ABSTRACT
Competence for genetic transformation in Streptococcus pneumoniae develops in response to accumulation of a secreted peptide pheromone and was one of the initial examples of bacterial quorum sensing. Activation of this signaling system induces not only expression of the proteins required for transformation but also the production of cellular chaperones and proteases. We have shown here that activity of this pathway is sensitively responsive to changes in the accuracy of protein synthesis that are triggered by either mutations in ribosomal proteins or exposure to antibiotics. Increasing the error rate during ribosomal decoding promoted competence, while reducing the error rate below the baseline level repressed the development of both spontaneous and antibiotic-induced competence. This pattern of regulation was promoted by the bacterial HtrA serine protease. Analysis of strains with the htrA (S234A) catalytic site mutation showed that the proteolytic activity of HtrA selectively repressed competence when translational fidelity was high but not when accuracy was low. These findings redefine the pneumococcal competence pathway as a response to errors during protein synthesis. This response has the capacity to address the immediate challenge of misfolded proteins through production of chaperones and proteases and may also be able to address, through genetic exchange, upstream coding errors that cause intrinsic protein folding defects. The competence pathway may thereby represent a strategy for dealing with lesions that impair proper protein coding and for maintaining the coding integrity of the genome.
IMPORTANCE
The signaling pathway that governs competence in the human respiratory tract pathogen Streptococcus pneumoniae regulates both genetic transformation and the production of cellular chaperones and proteases. The current study shows that this pathway is sensitively controlled in response to changes in the accuracy of protein synthesis. Increasing the error rate during ribosomal decoding induced competence, while decreasing the error rate repressed competence. This pattern of regulation was promoted by the HtrA protease, which selectively repressed competence when translational fidelity was high but not when accuracy was low. Our findings demonstrate that this organism is able to monitor the accuracy of information used for protein biosynthesis and suggest that errors trigger a response addressing both the immediate challenge of misfolded proteins and, through genetic exchange, upstream coding errors that may underlie protein folding defects. This pathway may represent an evolutionary strategy for maintaining the coding integrity of the genome.
Introduction
Quality control processes during protein biosynthesis ensure the production of functional cellular proteins and prevent the accumulation of toxic aggregates (1) of misfolded proteins. Starting with DNA replication and continuing through ribosomal synthesis of nascent polypeptides, proofreading mechanisms monitor the accurate transmission of information required for protein production (2–4), while cellular chaperones and proteases facilitate proper protein folding and degradation of misfolded proteins (5–9). To deal with conditions of elevated protein folding stress, coordinated responses, including the unfolded protein response (5), heat shock response (8, 10), and extracytoplasmic stress response (9), are employed by both bacteria and eukaryotes to promote production of chaperones and proteases. Although these responses address the downstream folding aspects of protein biosynthesis, they do not address the quality of the upstream information that drives protein production.
The competence response of Streptococcus pneumoniae is intriguing in this regard because it combines chaperone and protease production with genetic recombination. Although characterized initially as regulating transformation, the S. pneumoniae competence signaling pathway controls a broader phenotype, recently also designated the pneumococcal X-state (11), that includes induction of such stress response proteases and chaperones as those encoded by clpL, htrA, grpE, dnaK, dnaJ, groEL, and groES (12). Competence in S. pneumoniae is induced in response to detection by a two-component signaling system of a peptide pheromone (CSP) secreted by the bacterium. The resulting sharp peak of competence expression during early or mid-exponential-phase growth has often been considered an example of quorum sensing, or a prokaryotic display of a density-dependent multicellular behavior. This view recently has been questioned (11, 13) based on observations showing that the development of competence is modulated by other factors beyond density, including activation by the antibiotics streptomycin and kanamycin (14). Although the mechanism of this antibiotic effect was uncertain, these agents increase the rate of decoding errors during translation (15). We therefore tested whether the effect of these agents on competence was due specifically to the induction of ribosomal errors. Our observations support a role for competence as a multifaceted response to errors during protein biosynthesis.
RESULTS
Modulation of ribosomal decoding errors and competence by antibiotics.
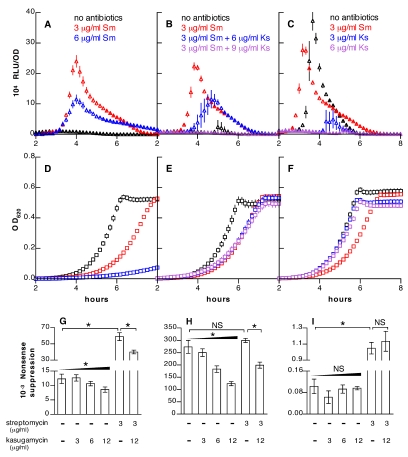
Competence was monitored using an ssbB′-luc transcriptional fusion, activation of which correlates with transformability of pneumococcal cultures (14). Development of spontaneous competence in untreated, wild-type cultures is sensitively dependent on pH, and use of permissive conditions (pH near 7.30; Fig. 1C) or nonpermissive conditions (pH near 7.20; Fig. 1A and B) facilitates assays for either enhancement or repression of competence. Streptomycin induced competence at concentrations of 3 or 6 µg/ml (Fig. 1A), while a similar effect was seen with 6 or 12 µg/ml of kanamycin (Fig. 2A).
FIG 1 .
Modulation of competence and nonsense suppression by streptomycin and kasugamycin. (A) Stimulation of competence with streptomycin (Sm). (B) Repression of streptomycin-induced competence with kasugamycin (Ks). (C) Repression of spontaneous competence with kasugamycin. Luciferase activity (expressed as relative light units/optical density [RLU/OD]) of strain R895 (ssbB′-luc) grown under conditions nonpermissive (A and B) or permissive (C) for development of competence in untreated samples is shown. Growth curves (OD at 620 nm) for the samples in panels A to C are shown in panels D to F, respectively. Symbols for luminescence and growth represent means ± SEM for 6 to 18 replicate cultures here and in subsequent figures. (G to I) Effects of streptomycin and kasugamycin on pneumococcal decoding accuracy as measured by suppression of TAA (G), TGA (H), and TAG (I) stop codons in a lacZ reporter. Bars represent means ± SD for 3 to 6 replicate measurements here and in subsequent figures. *, P < 0.001 by ANOVA with posttests for trends with increasing antibiotic doses (wedges) or Bonferroni’s correction for comparison of individual values (lines). “NS” indicates P > 0.05.
