Abstract
The efficient synthesis and biological evaluation of both the reported and revised structures of tyroscherin have been achieved. Central to our synthesis is a cross metathesis reaction that generated the trans-olefin regioselectively. This synthetic strategy enabled the facile manipulation of tyroscherin stereochemistry facilitating the generation of all 16 tyroscherin diastereomers and a photoactivatable tyroscherin-based affinity probe for future mode of action studies.
Natural products continue to provide promising leads for drug candidates as well as a powerful arsenal of small molecule probes to dissect complex biological processes.1 The potent cytotoxic natural product tyroscherin (1, Figure 1) was isolated from the culture of a fungus identified as Pseudallescheria sp. by the research groups of Hayakawa and Watanabe in 2004.2 Tyroscherin was originally reported to inhibit insulin-like growth factor-1 (IGF-1)-induced and fetal bovine serum (FBS)-induced growth of MCF-7 human breast cancer cells with IC50 values of 30 nM and 6.2 µM, respectively.2,3
Figure 1.
Retrosynthetic analysis of the reported tyroscherin 1.
The reported highly selective inhibition of IGF1-induced growth of MCF-7 cancer cells and unique structural features of tyroscherin led us to embark on its total synthesis independently of, and in parallel to, the more recently reported studies by the Watanabe group and the Maier group.4,5 Our motivation was to investigate the structure-activity relationship (SAR) of tyroscherin, followed by efforts to understand its mode of action and IGF-specificity.
Herein, we report a concise total synthesis of the originally-proposed tyroscherin structure, whose spectral data did not match that of the reported natural product. Subsequent syntheses of the other 15 stereoisomers of tyroscherin allowed for the correct structural assignment of tyroscherin, consistent with a recent re-assignment by Watanabe and coworkers.4 A full biological evaluation of the correct tyroscherin was performed, revealing discrepancies with the previously observed selectivity for IGF-induced growth. In addition, we report the synthesis of a photo-activatable tyroscherin-based affinity reagent that will allow for the identification of tyroscherin binding protein(s). Similar affinity chromatographic approaches have proven successful in mode of action studies of other biologically active natural products.6
The originally reported structure of tyroscherin (1) was retrosynthetically disconnected at the C6–C7 bond, yielding two olefinic precursors, 5 and 6, which we envisioned could be coupled via a cross metathesis (Figure 1).7 The target would be synthesized through a cross metathesis of two key fragments 5 and 6, which were chosen to provide convenient access to the minimum stereoarray needed to support future structure-activity relationship (SAR) and mode of action studies.
The fragment 5 was obtained via a five-step sequence starting with Boc-d-Tyr(t-Bu)-OH (Scheme 1). N-Methylation, EDCI-mediated coupling of the N-methylated acid with N,O-dimethylhydroxylamine, and the addition of 3-butenylmagnesium bromide to the resulting Weinreb amide gave the ketone 7 in excellent yield. Reduction of the ketone in 7 with L-selectride resulted in a single diastereomer of the desired secondary alcohol 8. Protection of the hydroxyl group as an acetate afforded the desired fragment 5. The configuration of the newly installed hydroxyl group at C3 was determined by NOE experiments on the oxazolidinone obtained after reaction of 8 with KHMDS, and the absolute configuration was confirmed using the modified Mosher’s method.8
Scheme 1.
