Abstract
In this paper we report the synthesis, purification, 13C NMR and other characterization studies of Y3N@C88. The 13C NMR, UV-vis and chromatographic data suggest an Y3N@C88 having IPR allowed cage with D2(35)-C88 symmetry. In earlier density functional theory (DFT) computational and X-ray crystallographic studies, it was reported that lanthanide (A3N)6+ clusters are stabilized in D2(35)-C88 symmetry cages and have reduced HOMO-LUMO gaps relative to other trimetallic nitride endohedral metallofullerene cage systems, for example, A3N@C80. In this paper, we report that the non-lanthanide (Y3N)6+ cluster in the D2(35)-C88 cage exhibits a HOMO-LUMO gap consistent with other lanthanide A3N@C88 molecules based on electrochemical measurements and DFT computational study. These results suggests that the reduced HOMO-LUMO gap of A3N@C88 systems is a property dominated by the D2(35)-C88 carbon cage and not f-orbital lanthanide electronic metal cluster (A3N)6+ orbital participation.
Introduction
The separation and characterization of higher fullerenes beyond C80 is very difficult, not only because of their extremely low yield, but also the possible presence of multiple isomers, instability, and lower solubility.1 For example, Yang et al. reported the separation of Di-and Tridysprosium Endohedral Metallofullerenes in the fullerene cages from C94 to C100.2 Due to the limited available amount of sample, they did not obtain any structural information of those large cages. 2 Among the large fullerene cages, the C88 cage has been widely studied. As early as 1995, C88 empty cage fullerenes were separated from soot generated in a Kratschmer-Huffman electric-arc generator and subsequently characterized using 13C NMR by Achiba et al.3 In a later report, Miyake et al.4 described a more detailed 13C NMR study for the C88 empty cage fullerene. However, the structural assignments were ambiguous because at least three C88 isomers exist.4 Due to the difficulty in experimental part, the extensive theoretical studies were performed by several groups to aid the structural determination.5,6 It was predicted that C2(7), Cs(17) and C2(33) isomers are the mostly likely to be experimentally-isolated isomers among the 35 IPR-obeying isomers of C88.5,6 Derivatization of empty fullerene cages has proved to be an effective tool for cage stabilization and meanwhile provides a path for further structural characterization. For example, Troyanov and Tamm 7 synthesized and characterized trifluoromethyl derivatives of C88, C88(CF3)18. Based on their X-ray investigation, they determined that C88(CF3)18 processes a C2(33)-C88 cage, which is one of the three most stable isomers predicted on a theoretical basis. Encapsulation of atoms or clusters into fullerene cages is another method used to stabilize empty fullerene cages by electron transfer processes between the encapsulated and the carbon cage.
Trimetallic nitride template (TNT) endohedral metallofullerenes (EMFs) are particularly important not only because of their relatively high yields, but also because of their intriguing potential applications in biomedical, optoelectronic, and photovoltaic fields.8,9 Insertion of a TNT cluster into a C88 cage (M3N@C88, M= Gd, Tm, Dy, Tb, Nd, Pr, Ce, La) has been reported by several laboratories.10–13 Echegoyen and coworkers suggested that the C88 cage is preferentially templated by a TNT cluster for metal ions with ionic radius larger than gadolinium, such as, Nd, Pr, Ce.10,11 However, most of the TNT EMFs with the C88 cage have been characterized by mass spectroscopy without definitive structural characterization. Notable exceptions are the single crystal X-ray studies reported for Tb3N@C88 which have been found to have an IPR-obeying D2(35) structure.12 This is in contrast with the theoretical predictions for the empty C88 cage. Thus, it is interesting to examine whether clusters with different metals (A3N)6+ encapsulated within a C88 cage have the same cage structure as IPR-obeying Tb3N@D2(35)-C88.
In this paper, we report the synthesis, separation and structural characterization of diamagnetic Y3N@C88. This diamagnetic molecule allows high-resolution 13C NMR structural studies, which are generally not feasible for the paramagnetic lanthanide C88 cage TNT EMFs reported above. Density function theory calculations were utilized to augment the structural determination. The electronic properties of Y3N@C88 were also studied by both electrochemical and DFT computational approaches to compare a non-lanthanide TNT EMF with the corresponding lanthanide TNT EMFs reported to date.
Experimental Section
A sample of Y3N@C88 was synthesized in an electric arc-discharge reactor by vaporizing graphite rods containing a mixture of Y2O3 and graphite powder and using Cu as catalyst with a weight ratio of 1.1:1.0:2.1 in a dynamic flow of N2 and He (flow rate ratio of N2/He=3:100).14 The toluene extract from the raw soot was applied to a cyclopentadiene-functionalized Merrifield peptide resin. The eluent was further separated by two-stage HPLC. The first stage was carried out on a 5PBB column and the sixth fraction contains Y3N@C88, which was further purified by a 5PYE column.