FIG 2 .
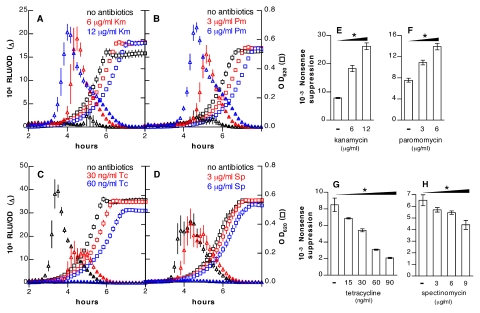
Effects of antibiotics on competence correlate with effects on translational errors. (A to D) Luciferase activity (triangles) and OD620 (squares) of strain R895 (ssbB′-luc) in samples treated with (A) kanamycin (Km), (B) paromomycin (Pm), (C) tetracycline (Tc), or (D) spectinomycin (Sp). The culture medium was nonpermissive for competence in untreated samples in panels A and B but permissive in panels C and D. (E to H) Effects of antibiotics on suppression of the TAA stop codon in a lacZ reporter. *, P < 0.001 by ANOVA with posttests for trends with increasing antibiotic doses (wedges).
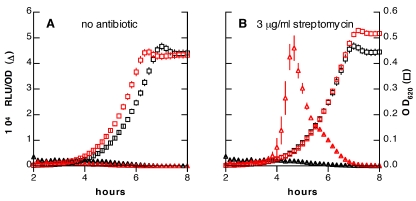
Prudhomme et al. previously reported that a comA deletion mutant failed to induce competence in response to streptomycin (14). This observation suggested that antibiotic induction of competence resembled the spontaneous pneumococcal competence displayed under permissive conditions in that it depended on secretion of CSP by the ComAB transporter system. Consistent with a role for CSP in streptomycin-induced competence, we found that stimulation of a sensitive strain by streptomycin could indirectly activate expression of the ssbB′-luc reporter in a streptomycin-resistant strain when grown in mixed culture (Fig. 3). Together these observations suggest that induction of competence by streptomycin proceeds through the peptide pheromone system rather than bypassing this pathway.
FIG 3 .
Activation of competence in mixed culture. Luciferase activity (triangles) and OD620 (squares) of strain KSP86 (streptomycin-resistant, ssbB′-luc) grown alone (black symbols) or cocultured (red symbols) in a 1:1 ratio with strain R800 (streptomycin-sensitive, without ssbB′-luc). Cultures were grown without antibiotics (A) or with 3 µg/ml of streptomycin (B) in medium nonpermissive for development of competence in untreated samples.
To measure the rate of translational errors in S. pneumoniae, we utilized a nonsense suppression assay in which a premature stop codon interrupts a constitutive lacZ reporter. Streptomycin induced translational misreading of 2 stop codons (TAA and TAG) at the concentrations required to trigger competence (Fig. 1G and I). The third stop codon (TGA) displayed an elevated basal level of misreading that was not further increased by streptomycin (Fig. 1H). Elevated suppression rates for the TGA codon have also been found in Bacillus subtilis and Staphylococcus aureus (16, 17) and may be a general feature of Gram-positive bacteria. As seen with streptomycin, concentrations of kanamycin that promoted competence were also effective in increasing nonsense suppression (Fig. 2E).
Although most aminoglycoside antibiotics increase the ribosomal error rate, kasugamycin has been shown to decrease decoding errors in an Escherichia coli cell-free translation system (18). In S. pneumoniae, kasugamycin decreased spontaneous translational errors involving TAA and TGA stop codons (Fig. 1G and H). The error rate associated with the TAG codon, which was already read more accurately at baseline, did not fall further with kasugamycin (Fig. 1I). Kasugamycin also reduced decoding errors in streptomycin-treated samples for TAA and TGA codons (Fig. 1G and H) but not for TAG (Fig. 1I). Although the enhancement of translational fidelity for the TAA codon was modest, the effect on accuracy for the TGA codon was strong. Because the absolute error rate for the TGA codon is particularly high, improving the accuracy with which this codon is interpreted is likely to have a substantial effect on the overall level of translational errors. Consistent with a role for translational errors in triggering competence, kasugamycin blocked both spontaneous and streptomycin-induced competence (Fig. 1B and C). Although kasugamycin caused a slight reduction in the growth rate (Fig. 1D to F), this effect was small compared to that of streptomycin. These data indicated that a specific difference in the modes of action of streptomycin and kasugamycin—rather than a general effect on bacterial growth rates or inhibition of protein synthesis—was critical for the regulation of competence.
In addition to streptomycin and kanamycin, we found that paromomycin, which promotes decoding errors by binding to the 16S rRNA (19, 20), induces competence (Fig. 2B) at the same concentrations required to cause decoding errors (Fig. 2F). Conversely, spectinomycin and tetracycline both increased the accuracy of ribosomal decoding (Fig. 2G and H) and repressed competence (Fig. 2C and D). These last antibiotics, like kasugamycin, also reduced translational errors caused by streptomycin and prevented induction of competence by streptomycin (see Fig. S1 in the supplemental material).
Effects of ribosomal mutations on decoding accuracy and competence regulation.