Synthesis of fragment 5
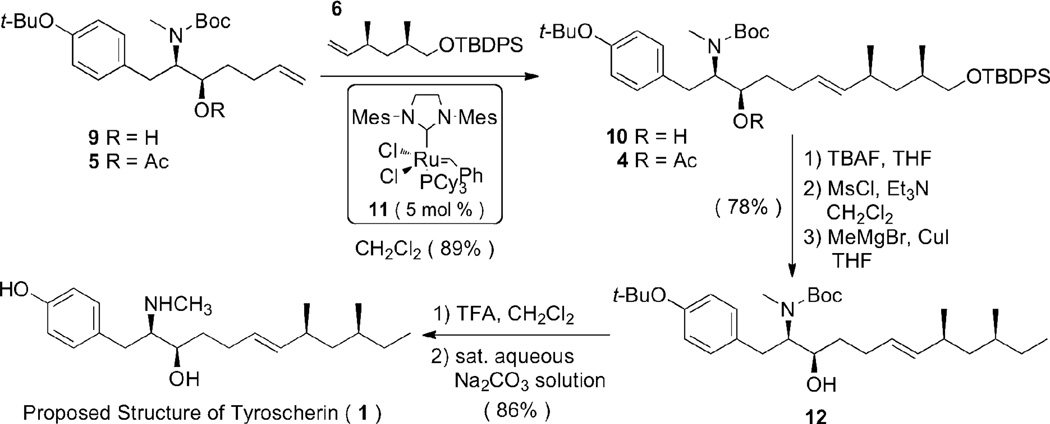
Fragment 6 was prepared with readily available (1S, 2S)-(+)-pseudoephedrine propionamide according to the procedure of Smith and coworkers.9 Initial attempts to obtain the trans-olefin 10 at C6–C7 by cross metathesis between alcohol 9 and olefin 6 resulted in an unsatisfactory (less than 25%) yield (Scheme 2). At the same time, we undertook cross metathesis reactions of 5 with 6 to construct the E-olefin framework of 1. By screening various reaction conditions, it was found that the use of 1.0 equivalent of olefin 5 and 2.0 equivalents of olefin 6 in the presence of 5 mol% of the second generation Grubbs catalyst 11 afforded the best results (Supporting Information).10
Scheme 2.
Synthesis of the Proposed Structure of Tyroscherin 1
Having established suitable conditions for the cross metathesis, we proceeded next towards the completion of the synthesis of 1. Cross metathesis of fragment 5 with fragment 6 gave exclusively the E-isomer of the adduct 4 in 89% yield (Scheme 2). Treatment of TBDPS ether 4 with TBAF afforded the alcohol, and mesylation of this primary alcohol followed by treatment with MeMgBr (2.5 equi.) and CuI (0.4 equi.) resulted in the addition of the terminal methyl group and the simultaneous cleavage of the acetyl group. Finally, global deprotection using TFA afforded the originally-proposed structure of tyroscherin 1. Although similar, the 1H and 13C NMR spectra of synthetic tyroscherin 1 did not match the reported data for the natural product, with notable differences for H2 and H3 (tyroscherin numbering). Further, the optical rotation obtained for 1, (MeOH, c = 0.346), was very similar in magnitude but opposite in sign to the value for the reported structure of the natural product, (MeOH, c = 0.365).11
At this point, we hypothesized that the originally reported structure of tyroscherin 1 was incorrect; indeed, this has been subsequently corroborated in the literature.4 We suspected that the error lay in the assignment of the stereochemistry and therefore turned our focus to the syntheses of diastereomers of 1. To assign the correct structure of tyroscherin and to establish extensive SAR studies, we synthesized all possible diastereomers of 1 using the same synthetic strategy we employed to generate tyroscherin 1.12 This was achieved with eight stereochemically different fragments. Tyroscherin 2 was synthesized by the same methods as tyroscherin 1 (Scheme 3). Tyroscherin 2 matched in all spectral and physical data to the published natural product.2,4
Scheme 3.
Synthesis of the Revised Structure of Tyroscherin 2
Interestingly, while we confirmed the recently revised structure of tyroscherin 2, we were unable to corroborate its reported IGF-selective inhibitory activity. As shown in Figure 2 (upper panel), we found that tyroscherin 2 inhibits IGF- and FBS-induced cell proliferation with nearly equivalent IC50 values (2.23 µM vs. 7.13 µM) as opposed to the reported 1800-fold selectivity for IGF-induced growth over FBS-stimulated proliferation.3
Figure 2.
Activity of Tyroscherin 2 on MCF-7 cells.