The 13C NMR measurements (150 MHz) were performed on a Bruker Advance spectrometer (600 MHz, 1H). The sample was dissolved in CS2 with Cr(acac)3 as the relaxation agent and acetone-d6 as the internal lock at 25 °C. Cyclic voltammetry was conducted using a CH Instruments 600A potentiostat (Austin, TX) with a single-compartment, three electrode, electrochemical cell. A 2.0 mm glassy carbon working electrode, platinum wire auxiliary, and a silver wire pseudo-reference electrode was used; ferrocene was used as an internal standard.
Density functional theory (DFT) computations were performed using the Gaussian 03 program package. All of the molecules were geometrically optimized at the UB3LYP level with a DZVP basis set for yttrium atoms and a 6-31G* basis set for carbon and nitrogen atoms.14 DFT-optimized energy values were obtained starting from the X-ray crystallographic structures of the corresponding Tb3N@D2(35)-C88.11
Results and Discussion
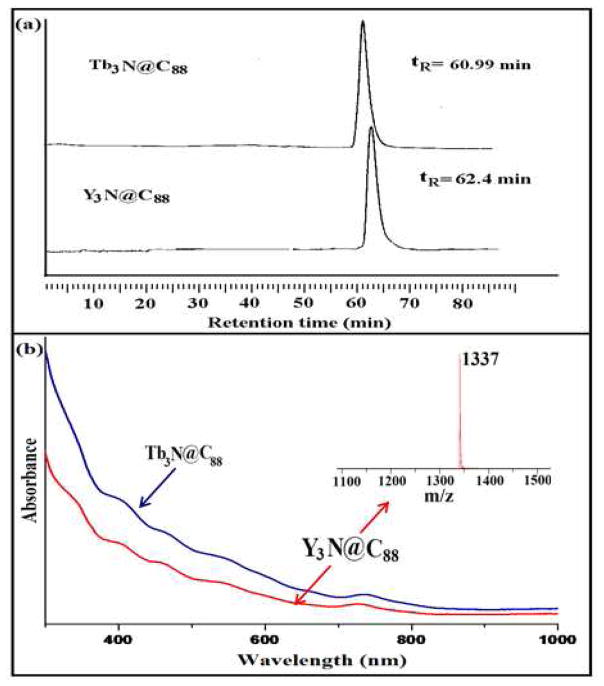
As illustrated in Figure 1, the HPLC, positive ion LD-TOF MS, and UV-vis spectra for Y3N@C88 is compared with data that was previously reported for Tb3N@C88.12 The close correspondence of the data in Figure 1 strongly suggests that Y3N@C88 has the same D2(35)cage structure as Tb3N@D2(35)-C88.12 Because the Y3N@C88 molecule is diamagnetic, we were able to obtain high-resolution 13C NMR data for Y3N@C88 to further confirm the cage structure.
Figure 1.
(a) HPLC chromatogram of the purified Y3N@C88 and Tb3N@C88 (10 × 250 mm 5PYE column; λ=390 nm; flow rate 2.0 mL/min; toluene as eluent; 25 °C. (b) UV-vis spectra of Y3N@C88 and Tb3N@C88 in toluene and positive ion LD-TOF MS for purified Y3N@C88.