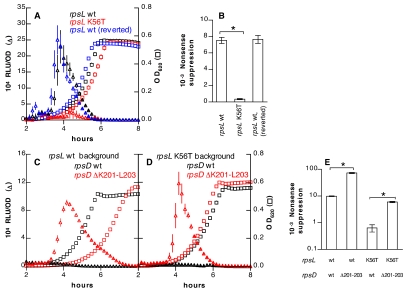
Intrinsic decoding accuracy is affected by mutations in components of the 30S ribosomal subunit. These mutations may produce either a restrictive phenotype with increased translational fidelity or a ram (ribosomal ambiguity) phenotype with an elevated rate of decoding errors (21–23). The S12 ribosomal protein, encoded by rpsL, is a frequent site of restrictive mutations that confer streptomycin resistance and increase decoding accuracy (21, 24). The rpsL(K56T) mutation in S. pneumoniae (homologous to K42T in E. coli numbering) reduced nonsense suppression to 4.7% of the wild-type level (Fig. 4B) and repressed development of spontaneous competence (Fig. 4A). Although ram mutations have not been previously identified in S. pneumoniae, ram mutations in other organisms frequently map to the interface between the S4 and S5 proteins (25). A pneumococcal rpsD(K201T) mutation [homologous to rpsD(K205T) in E. coli numbering] produced a weak ram phenotype with elevation of nonsense suppression by only 15% and did not affect competence (data not shown). A small C-terminal deletion in the S4 protein [rpsD(∆K201-L203)], however, produced a strong ram effect (Fig. 4E) and promoted development of competence even under nonpermissive conditions (Fig. 4C). In the restrictive rpsL(K56T) background, this ram mutation increased the translational error rate back nearly to the wild-type level and restored competence under permissive conditions, which had been lost in the restrictive mutant (Fig. 4D).
FIG 4 .
Ribosomal mutations affecting translational accuracy and competence. (A) A strain with an rpsL(K56T) allele (KSP86) displays repressed luciferase activity (triangles) compared to isogenic strains with wild-type (wt) rpsL before the K56T mutation (R895) or after reversion (KSP107). OD620s of cultures are shown with squares of matching colors. (B) The rpsL(K56T) mutation decreases suppression of a TAA codon. (C and D) An rpsD(∆K201-L203) ram mutation (strains DCP32 in panel C and DCP31 in panel D) increases luciferase activity (triangles) compared with that of wild-type rpsD (strains DCP19 in panel C and KSP190 in panel D) in the background of wild-type rpsL (C) or rpsL(K56T) (D) alleles. All strains in these panels express the ssbB′-luc competence reporter. (E) Effects of rpsL and rpsD mutations on suppression of a TAA codon. *, P < 0.001 by ANOVA with Bonferroni’s correction.
Error-sensitive modulation of competence by the HtrA protease.
Having observed the sensitivity of the pneumococcal competence pathway to translational errors, we considered how this pattern of regulation might be generated and in particular whether the pneumococcal HtrA protease might be involved. This surface-associated serine protease is the sole member of the HtrA family found in S. pneumoniae, and activity of this protease has been shown to repress competence, although the molecular target of its activity is uncertain (26). The observation that DegP (one of three HtrA proteases in E. coli) degrades and refolds denatured periplasmic proteins by means of its dual protease and chaperone activities (27–29) suggested that HtrA might interact with misfolded proteins in addition to regulating competence. If processing of misfolded proteins were to compete with the ability of HtrA to repress competence, we reasoned that this interaction might result in derepression of competence in response to errors during protein synthesis, some of which would produce proteins with intrinsic folding defects.
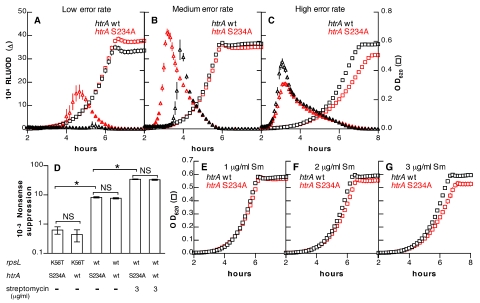
We predicted, therefore, that the ability of HtrA to repress competence would decline as the frequency of translational errors rose. Strains with wild-type htrA were compared under conditions of low, medium, and high error rates (Fig. 5D) with isogenic strains in which an htrA(S234A) mutation (26) inactivates the catalytic site of the protease. As anticipated, wild-type htrA caused repression of competence when decoding errors were rare but had less effect as decoding errors became more common (Fig. 5A to C; see also Fig. S2 in the supplemental material). At the highest level of mistranslation, competence profiles of the mutant and wild-type strains were nearly identical. Similar results were seen when an htrA deletion strain was examined (see Fig. S3).
FIG 5 .
Competence is repressed by the HtrA protease when translational errors are rare. (A to C) Effects of an htrA(S234A) mutation (strains KSP88 in panel A and KSP122 in panels B and C) compared with wild-type htrA (strains KSP90 in panel A and KSP148 in panels B and C) on activity of an ssbB′-luc competence reporter (triangles) in the context of a low translational error rate [rpsL(K56T) background] (A), a medium error rate (wild-type rpsL) (B), or a high error rate (wild-type rpsL plus streptomycin at 3 µg/ml) (C). OD620s (squares) of cultures are shown for comparison. Cultures for panels A to C were grown under conditions permissive for competence in the strain with wild-type htrA and rpsL. (D) Suppression of a TAA stop codon is not influenced by mutation of htrA under the low, medium, and high translational error conditions used in panels A to C. *, P < 0.001 by ANOVA with Bonferroni’s correction. “NS” indicates P > 0.05. (E to G) Growth of KSP148 (wild-type htrA) and KSP122 [htrA (S234A)] in the presence of streptomycin at 1 µg/ml (E), 2 µg/ml (F), or 3 µg/ml (G). Symbols represent means ± SEM for 6 replicate cultures grown under competence-permissive conditions.
We observed, furthermore, that the htrA(S234A) strain displayed a growth defect compared to the wild type in the presence of streptomycin (Fig. 5E to G) under permissive conditions in which both strains developed competence with similar kinetics. This conditional reduction in the growth rate became evident only when the concentration of streptomycin was increased from 1 to 3 µg/ml (P = 0.13, 0.017, and 0.0009 at 1, 2, and 3 µg/ml of streptomycin, respectively). The faster growth of the wild-type strain indicates that in addition to its role as a regulator of competence, htrA functions to alleviate the stress imposed by mistranslated proteins. This finding is consistent with a model of dual regulatory and stress-response activities for pneumococcal HtrA, and competition between such dual activities may be responsible for the derepression of competence observed in response to translational errors. To exclude the possibility that streptomycin might induce competence by lowering HtrA expression rather than by modulating its proteolytic effects, we assessed the impact of this antibiotic on HtrA protein levels. Neither streptomycin nor other competence-inducing antibiotics reduced HtrA levels (see Fig. S4 in the supplemental material), indicating that under high translational error conditions this protease continues to be expressed but is no longer effective in repressing competence.