Results obtained using [3H]-thymidine incorporation assay (upper panel) and using colorimetric MTS conversion assay (lower panel) are shown. Similar IC50 values were obtained with IGF and Fetal Bovine Serum (2.23 µM vs. 7.13 µM, respectively) (upper panel). Tyroscherin 2 displayed equivalent potency to inhibit cell growth driven by IGF, basic FGF, EGF, or Fetal Bovine Serum (lower panel).
While our results were obtained using the more rigorous and sensitive method of [3H]-thymidine incorporation to specifically measure MCF-7 cell passage through S phase to quantify cell proliferation, we obtained similar results redundant using a tetrazolium salt-based assay (MTS assay) (Figure 2, lower panel). In addition, to eliminate the possibility of differences between the different MCF-7 cell lines, we tested tyroscherin 2 in our MTS assay using the same MCF-7 cells that were used previously (kindly provided by Dr. Shin-Ichiro Takahashi) under identical mitogen and culture conditions as reported.4
Nevertheless, we failed to observe any selective growth inhibition when cells were incubated with IGF vs. FBS. Finally, given the heterogeneous and relatively undefined nature of serum as a mitogen, we chose to test tyroscherin 2 further against other purified growth factors, namely bFGF and EGF. However, tyroscherin 2 exhibited the same potency to inhibit bFGF- and EGF-driven proliferation (2.77 µM and 3.02 µM, respectively) as it did against IGF-driven proliferation. Having identified a correct structure of tyroscherin 2, the next aim was to design a chemical probe for identification of its binding protein(s). In general, three structural components are important in the successful design of affinity probes: the potential ligand structure, an appropriate linker, and a tag suitable for detection. On the basis of our preliminary SAR analysis (data not shown) and optimization of linker length, we chose to synthesize a biotinylated tyroscherin 3 using the same synthetic strategy we employed to generate reported tyroscherin 1 and revised tyroscherin 2.
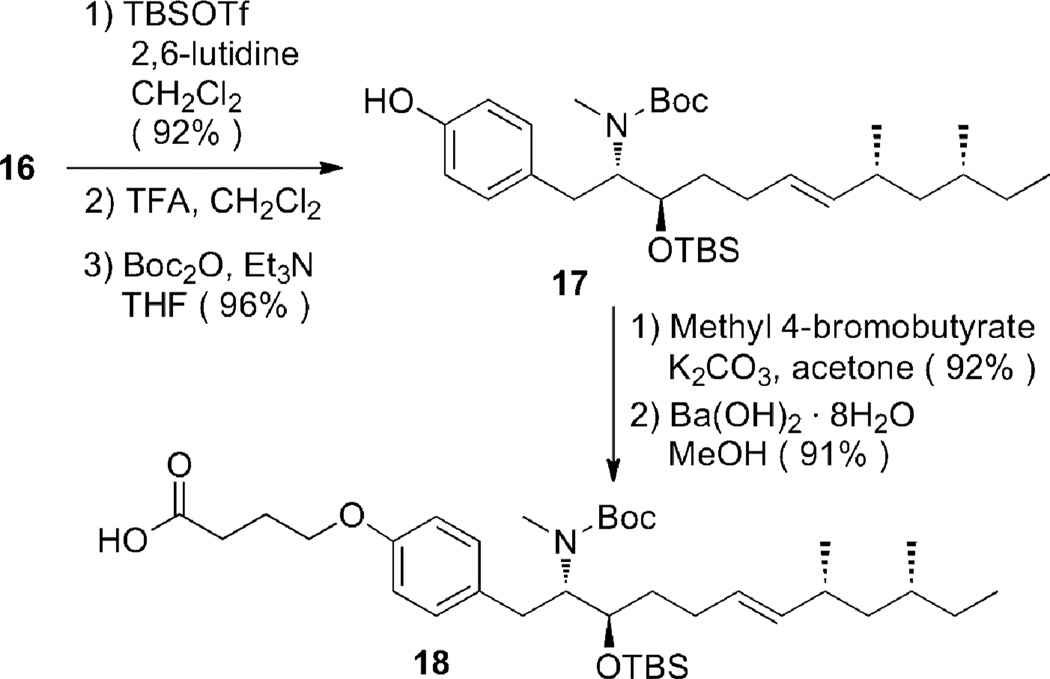
The synthesis of acid 18 proceeded smoothly (Scheme 4). Sequential protection of secondary alcohol, simultaneous removal of Boc and t-Bu groups, and selective protection of the amine gave phenol 17. Alkylation of 17 with methyl 4-bromobutyrate and hydrolysis of the ester provided acid 18.