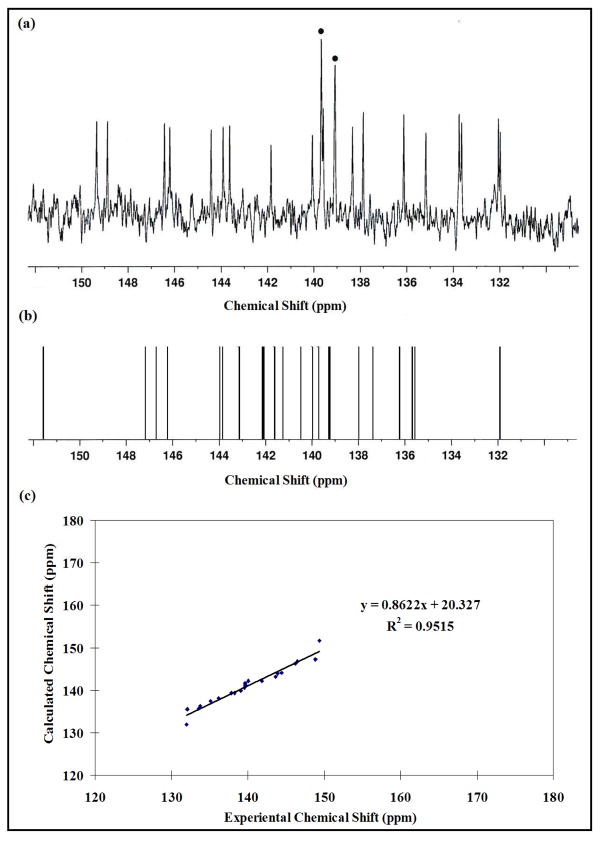
The 13C NMR spectrum (Figure 2a) for Y3N@C88 exhibits a total of 22 lines (lines at 139.10 and 139.69 ppm, double intensity) with a shift range from 131.0 to 150.0 ppm. This result is completely consistent with the 22 13C NMR signals observed for Y3N@C88. An IPR-allowed isomer is suggested because of the absence of a 13C NMR signal above 155 ppm, which is characteristic of non-IPR pentalene motifs.14 There are 35 IPR-allowed C88 isomers and two isomers (1) and (35) that have D2 symmetry (22 × 4 lines) which is consistent with the observed spectrum.15 Among the 35 IPR-obeying isomers for the C88 cage, no other IPR-allowed structure would exhibit fewer than 22 lines except for isomer (34) with T symmetry that would exhibit 8 13C NMR resonances (1×4 and 7×12). All other C88 IPR isomers have lower symmetry and would exhibit more than 22 spectral lines. In addition, there are 7 pyrene 6,6,6 type carbons that range from 132.0–137.9 ppm, which is in reasonably good agreement with 7 DFT predicted values (Figure 2b) ranging from 131.9–139.2 ppm.14 The correlation between experimental 13C and the DFT predicted chemical shielding values are shown in Figure 2c. Tb3N@D2(35)-C88 exhibits a carbon cage with D2 symmetry as determined by previous single-crystal X-ray diffraction studies. Thus, our current results are consistent with a Y3N@D2(35)-C88 structure in analogous fashion to Tb3N@D2(35)-C88.12
Figure 2.
(a)The 13C NMR spectrum of Y3N@D2-C88 in CS2 with 10 mg Cr(acac)3 relaxant, acetone-d6 lock) after 64,000 scan at 25 °C, showing the 22×4 pattern (number of NMR lines × relative intensity). The • corresponds to signal with double intensity. (b) Computational 13C NMR spectrum for Y3N@D2(35)-C88. The experimental and calculated 13C shifts are provided in the SI. (c) Correlation between experimental and computational 13C NMR results.
As previously indicated, there are three isomers of empty-cage C88 cage, C2(7), Cs(17) and C2(33), that are predicted to be thermodynamically and kinetically stable.5,6 However, C88 with D2(35) symmetry is not included in this group. Computational calculations (DFT) summarized in Figure 3 suggest that the neutral IPR D2(35)-C88 has a small HOMO-LUMO gap (1.27 eV), indicating the lower stability of the neutral D2(35)-C88 cage. However, upon accepting six electrons, the HOMO-LUMO gap becomes significantly larger (1.77 eV), consistent for higher stability of the Y3N@D3(35)-C88 molecule. To our knowledge, neither the Y3N cluster or the D2(35)-C88 cage have been isolated, but when associated together, they form a stable Y3N@D3(35)-C88 structure by electron transfer between the cluster and the cage.
Figure 3.
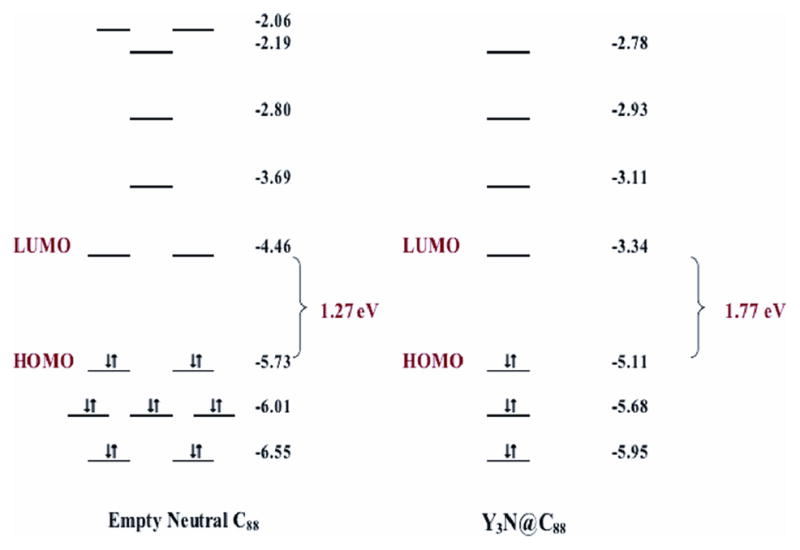
DFT computational HOMO-LUMO levels for the neutral IPR D2(35)-C88 and Y3N@D2(35)-C88 cages.