DISCUSSION
In this work, we have shown that development of competence in S. pneumoniae is sensitively modulated in response to the rate of biosynthetic errors during protein production. This pattern of activation in response to decoding errors provides an explanation for the observation that cellular chaperones and proteases are induced as part of the pneumococcal competence regulon and suggests that competence functions as a response to misfolded proteins arising from such synthetic errors. Although this response may help the bacterium prevent the accumulation of misfolded proteins, generalized stress per se from aberrant protein production does not appear to be required for competence induction. Rather, this pathway is able to monitor changes in translational fidelity that even represent improvements over the performance of the wild type and to activate competence before the level of miscoding is sufficient to impair growth [in Fig. 4A, compare the enhanced growth of the competent wild-type rpsL strains with that of the rpsL(K56T) strain that does not develop competence].
We have observed that the pneumococcal HtrA protease represses competence selectively under conditions where the translational error rate is low. This protease appears thereby to function as an indirect sensor to modulate the development of competence in response to the level of mistranslated proteins. Because the molecular target through which HtrA represses competence is uncertain, the mechanism of this error-sensitive competence modulation remains the subject of investigation. A model that appears consistent with our observations without invoking the existence of new components of the signaling pathway would involve direct degradation of CSP by HtrA. In addition to its parsimony, this model is attractive because the hydrophobic patch on CSP that is required for its signaling function (30) appears likely to resemble the hydrophobic regions of unfolded proteins that become substrates for the DegP protease when exposed to a solvent by denaturation (31). The conditional reduction in the growth rate of the htrA(S234A) mutant when exposed to increasing concentrations of streptomycin suggests that, like DegP, pneumococcal HtrA may also serve to degrade generic misfolded proteins. Competition among substrates for limited HtrA proteolysis may then cause derepression of competence as a consequence of reduced degradation of CSP when the abundance of mistranslated proteins rises. This model would be consistent with the observation that streptomycin-induced competence requires the CSP transport protein ComA (14) as well as our finding that streptomycin induces competence in a manner that is capable of spreading between cells in a mixed culture. At a more general level, it is conceivable that HtrA regulates competence through a substrate other than CSP but that an analagous competitive mechanism (i.e., between competence-related and generic substrates for HtrA) may still account for the induction of competence by translational errors.
The localization of HtrA to the cell envelope of S. pneumoniae, where it fractionates primarily with the membrane and to a lesser degree with the cell wall (26), suggests that HtrA interacts selectively with surface-associated or secreted proteins and regulates competence in response to mistranslation of these proteins. Factors in addition to HtrA, however, must participate in the regulation of competence in response to decoding errors because changes in the error rate impact competence even when htrA is inactivated, although the response is less steep. One candidate for mediating this residual response is the multisubunit cytoplasmic Clp protease, which also degrades aberrant proteins (32) and represses competence through degradation of the competence sigma factor ComX (33). Direct investigation of the role of Clp proteases in the error-induced competence response has been impaired by a severe growth defect observed following deletion of clpP, encoding the catalytic subunit, and the acquisition of a secondary mutation(s) that affects competence development.
An alternative to the model of conditional degradation of CSP by HtrA is the possibility that HtrA might function exclusively to relieve the stress of misfolded surface proteins and that accumulation of these misfolded proteins when not cleared by HtrA might trigger competence through another pathway that has yet to be described. Although this second model cannot be excluded, we favor the former mechanism for the reasons discussed above and because the loss of htrA activity stimulated competence even under conditions where the mutation was not associated with a growth defect (Fig. 5A). This observation suggested that induction of competence in the htrA mutant is unlikely to be an indirect effect of stress from accumulation of misfolded proteins. Whether misfolded proteins stimulate competence directly or by reducing degradation of CSP, these findings also raise the question of whether external stresses that damage and denature surface proteins might activate competence in a manner analogous to what we have seen with translational errors. The possibility that such external interactions—with either the immune system or other bacteria—may trigger competence remains to be investigated. It should also be noted that this work has been conducted using the unencapsulated laboratory strain R6 and that more definitive conclusions regarding the biological relevance of these observations will require confirmation with wild-type strains.
In addition to streptomycin and kanamycin, it was previously reported that pneumococcal competence could be induced by either mitomycin C or norfloxacin (14). Because mitomycin C cross-links DNA (34) and fluoroquinolones such as norfloxacin inhibit DNA gyrase and topoisomerase IV (35), induction of competence by these agents would not appear to be the result of changes in translational accuracy. The mechanism by which these drugs stimulate competence is uncertain and may be unrelated to the effects of streptomycin and kanamycin. It is possible, however, that a similar process mediated by misfolded proteins may result indirectly following blockade of the transcriptional apparatus by the DNA cross-links that are generated by either mitomycin C or the quinolone-gyrase-DNA complex (35), causing incomplete transcripts and production of truncated proteins that cannot properly fold.