Scheme 4.
Synthesis of Acid 18
The benzophenone-biotin 21 was obtained via cycloaddition of benzophenone-alkyne 19 (prepared by alkylation of 4, 4’-dihydroxy benzophenone with propargyl bromide followed by Mitsunobu reaction of monoalkylated phenol with Boc-protected aminoethoxy ethanol) and biotinylated azide 20 (prepared by coupling of 11-azido-3,6,9-trioxaundecan-1-amine and biotin-ONp) (Scheme 5).13 The convergent step of the synthesis of biotinylated tyroscherin 3 is depicted in Scheme 6. The removal of Boc group in 21 using TFA provided the amine as a TFA salt, which was coupled to acid 18, using HATU and DIEA in DMF. Finally, subsequent TBS deprotection of the silyl ether, followed by the removal of Boc group by TFA in CH2Cl2, afforded biotinylated tyroscherin 3.13
Scheme 5.
Synthesis of Benzophenone-Biotin 2
Scheme 6.
Synthesis of Biotinylated Tyroscherin 3
In summary, we have accomplished a concise total synthesis of the proposed and revised structure of tyroscherin in 12 steps from commercially available materials, and we have synthesized a revised structure-based molecular probe for finding tyroscherin-binding proteins, using an efficient cross metathesis strategy to construct the trans-olefin. This convergent synthesis should be easily applicable to the construction of various analogs for extensive SAR studies. The main advantages of our synthesis are its brevity and its efficiency, as well as its flexibility: a unified strategy was used to access all desired diastereomers. Through our total synthesis of all tyroscherin diastereomers, we have provided additional evidence that the originally reported structure is incorrect, which has been recently acknowledged by the original team that reported the natural product structure. Our results confirm that tyroscherin 2 (2S,3R,8R,10R) matches the reported natural product spectral data. However, our findings contradict the reported IGF-specificity of growth inhibition previously reported for tyroscherin. Studies to identify tyroscherin’s in vivo biological targets and elucidate its mode of action are underway.
Supplementary Material
Acknowledgements
This work was supported by a Korea Research Foundation Grant (KRF-2005-214-C00218) (H.S.T.), the National Science Foundation (A.R.S.) and the National Institutes of Health (GM062120) (C.M.C.). The authors gratefully acknowledge Shin-Ichiro Takashashi (University of Tokyo) for providing MCF-7 cells, and David Spiegel (Yale University) and members of the Crews lab for helpful discussions throughout the preparation of this manuscript.
Footnotes
Supporting Information Available: Representative experimental procedures, spectral data, and analytical data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews on the natural products, see: Clardy J, Walsh C. Nature. 2004;432:829–837. doi: 10.1038/nature03194. Paterson I, Anderson EA. Science. 2005;310:451–453. doi: 10.1126/science.1116364. Ganesan A. Curr. Opin. Chem. Biol. 2008;12:306–317. doi: 10.1016/j.cbpa.2008.03.016. Li JW-H, Vederas JC. Science. 2009;325:161–165. doi: 10.1126/science.1168243. Harvey AL. Drug Disc. Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. Koehn FE, Carter GT. Nature Rev. Drug Disc. 2005;4:206–220. doi: 10.1038/nrd1657. Wilson RM, Danishefsky SJ. J. Org. Chem. 2006;71:8329–8351. doi: 10.1021/jo0610053. Driggers EM, Hale SP, Lee J, Terrett NK. Nature Rev. Drug Disc. 2008;7:608–624. doi: 10.1038/nrd2590. Tietze LF, Bell HP, Chandrasekhar S. Angew. Chem. Int. Ed. 2003;42:3996–4028. doi: 10.1002/anie.200200553. Schreiber SL. Nature Chem. Biol. 2005;1:64–66. doi: 10.1038/nchembio0705-64. Morton D, Leach S, Cordier C, Warriner S, Nelson A. Angew. Chem. Int. Ed. 2009;48:104–109. doi: 10.1002/anie.200804486. Nicolaou KC, Chen JS, Edmonds DJ, Estrade AA. Angew. Chem. Int. Ed. 2009;48:660–719. doi: 10.1002/anie.200801695.
