Abstract
Background
Exercise has been shown to improve many health outcomes and well-being of people of all ages. Long-term studies in older adults are needed to confirm disability and survival benefits of exercise.
Methods
Annual self-administered questionnaires were sent to 538 members of a nationwide running club and 423 healthy controls from Northern California aged 50 years and older beginning in 1984. Data included running and exercise frequency, body mass index (BMI), and disability assessed by the Health Assessment Questionnaire Disability Index (HAQ-DI scored 0–3) through 2005. 284 runners and 156 controls completed 21-years of follow-up. Causes of death through 2003 were ascertained using the National Death Index. Multivariable regression techniques compared groups on disability and mortality.
Results
At baseline, runners were younger, leaner, and less likely to smoke than controls. HAQ-DI was higher for controls than runners at all time points and increased with age in both groups, but to a lesser degree in runners (0.17, SD 0.34) than controls (0.36, SD 0.55, p<0.001). Multivariable analyses showed that runners had significantly lower risk of HAQ-DI=0.5 (HR 0.62, 95% CI 0.46–0.84). At 19 years, 15% of runners had died compared to 34% of controls. After adjustment for covariates, runners demonstrated a survival benefit (HR 0.61, 95% CI 0.45–0.82). Disability and survival curves continued to diverge between groups after 21-years of follow-up as participants approached their ninth decade of life.
Conclusions
Vigorous exercise (running) at middle and older ages is associated with reduced disability in later life and a notable survival advantage.
Keywords: exercise, disability, aging, mortality, running, longitudinal study
Introduction
Age-adjusted death rates have reached record lows and life expectancy has reached record highs in recent years [1], likely due to a combination of behavior and societal changes as well as improved medical and surgical therapies. With the rise in life expectancy, it becomes necessary to focus on improving the quality of life and functional abilities as people reach older ages. Regular exercise, including running, may contribute to improved health among older adults. [2–7].
The Compression of Morbidity hypothesis posits that preventive lifestyle behaviors, including regular exercise, will postpone disability by at least as much as mortality, thus compressing morbidity between a later onset and the age at death [8,9]. Evaluation of this hypothesis requires that cohorts of subjects be followed over long periods of time in order to compare cumulative disability and mortality between groups. We have previously reported 8- [10] and 13-year [11] results of a longitudinal study comparing disability and mortality outcomes between cohorts of runners and control subjects initially aged 50 to 72 years suggesting that reduction of disability among runners continued to increase over time. In this study, we report the outcomes of disability and mortality of this cohort after 21 years.
Methods
Study Subjects
Subjects were recruited in January 1984 to participate in a longitudinal study of the effect of long-distance running on health outcomes. Runners 50 years and older were enrolled from a nationwide running club, the 50+ Runners Association, Control subjects were recruited from the roster of the Stanford University Lipid Research Clinics Prevalence Study (LRC) [12], that identified all permanent university staff and faculty between ages 26 and 70 years. This group was chosen to provide a sample of subjects with demographic characteristics similar to those of the running club. Details regarding the development of the cohort have been described elsewhere [13–15]. Briefly, study descriptors were sent to all 1,311 runners club and 2181 Stanford LRC participants in January 1984. Of these, 654 members of the Runners Club (runners) and 568 LRC participants (controls) expressed interest in the study, met eligibility requirements (age ≥ 50 years, high school graduate, and English as primary language), and were sent consent forms and questionnaires.
Five hundred thirty eight runners and 423 controls returned completed consent documents and questionnaires indicating agreement to participate. Subjects were not excluded based upon lipid levels. Participants completed annual self-administered questionnaires containing information on demographic variables, medical history, exercise habits (running and other vigorous exercise including biking, aerobic dance, and swimming), and the Health Assessment Questionnaire Disability Index (HAQ-DI) [13,16,17]. Validation studies in a subset of runners and controls, performed at the baseline visit, showed excellent correlation between self-reported data and that obtained by physicians or trained observers [13]. LRC subjects who reported regular vigorous exercise, including running, were retained as controls so that the group represented an average of exercise habits in the community. Baseline variables and disability of subjects who withdrew from the study were compared to those who completed 21 years of follow-up. All subjects provided informed consent prior to participation.
Group assignment by running history
The primary longitudinal analysis focused on the 284 runners and 156 controls who continued to participate in 2005. However, to control for potential self selection bias based on group membership rather than running status and to include those who began to run but discontinued after a period of time, we created groups of Ever Runners and Never Runners based on the following question, “ Have you ever run for exercise for a period of greater than 1 month?” Here, the Ever Runners group includes subjects who currently run regularly but are not necessarily members of the Runners Club or those who may have run in the past but discontinued before study onset. This procedure shifted 143 participants (73 participating in 2005) from the control group to the Ever Runners group. Over time, all groups decreased running activity, but the ‘Runners’ groups continued to accumulate more vigorous activity minutes of all kinds per week.
Assessment of Outcomes
Disability
The HAQ-DI is a self-reported instrument assessing functional ability in 8 areas: rising, dressing and grooming, hygiene, eating, walking, reach, grip, and activities. Each area is scored from 0 (no difficulty) to 3 (unable to perform) accounting for the use of special aids or devices or the assistance of another person. The HAQ-DI score is the average scores of the 8 areas. It has been validated in numerous studies, is sensitive to change, and is widely used in observational studies and clinical trials [16,18].
Mortality
Mortality data was obtained for 100% of original participants from annual searches of the National Death Index through 2003. Data on death was ascertained even if participants had previously withdrawn from the study. The principal cause of death was determined using the National Death Index Plus service, [19, 20] blinded to group status.