The sensitivity of pneumococcal competence regulation to pH is an aspect of this signaling system that is not well understood despite having been recognized long before the molecular characterization of CSP. Early studies speculated that the competence-stimulating factor showed increased stability (36) or increased bioactivity (37) at a slightly alkaline pH. In the context of our work on mistranslation-induced competence, it is interesting that the accuracy of amino acid incorporation by E. coli ribosomes in vitro has been shown to decrease as the pH rises over a range from 6.5 to 8 (38). A similar effect on the accuracy of translation in S. pneumoniae would appear to present a potential explanation for the pH sensitivity of competence. We therefore tested whether rates of nonsense suppression varied between competence-permissive and nonpermissive media for our 3 stop codon reporters and for an additional reporter construct with a missense mutation at the catalytic site of LacZ. These assays, however, did not reveal a difference in translational accuracy with pH (data not shown). Because our missense reporter assesses only the frequency of one particular amino acid substitution (glutamate for glycine), it remains possible that misincorporation of other amino acids increases with pH and may account for the induction of competence under these conditions. It is also notable that the enzymatic activity of another HtrA protein, the E. coli DegQ protease, has recently been shown to increase at slightly acidic pH (39). Although the effect of pH on activity of pneumococcal HtrA remains to be determined, a similar increase in activity might contribute to the inhibition of competence at lower pH.
Whereas the DegP and DegS proteases in E. coli—among the best-characterized members of the HtrA family—have been implicated previously in stress responses to misfolded proteins, the role of HtrA in S. pneumoniae appears to be distinct. Unlike the sensor protein DegS, which activates a proteolytic cascade leading to σE induction when triggered by unfolded or misfolded outer-membrane porins (40), it is loss of HtrA function instead of its activation that promotes the pneumococcal competence response. In contrast to the regulatory function of DegS, DegP was initially described as an effector of the extracytoplasmic stress response. More recently, however, it has been shown that DegP also degrades the small periplasmic protein CpxP, which functions as a repressor of periplasmic stress signaling by the CpxA sensor kinase (41, 42). This system, in which deletion of degP leads to a reduction in Cpx stress signaling (41), also appears to differ from the regulation we have observed for pneumococcal competence, in which a loss of htrA causes an increase in competence signaling. Intriguingly, the Cpx system has also been shown to affect conjugation in E. coli. However, this form of genetic exchange is repressed rather than induced by activation of Cpx signaling (43). Such divergent functions should perhaps not be surprising considering that the protease domain of pneumococcal HtrA has no more sequence similarity with either DegS (56%) or DegP (64%) than these proteases have with each other (65%) or with the third E. coli protease in the HtrA family, DegQ (63% similar to pneumococcal HtrA, 67% to DegS, and 86% to DegP). The broad distribution of the HtrA family of proteases, however, in both bacteria and eukaryotes—where HtrA proteases have been linked to protein misfolding diseases such as Alzheimer’s (44) and Parkinson’s (45, 46) diseases—raises the question of whether HtrA proteases in other organisms may also function as biosynthetic error sensors, albeit with outputs potentially distinct from transformation.
The finding that competence is induced by ribosomal decoding errors additionally suggests a mechanism by which genetic damage—in the form of miscoding at the level of the genome rather than the ribosome—may trigger the same competence response. A pathway sensing such informational lesions in the genome would contrast with previously characterized DNA damage responses that are activated by physical damage. The pneumococcal competence system may thereby function to address the stress of protein misfolding both by activating proteases and chaperones and by initiating transformation to repair underlying genetic damage.
MATERIALS AND METHODS
Bacterial growth conditions, strains, and mutagenesis.
Broth cultures of S. pneumoniae strain R6 and derivatives were grown in C+Y medium prepared using 2 distinct recipes that have both been described by that name. An early reference to this medium used Difco Casamino Acids in its formulation (47), whereas modifications later substituted individual amino acids among other changes (48, 49). We have designated the first recipe as C+YCAA (for Casamino Acids) and the second as C+YYB (for its increased concentration of yeast extract, as well as bovine serum albumin). The compositions of these media are given in Table S1 in the supplemental material. We found that C+YYB but not C+YCAA supported development of spontaneous competence in the microtiter plate assay described below. The final pH of C+YYB medium was adjusted to near 7.30 or 7.20 to produce medium either permissive or nonpermissive, respectively, for the development of spontaneous competence in untreated, wild-type cultures. S. pneumoniae was grown in C+YCAA medium for mutagenesis and routine propagation. Antibiotics were used in the following concentrations for selection: erythromycin, 2 µg/ml (S. pneumoniae) and 500 µg/ml (E. coli); streptomycin, 500 µg/ml; and kanamycin, 500 µg/ml.
S. pneumoniae and E. coli strains used in this study are described in Tables S2 and S3 in the supplemental material, respectively. Targeted mutations were introduced into S. pneumoniae by PCR ligation mutagenesis (50) using primers listed in Table S4. Unmarked point mutations and deletions in htrA and bgaA were produced using the counterselectable Janus cassette (51). Because disruption of rpsD with the Janus cassette would have been lethal, mutations in this gene were generated by introducing aph3 (conferring kanamycin resistance) downstream of rpsD by PCR ligation mutagenesis using primers designed to introduce changes into the 3′ end of rpsD adjacent to aph3. Control strains were generated with aph3 in the same location downstream of wild-type rpsD. Mutations in rpsL were isolated by direct screening or selection for changes in streptomycin susceptibility following transformation.
lacZ reporters for measuring nonsense suppression were constructed by amplifying the promoterless copy of lacZ from pEVP3 (52) using primers encoding the pneumococcal amiA promoter. This amplicon was cloned into the BamHI site of the shuttle plasmid pMU1328 (53). Premature stop codons were generated using QuikChange mutagenesis (Agilent Technologies, Santa Clara, CA). Plasmids with lacZ reporters were transformed into S. pneumoniae strains with deletions in bgaA, inactivating this endogenous galactosidase and reducing background activity in LacZ assays.
Nonsense suppression assays.