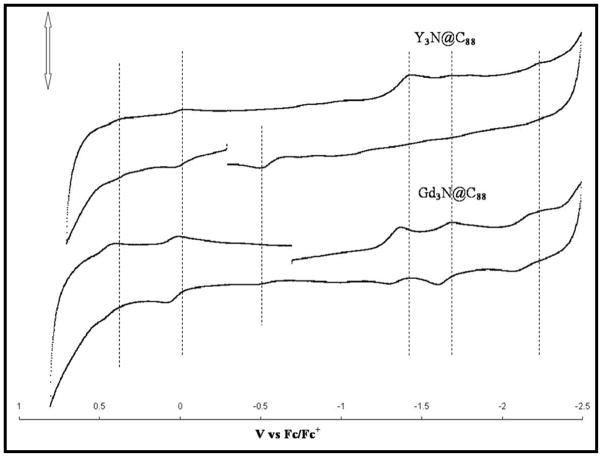
As illustrated in Figure 4, the CV electrochemistry of Y3N@C88 and Gd3N@C88 are nearly equivalent. The Gd3N@C88 sample for this CV electrochemistry comparison was isolated and purified (see SI) in the same fashion as described for Y3N@C88 vide supra and is consistent with previously reported data by Echegoyen and coworkers.16 The electrochemistry of Y3N@C88 and Gd3N@C88 can be described as having two distinct oxidative processes accompanied by a first and second reduction peak potential of −1.43 V and −1.70V, respectively. Redox potentials and resulting electrochemical gap (ΔEgap) for Y3N@C88 are in good agreement with reported literature values for other M3N@C88 systems (Table 1). This is consistent with a single (non-degenerate) LUMO level for Y3N@C88 obtained from the DFT calculations described above. The resulting electrochemical bandgap (oxE1-redE1) for the Y3N@C88 is 1.46 V, reasonably consistent with the DFT predictions above. Although (oxE1-redE1) for Y3N@C88 is smaller than the more stable Y3N@C80 case,16 this bandgap is similar to other M3N@C88 family members, such as, Gd3N@C88 (1.49 V),17 Nd3N@C88 (1.43 V),11 Pr3N@C88 (1.43 V),9 Ce3N@C88(1.38 V)9 and La3N@C88(1.57 V)9. These results suggests that the (oxE1-redE1) bandgap (and corresponding HOMO-LUMO gap) of A3N@C88 trimetallic nitride endohedral metallofullerenes is a property dominated by the properties of the D2(35)-C88 carbon cage and not the nature of the yttrium or lanthanide electronic metal cluster (A3N)6+.
Figure 4.
Cyclic voltammogram of Y3N@C88 and Gd3N@C88; 100 mV/s; 0.1 M TBABF4 in o-DCB.
Table 1.
Redox potential of the A3@C2n Endohedral Metallofullerenes
| E1/2ox1 | E1/2ox2 | Epcred1 | Epcred2 | ΔEgap | |
|---|---|---|---|---|---|
| Y3N@Ih-C8015 | 0.64 | −1.41 | −1.83 | 2.05 | |
| Y3N@C88 | 0.03 | 0.43 | −1.43 | −1.70 | 1.46 |
| Gd3N@C88 | 0.05 | 0.45 | −1.39 | −1.71 | 1.44 |
| Gd3N@C8816 | 0.06 | 0.49 | −1.43 | −1.74 | 1.49 |
| Nd3N@C8810 | 0.07 | 0.53 | −1.36 | −1.75 | 1.43 |
| Pr3N@C889 | 0.09 | 0.54 | −1.34 | −1.72 | 1.43 |
| Ce3N@C889 | 0.08 | 0.63 | −1.30 | −1.57 | 1.38 |
| La3N@C889 | 0.21 | 0.66 | −1.34 | −1.67 | 1.57 |
Conclusion
In summary, we have synthesized, purified and characterized diamagnetic Y3N@C88 for the first time. The 13C NMR study indicates that Y3N@C88 exhibits an IPR-obeying D2(35)-C88 cage. The electrochemical data suggested that Y3N@C88 has a smaller HOMO-LUMO gap than Y3N@C80 which is consistent with the computational DFT study. Also, our results show that encapsulation of an Y3N cluster does not significantly alter the electrochemical properties of these trimetallic nitride endohedral metallofullerenes and this result is consistent with other lanthanide M3N clusters in D2(35)-C88 cages. This suggests that the unique D2(35)-C88 cage properties strongly influence the electrochemical and electronic properties of these trimetallic nitride endohedral metallofullerenes.
