E, Zaharia M, Misad O, Bravo F, et al. Hydroa-like cutaneous T-cell lymphoma: a clinicopathologic and molecular genetic study of 16 pediatric cases from Peru. Appl Immunohistochem Mol Morphol. 2002;10(1):7–14. doi: 10.1097/00129039-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sato E, Ohga S, Kuroda H, Yoshiba F, Nishimura M, Nagasawa M, et al. Allogeneic hematopoietic stem cell transplantation for Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disease in Japan. Am J Hematol. 2008;83(9):721–7. doi: 10.1002/ajh.21247. [DOI] [PubMed] [Google Scholar]
- 5.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, Khalil M, Wu MF, Huls MH, Chang CC, Gresik MV, Gee AP, Brenner MK, Rooney CM, Heslop HE. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007 Oct 15;110(8):2838–45. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corradini P, Dodero A, Zallio F, Caracciolo D, Casini M, Bregni M, et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin’s lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol. 2004;22(11):2172–6. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 7.Focosi D, Caracciolo F, Galimberti S, Papineschi F, Petrini M. False positive PET scanning caused by inactivated influenza virus vaccination during complete remission from anaplastic T-cell lymphoma. Ann Hematol. 2008;87(4):343–4. doi: 10.1007/s00277-007-0413-4. [DOI] [PubMed] [Google Scholar]