Nonsense suppression was measured by determining beta-galactosidase activity in cultures of pneumococcal strains bearing lacZ reporters with and without premature stop codons early in the coding sequence. The ratio of LacZ activity in the presence of the stop codon compared to that from an uninterrupted reporter provided a measure of ribosomal accuracy. Strains to be tested were initially inoculated into C+YYB medium and grown to an optical density at 620 nm (OD620) of 0.26 before being concentrated by centrifugation and resuspended in 0.75 volumes fresh C+YYB medium with 16% glycerol. Aliquots were frozen at −75°C for later use. From these frozen stocks, cultures were diluted 1:50 into C+YYB medium containing antibiotics as indicated and grown at 37°C to an OD600 near 0.25. Bacteria were then lysed by adding Triton X-100 to a concentration of 0.1% and incubating for 10 min at 37°C. To each sample, 0.25 volumes of a reaction buffer containing 5 mM MgCl2, 50 mM KCl, 0.3 M Na2HPO4, 0.2 M NaH2PO4, 4 mg/ml 2-nitrophenyl-β-d-galactopyranoside, and 250 mM 2-mercaptoethanol was then added. Samples were incubated at room temperature, after which color development was stopped by addition of 0.4 volumes 1 M sodium carbonate. Absorbance was measured at 420 and 550 nm, and Miller units were calculated as previously described (54). Statistical comparisons were made by analysis of variance (ANOVA) testing, with posttests for linear trends for analyses involving multiple doses of the same antibiotic, using the Prism 4.0 software program (GraphPad Software, La Jolla, CA).
Competence assays.
Competence was determined by measuring the activity of a luciferase reporter in strains with an ssbB′-luc transcriptional fusion (55). Pneumococcal ssbB is induced specifically during competence, and activity of this fusion has been shown to reflect competence for transformation (14). Strains to be tested were initially inoculated into CAT medium (56) and grown to an OD620 of 0.26 before being concentrated by centrifugation and resuspended in 0.75 volumes fresh CAT medium with 16% glycerol. Aliquots were frozen at −75°C for later use. From these frozen stocks, cultures were diluted 1:400 into C+YYB medium containing 0.65 mM d-luciferin. Antibiotics were added as indicated. Samples were grown in 200-µl aliquots in white NBS 96-well microplates (Corning Inc., Corning, NY). Luminescence and the OD620 were measured every 5 min during incubation at 37°C in a Synergy2 plate reader (Bio-Tek, Winooski, VT). Activity of the ssbB′-luc fusion was normalized to the density of the culture and reported as relative light units (RLU)/OD620. For clarity, data are presented showing only measurements taken at 10-min intervals. This reduction did not affect the patterns of competence induction observed. For Fig. 4, in which the difference in timing of competence development between wild-type and htrA(S234A) cultures is of interest, data are shown at 5-min intervals during the induction phase. Statistical comparisons for RLU/OD620 data and growth curves were performed by 2-way repeated-measures ANOVA with Bonferroni’s correction using Prism 4.0 software.
Western immunoblotting.
Western immunoblotting was performed using anti-HtrA antiserum (26) at a 1:500 dilution and a secondary monoclonal anti-rabbit immunoglobulin alkaline phosphatase conjugate, following standard protocols. Membranes were blocked with 5% nonfat dry milk in Tris-saline blotting buffer (10 mM Tris-Cl, pH 8.0, 0.5 M NaCl, 0.5% Tween 20).
SUPPLEMENTAL MATERIAL
Tetracycline and spectinomycin counteract streptomycin-induced competence. (A and B) Inhibition of streptomycin-induced competence by tetracycline (Tc) (A) or spectinomycin (Sp) (B). Luciferase activity and OD620 of strain R895 (ssbB′-luc) under different treatments are shown with triangles and squares, respectively. Samples treated with streptomycin (Sm) alone or no antibiotics are shown in both panels for comparison. (C and D) Effects of tetracycline (C) or spectinomycin (D) on suppression of the TAA stop codon in a lacZ reporter in samples also treated with streptomycin. Download Figure S1, EPS file, 0.4 MB.
Competence is repressed by the HtrA protease when translational errors are rare. Effects of an htrA(S234A) mutation (strains KSP88 in panel A and KSP122 in panels B and C) compared with wild-type htrA (strains KSP90 in panel A and KSP148 in panels B and C) on activity of an ssbB′-luc competence reporter in the context of a low translational error rate [rpsL(K56T) background] (A), medium error rate (wild-type rpsL) (B), or high error rate (wild-type rpsL plus streptomycin at 3 µg/ml) (C). The data here are from the same experiments shown in Fig. 4 but are plotted as RLU/OD versus OD rather than time in order to adjust for differences in growth rates of the samples. These graphs are truncated to show only data points with OD readings that can be reliably quantified in this assay (OD620 ≥ 0.02). Download Figure S2, EPS file, 0.3 MB.
Deletion of htrA enhances competence under low translational error conditions. Effects of an htrA(∆286–1140) mutation (strains KSP89 in panels A and D and KSP172 in panels B, C, E, and F) compared with wild-type htrA (strains KSP90 in panels A and D and KSP148 in panels B, C, E, and F) on activity of an ssbB′-luc competence reporter (triangles) in the context of a low translational error rate [rpsL(K56T) background] (A and D), a medium error rate (wild-type rpsL) (B and E), or a high error rate (wild-type rpsL plus streptomycin at 3 µg/ml) (C and F). Data from the same experiments are plotted in panels A to C as RLU/OD versus time and in panels D to F as RLU/OD versus OD. OD620s of cultures are shown separately (squares) in panels A to C. Download Figure S3, EPS file, 0.7 MB.
HtrA expression in antibiotic-treated cultures. Western blot for HtrA expression in strain JKP65 treated with antibiotics at the indicated concentrations. Samples were grown in C+YYB medium at a nonpermissive pH for development of competence in untreated samples. Download Figure S4, EPS file, 0.2 MB.
Composition of growth media for S. pneumoniae.
S. pneumoniae strains used in this study.
E. coli strains used in this study.
PCR primers used in this study.
ACKNOWLEDGMENTS
We thank J.-P. Claverys for providing strains R800 and R895 and J. Bergelson, P. Offit, E. Robertson, and J. Weiser for valuable discussions and critical reading of the manuscript during its preparation.
This work was supported by Public Health Service grant AI075194 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Citation Stevens KE, Chang D, Zwack EE, Sebert ME. 2011. Competence in Streptococcus pneumoniae is regulated by the rate of ribosomal decoding errors. mBio 2(5):e00071-11. doi:10.1128/mBio.00071-11.