- 2.Hayakawa Y, Yamashita T, Mori T, Nagai K, Shin-Ya K, Watanabe H. J. Antibiot. 2004;57:634–638. doi: 10.7164/antibiotics.57.634. [DOI] [PubMed] [Google Scholar]
- 3.Yamanouchi Pharmaceutical Co., Ltd., Jpn. JP. 2005-272357. [Google Scholar]
- 4.(a) Katsuta R, Shibata C, Ishigami K, Watanabe H, Kitahara T. Tetrahedron Lett. 2008;49:7042–7045. [Google Scholar]; (b) Ishigami K, Katsuta R, Shibata C, Hayakawa Y, Watanabe H, Kitahara T. Tetrahedron. 2009;65:3629–3638. [Google Scholar]
- 5.Ugele M, Maier ME. Tetrahedron. 2010;66:2653–2641. [Google Scholar]
- 6.(a) Chen L, Yang S, Zhang JJ, Huang X-Y. Nature. 2010;464:1062–1066. doi: 10.1038/nature08978. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ulanovskaya OA, Janjic J, Suzuki M, Sabharwal SS, Schumacker PT, Kron SJ, Kozmin SA. Nature Chem. Biol. 2008;4:418–421. doi: 10.1038/nchembio.94. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kotake Y, Sagane K, Owa T, Mimori-Kiyosue Y, Shimizu H, Uesugi M, Ishihama Y, Iwata M, Mizui Y. Nature Chem. Biol. 2007;3:570–575. doi: 10.1038/nchembio.2007.16. [DOI] [PubMed] [Google Scholar]; (d) Wulff JE, Siegrist R, Myers AG. J. Am. Chem. Soc. 2007;129:14444–14451. doi: 10.1021/ja075327f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sato S-i, Murata A, Shirakawa T, Uesug M. Chem & Biol. 2010;17:616–623. doi: 10.1016/j.chembiol.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 7.(a) Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]; (b) Connon SJ, Blechert S. Angew. Chem. Int. Ed. 2003;42:1900–1923. doi: 10.1002/anie.200200556. [DOI] [PubMed] [Google Scholar]; (c) Harrison BA, Gierasch TM, Neilan C, Pasternak GW, Verdine GL. J. Am. Chem. Soc. 2002;124:13352–13353. doi: 10.1021/ja027150p. [DOI] [PubMed] [Google Scholar]
- 8.see Supporting Information.
- 9. Smith AB, III, Basu K, Bosanac T. J. Am. Chem. Soc. 2007;129:14872–14874. doi: 10.1021/ja077569l. Smith AB, III, Basu K, Bosanac T. J. Am. Chem. Soc. 2009;131:2348–2358. doi: 10.1021/ja8084669. (c) For more details on the synthesis results of 6, see Supporting Information.
- 10.This protecting group on the C3 alcohol had an important influence on the selectivity and yield of the cross metathesis reaction. The best E:Z ratio was obtained with the C3 acetate compared to other esters or the free hydroxyl.
- 11.For 1 as free amine, (MeOH, c = 0.346), whereas 1 as HCl salt, (MeOH, c = 0.325) and 1 as TFA salt, (MeOH, c = 0.330). The large discrepancy in optical rotation values is perhaps due to the equivalent of amine salt in C2.
- 12.The syntheses of 8 key fragments and 16 stereoisomers and the biological results will be reported elsewhere.
- 13.For more details on the syntheses of 19, 20, 21 and 3, see Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.