Supplementary Material
Acknowledgments
We gratefully acknowledge support by the National Science Foundation [CHE-0443850 (H.C.D.), DMR-0507083 (H.C.D.)] and the National Institutes of Health [1R01-CA119371-01 (H.C.D.)]. We would also like to acknowledge Dr. Jiechao Ge for his help with some of the preparation for this work.
References
- 1.Popov AA, Dunsch L. J Am Chem Soc. 2007;129:11835–11849. doi: 10.1021/ja073809l. [DOI] [PubMed] [Google Scholar]
- 2.Yang S, Dunsch L. Angew Chem Int Ed. 2006;45:1299–1302. doi: 10.1002/anie.200502417. [DOI] [PubMed] [Google Scholar]
- 3.Achiba Y, Kikuchi K, Aihara Y, Wakabayashi Miyake Y, Kainosho M. Science and Technology of Fullerene Matrials. In: Bernier P, Bethune DS, Chiang LY, Ebbesen TW, Metzger RM, Mintmire JW, editors. MRS Symposia Proceedings No. 359. 1995. pp. 3–9. [Google Scholar]
- 4.Miyake Y, Minami T, Kikuchi K, Kainosho M, Achiba Y. Mol Cryst Liq Cryst. 2000;340:553–558. [Google Scholar]
- 5.Watanabe M, Ishimaru D, Mizorogi N, Kiuchi M, Aihara JI. Theochem. 2005;726:11–16. [Google Scholar]
- 6.Sun GY. Chem Phys Lett. 2003;367:26–33. [Google Scholar]
- 7.Troyanova S, Tamm NB. Chem Comm. 2009:6035–6037. doi: 10.1039/b912839e. [DOI] [PubMed] [Google Scholar]
- 8.Fatouros PP, Corwin FD, Chen ZJ, Broaddus WC, Tatum JL, Kettenmann B, Ge ZX, Gibson HW, Russ JL, Leonard AP, Duchamp JC, Dorn HC. Radiology. 2006;240:756–764. doi: 10.1148/radiol.2403051341. [DOI] [PubMed] [Google Scholar]
- 9.Ross RB, Cardona CM, Guldi DM, Sankaranarayanan SG, Reese MO, Kopidakis N, Peet J, Walker B, Bazan GC, Van Keuren E, Holloway BC, Drees M. Nature Mater. 2009;8:208–212. doi: 10.1038/nmat2379. [DOI] [PubMed] [Google Scholar]
- 10.Chaur MN, Melin F, Elliott B, Kumbhar A, Athans AJ, Echegoyen L. Chem Eur J. 2008;14:4594–4599. doi: 10.1002/chem.200800044. [DOI] [PubMed] [Google Scholar]
- 11.Melin F, Chaur MN, Engmann S, Elliott B, Kumbhar A, Athans AJ, Echegoyen L. Angew Chem Int Ed. 2007;46:9032–9035. doi: 10.1002/anie.200703489. [DOI] [PubMed] [Google Scholar]
- 12.Zuo TM, Beavers CM, Duchamp JC, Campbell A, Dorn HC, Olmstead MM, Balch AL. J Am Chem Soc. 2007;129:2035–2043. doi: 10.1021/ja066437+. [DOI] [PubMed] [Google Scholar]
- 13.Yang SF, Dunsch L. J Phys Chem B. 2005;109:12320–12328. doi: 10.1021/jp051597d. [DOI] [PubMed] [Google Scholar]
- 14.Fu WJ, Xu LS, Azurmendi H, Ge JC, Fuhrer T, Zuo TM, Reid J, Shu CY, Harich K, Dorn HC. J Am Chem Soc. 2009;131:11762–11769. doi: 10.1021/ja902286v. [DOI] [PubMed] [Google Scholar]
- 15.Fowler PW, Manolopoulos DE. An Atlas of Fullerenes. Clarendon Press; Oxford: 1995. [Google Scholar]
- 16.Cardona CM, Elliott B, Echegoyen L. J Am Chem Soc. 2006;128:6480–6485. doi: 10.1021/ja061035n. [DOI] [PubMed] [Google Scholar]
- 17.Charu MN, Melin F, Elliott B, Athans AJ, Walker K, Holloway BC, Echegoyen L. J Am Chem Soc. 2007;129:14826–14829. doi: 10.1021/ja075930y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.