REFERENCES
- 1. Bucciantini M, et al. 2002. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416:507–511 [DOI] [PubMed] [Google Scholar]
- 2. Kunkel TA. 1988. Exonucleolytic proofreading. Cell 53:837–840 [DOI] [PubMed] [Google Scholar]
- 3. Thomas MJ, Platas AA, Hawley DK. 1998. Transcriptional fidelity and proofreading by RNA polymerase II. Cell 93:627–637 [DOI] [PubMed] [Google Scholar]
- 4. Zaher HS, Green R. 2009. Quality control by the ribosome following peptide bond formation. Nature 457:161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schröder M, Kaufman RJ. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74:739–789 [DOI] [PubMed] [Google Scholar]
- 6. Meusser B, Hirsch C, Jarosch E, Sommer T. 2005. ERAD: the long road to destruction. Nat. Cell Biol. 7:766–772 [DOI] [PubMed] [Google Scholar]
- 7. Kubota H. 2009. Quality control against misfolded proteins in the cytosol: a network for cell survival. J. Biochem. 146:609–616 [DOI] [PubMed] [Google Scholar]
- 8. Young JC, Agashe VR, Siegers K, Hartl FU. 2004. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 5:781–791 [DOI] [PubMed] [Google Scholar]
- 9. Ruiz N, Silhavy TJ. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122–126 [DOI] [PubMed] [Google Scholar]
- 10. Guisbert E, Yura T, Rhodius VA, Gross CA. 2008. Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol. Mol. Biol. Rev. 72:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Claverys J-P, Prudhomme M, Martin B. 2006. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu. Rev. Microbiol. 60:451–475 [DOI] [PubMed] [Google Scholar]
- 12. Peterson SN, et al. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 51:1051–1070 [DOI] [PubMed] [Google Scholar]
- 13. Yang J, Evans BA, Rozen DE. 2010. Signal diffusion and the mitigation of social exploitation in pneumococcal competence signalling. Proc. Biol. Sci. 277:2991–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prudhomme M, Attaiech L, Sanchez G, Martin B, Claverys J-P. 2006. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science 313:89–92 [DOI] [PubMed] [Google Scholar]
- 15. Davies J, Gilbert W, Gorini L. 1964. Streptomycin, suppression and the code. Proc. Natl. Acad. Sci. U. S. A. 51:883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karow ML, Rogers EJ, Lovett PS, Piggot PJ. 1998. Suppression of TGA mutations in the Bacillus subtilis spoIIR gene by prfB mutations. J. Bacteriol. 180:4166–4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lovett PS, Ambulos J, Mulbry W, Noguchi N, Rogers EJ. 1991. UGA can be decoded as tryptophan at low efficiency in Bacillus subtilis. J. Bacteriol. 173:1810–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Buul CPJJ, Visser W, van Kippenberg PH. 1984. Increased translational fidelity caused by the antibiotic kasugamycin and ribosomal ambiguity in mutants harbouring the ksgA gene. FEBS Lett. 177:119–124 [DOI] [PubMed] [Google Scholar]
- 19. Carter AP, et al. 2000. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407:340–348 [DOI] [PubMed] [Google Scholar]
- 20. Fourmy D, Recht MI, Blanchard SC, Puglisi JD. 1996. Structure of the A site of Escherichia coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science 274:1367–1371 [DOI] [PubMed] [Google Scholar]
- 21. Ozaki M, Mizushima S, Nomura M. 1969. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature 222:333–339 [DOI] [PubMed] [Google Scholar]
- 22. Rosset R, Gorini L. 1969. A ribosomal ambiguity mutation. J. Mol. Biol. 39:95–112 [DOI] [PubMed] [Google Scholar]
- 23. Björkman J, Samuelsson P, Andersson DI, Hughes D. 1999. Novel ribosomal mutations affecting translational accuracy, antibiotic resistance and virulence of Salmonella typhimurium. Mol. Microbiol. 31:53–58 [DOI] [PubMed] [Google Scholar]
- 24. Bohman K, Ruusala T, Jelenc PC, Kurland CG. 1984. Kinetic impairment of restrictive streptomycin-resistant ribosomes. Mol. Gen. Genet. 198:90–99 [DOI] [PubMed] [Google Scholar]
- 25. Maisnier-Patin S, Berg OG, Liljas L, Andersson DI. 2002. Compensatory adaptation to the deleterious effect of antibiotic resistance in Salmonella typhimurium. Mol. Microbiol. 46:355–366 [DOI] [PubMed] [Google Scholar]
- 26. Sebert ME, Patel KP, Plotnick M, Weiser JN. 2005. Pneumococcal HtrA protease mediates inhibition of competence by the CiaRH two-component signaling system. J. Bacteriol. 187:3969–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clausen T, Southan C, Ehrmann M. 2002. The HtrA family of proteases: implications for protein composition and cell fate. Mol. Cell 10:443–455 [DOI] [PubMed] [Google Scholar]
- 28. Meltzer M, et al. 2009. Structure, function, and regulation of the conserved serine proteases DegP and DegS of Escherichia coli. Res. Microbiol. 160:660–666 [DOI] [PubMed] [Google Scholar]
- 29. Spiess C, Beil A, Ehrmann M. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339–347 [DOI] [PubMed] [Google Scholar]
- 30. Johnsborg O, Kristiansen PE, Blomqvist T, Håvarstein LS. 2006. A hydrophobic patch in the competence-stimulating peptide, a pneumococcal competence pheromone, is essential for specificity and biological activity. J. Bacteriol. 188:1744–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kolmar H, Waller PRH, Sauer RT. 1996. The DegP and DegQ periplasmic endoproteases of Escherichia coli: specificity for cleavage sites and substrate conformation. J. Bacteriol. 178:5925–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahlawat S, Morrison DA. 2009. ClpXP degrades SsrA-tagged proteins in Streptococcus pneumoniae. J. Bacteriol. 191:2894–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sung CK, Morrison DA. 2005. Two distinct functions of ComW in stabilization and activation of the alternative sigma factor ComX in Streptococcus pneumoniae. J. Bacteriol. 187:3052–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tomasz M, et al. 1987. Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science 235:1204–1208 [DOI] [PubMed] [Google Scholar]
- 35. Drlica K, Zhao X. 1997. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 61:377–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomasz A, Mosser JL. 1966. On the nature of the pneumococcal activator substance. Proc. Natl. Acad. Sci. U. S. A. 55:58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen JD, Morrison DA. 1987. Modulation of competence for genetic transformation in Streptococcus pneumoniae. J. Gen. Microbiol. 133:1959–1967 [DOI] [PubMed] [Google Scholar]
- 38. Grunberg-Manago M, Dondon J. 1965. Influence of pH and S-RNA concentration on coding ambiguities. Biochem. Biopyhs. Res. Commun. 18:517–522 [DOI] [PubMed] [Google Scholar]
- 39. Sawa J, et al. 2011. Molecular adaptation of the DegQ protease to exert protein quality control in the bacterial cell envelope. J. Biol. Chem. 286:30680–30690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. 2003. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell 113:61–71 [DOI] [PubMed] [Google Scholar]
- 41. Buelow DR, Raivio TL. 2005. Cpx signal transduction is influenced by a conserved N-terminal domain in the novel inhibitor CpxP and the periplasmic protease DegP. J. Bacteriol. 187:6622–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Isaac DD, Pinkner JS, Hultgren SJ, Silhavy TJ. 2005. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc. Natl. Acad. Sci. U. S. A. 102:17775–17779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lau-Wong IC, Locke T, Ellison MJ, Raivio TL, Frost LS. 2008. Activation of the Cpx regulon destabilizes the F plasmid transfer activator, TraJ, via the HslVU protease in Escherichia coli. Mol. Microbiol. 67:516–527 [DOI] [PubMed] [Google Scholar]
- 44. Grau S, et al. 2005. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. U. S. A. 102:6021–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strauss KM, et al. 2005. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum. Mol. Genet. 14:2099–2111 [DOI] [PubMed] [Google Scholar]
- 46. Jones JM, et al. 2003. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature 425:721–727 [DOI] [PubMed] [Google Scholar]
- 47. Lacks S, Hotchkiss RD. 1960. A study of the genetic material determining an enzyme in Pneumococcus. Biochim. Biophys. Acta 39:508–517 [DOI] [PubMed] [Google Scholar]
- 48. Tomasz A. 1964. Bacteriol. Proc. Abstr. 64th Annu. Meet., p 29 American Society for Microbiology, Washington, DC [Google Scholar]
- 49. Martin B, García P, Castanié M-P, Claverys J-P. 1995. The recA gene of Streptococcus pneumoniae is part of a competence-induced operon and controls lysogenic induction. Mol. Microbiol. 15:367–379 [DOI] [PubMed] [Google Scholar]
- 50. Lau PCY, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193–205 [DOI] [PubMed] [Google Scholar]
- 51. Sung CK, Li H, Claverys JP, Morrison DA. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Claverys J-P, Dintilhac A, Pestova EV, Martin B, Morrison DA. 1995. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene 164:123–128 [DOI] [PubMed] [Google Scholar]
- 53. Yother J, Handsome GL, Briles DE. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 55. Chastanet A, Prudhomme M, Claverys J-P, Msadek T. 2001. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 183:7295–7307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Porter RD, Guild WR. 1976. Characterization of some pneumococcal bacteriophages. J. Virol. 19:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tetracycline and spectinomycin counteract streptomycin-induced competence. (A and B) Inhibition of streptomycin-induced competence by tetracycline (Tc) (A) or spectinomycin (Sp) (B). Luciferase activity and OD620 of strain R895 (ssbB′-luc) under different treatments are shown with triangles and squares, respectively. Samples treated with streptomycin (Sm) alone or no antibiotics are shown in both panels for comparison. (C and D) Effects of tetracycline (C) or spectinomycin (D) on suppression of the TAA stop codon in a lacZ reporter in samples also treated with streptomycin. Download Figure S1, EPS file, 0.4 MB.
Competence is repressed by the HtrA protease when translational errors are rare. Effects of an htrA(S234A) mutation (strains KSP88 in panel A and KSP122 in panels B and C) compared with wild-type htrA (strains KSP90 in panel A and KSP148 in panels B and C) on activity of an ssbB′-luc competence reporter in the context of a low translational error rate [rpsL(K56T) background] (A), medium error rate (wild-type rpsL) (B), or high error rate (wild-type rpsL plus streptomycin at 3 µg/ml) (C). The data here are from the same experiments shown in Fig. 4 but are plotted as RLU/OD versus OD rather than time in order to adjust for differences in growth rates of the samples. These graphs are truncated to show only data points with OD readings that can be reliably quantified in this assay (OD620 ≥ 0.02). Download Figure S2, EPS file, 0.3 MB.
Deletion of htrA enhances competence under low translational error conditions. Effects of an htrA(∆286–1140) mutation (strains KSP89 in panels A and D and KSP172 in panels B, C, E, and F) compared with wild-type htrA (strains KSP90 in panels A and D and KSP148 in panels B, C, E, and F) on activity of an ssbB′-luc competence reporter (triangles) in the context of a low translational error rate [rpsL(K56T) background] (A and D), a medium error rate (wild-type rpsL) (B and E), or a high error rate (wild-type rpsL plus streptomycin at 3 µg/ml) (C and F). Data from the same experiments are plotted in panels A to C as RLU/OD versus time and in panels D to F as RLU/OD versus OD. OD620s of cultures are shown separately (squares) in panels A to C. Download Figure S3, EPS file, 0.7 MB.
HtrA expression in antibiotic-treated cultures. Western blot for HtrA expression in strain JKP65 treated with antibiotics at the indicated concentrations. Samples were grown in C+YYB medium at a nonpermissive pH for development of competence in untreated samples. Download Figure S4, EPS file, 0.2 MB.
Composition of growth media for S. pneumoniae.
S. pneumoniae strains used in this study.
E. coli strains used in this study.
PCR primers used in this study.