Statistical analyses
Progression of disability
Mean HAQ-DI scores over time were compared between runners and controls. Analyses by initial group assignment included all participants enrolled at study inception. Separate analyses were performed on the subset of runners and controls that completed the entire 21-years of observation. Differences between groups at each time point were compared using t-tests. Comparisons were considered statistically significant at p < 0.05.
The progression of disability (PD) over time for each group (analysis restricted to completers only) was expressed as a slope under the assumption that the rate of progression of disability is linear and constant over the time period of study. General linear mixed models were fitted to the data using compound symmetry as the correlation structure [21]. This assumes that the correlation between observations for a given participant is constant regardless of the distance between pairs of repeated measurements. PD for each group was adjusted for baseline HAQ-DI, age, gender, smoking, and BMI. PD was estimated as the difference between groups in the average time before a specified level of disability was attained. 95% confidence intervals were formed using 1000 bootstrap replications.
To identify the role of confounding variables upon the progression of disability, multivariable Cox proportional hazards models utilizing the subset of all initial participants (completers and non-completers) with an baseline HAQ-DI=0 and time-dependent covariates were developed. Endpoints of HAQ-DI=0.5 and 1.0 were chosen as clinically meaningful benchmarks of moderate and severe disability. All clinically relevant variables, including time-dependent BMI and weekly exercise (minutes/week), were included in univariable and multivariable analyses; final models were constructed of variables with statistical significance.
Survival analysis and cause of death
Survival analysis for runners compared to controls was performed using all 961 participants enrolled at study inception. Crude survival estimates were obtained using Kaplan-Meier methods. Cox proportional hazards regression models adjusted for baseline age, gender, BMI, smoking history, initial disability, and weekly aerobic exercise.
Causes of death were divided into five major causes: cardiovascular, malignancy, neurological, infectious, and other. Rates of death by cause were compared between the two groups.
Analyses were performed using SAS version 9.1. Institutional review board approval was obtained prior to initiating the study.
Results
Characteristics of study participants by group membership (runners vs. controls) are listed in Table 1. Values for all subjects at study inception are listed in the first columns. Baseline values (1984) for those subjects in each group who continued to participate through 2005 (completers) and for those who did not complete the follow-up period (non-completers) are listed in subsequent columns. The last columns list characteristics of the completers at 21-years of follow-up (2005). After 21-years of follow up, 284 runners and 156 controls remained in the study. Annual attrition rates among living subjects were approximately 3% for runners and 6% for controls. Differences between groups were observed both at baseline and at 21 years. Runners were younger, leaner, tended to be male, smoked less, and exercised more than controls. Mean education level and alcohol intake were statistically similar between the two groups. Both groups had little disability at the baseline, but runners had lower HAQ-DI scores and were more likely to have a HAQ-DI=0. Analysis of completers and non-completers showed that, among controls, completers tended to be younger (p<0.001), run more (p<0.001), have less baseline disability (p<0.05) and were more likely to have a baseline HAQ-DI score of 0 than non-completers. Among runners, the only statistically significant difference was the two-year age disparity between completers and non-completers (p<0.001).
Table 1.
Cohort Demographics (Runner’s Club vs. Community Controls)
| All subjects in 1984 | Non-Completers in 1984 | Completers in 1984 | Completers in 2005 | |||||
|---|---|---|---|---|---|---|---|---|
| Runners | Controls | Runners | Controls | Runners | Controls | Runners | Controls | |
| N | 538 | 423 | 254 | 267 | 284 | 156 | ||
| Age, years † | 58(5.6) *** | 62(7.2) | 59(6.4) +++ | 64(7.2)+++ | 57(4.4) *** | 59(5.8) | 78(4.4) *** | 80(5.8) |
| % Male | 84 *** | 56 | 87 | 57 | 81 *** | 56 | ||
| % of White | 97 | 96 | 95 | 97 | 98 | 95 | ||
| Education, years † | 16.6(2.5) | 16.6(2.7) | 16.5(2.6) | 16.5(2.8) | 16.6(2.5) | 16.8(2.4) | 16.6(2.5) | 16.8(2.4) |
| % Smokers | 1.9 *** | 9.5 | 2.8 | 9.0 | 1.1 *** | 10.3 | 0.7 | 1.3 |
| Body Mass Index (kg/m*2) † | 22.9(2.5) *** | 24.4(3.45) | 22.9(2.5) | 24.6(3.5) | 22.9(2.5) *** | 24.1(3.31) | 23.7(3.4) | 24.2(3.89) |
| Disability Index † | 0.029(0.10)*** | 0.095(0.18) | 0.043(0.12) | 0.112(0.18)+ | 0.022(0.07) *** | 0.068(0.16) | 0.20(0.35) *** | 0.43(0.57) |
| Running, minutes/week † | 237(144) *** | 15(49.4) | 234(141.2) | 9(38.0)+++ | 240(146.7) *** | 25.3(63.1) | 76.5(245) *** | 1.1(11.8) |
| Vigorous Exercise, min/week † | 311(196) *** | 87(123) | 315(207.7) | 79(124.7) | 307(185) *** | 100.1(117.8) | 287(398) *** | 138(189) |
| Alcoholic drinks per week † | 1.1(1.3) * | 1.3(1.5) | 1.2(1.5) | 1.4(1.6) | 1.1(1.2) | 1.3(1.2) | 0.86(1.2) | 0.97(1.1) |
| % with Disability Index of 0 | 86.6 *** | 61 | 84.7 | 54.3+++ | 88.4 *** | 72.4 | 62.3 *** | 46.2 |
mean (S.D)
p-value:
<0.05,
<0.01,
<0.001, comparing Runner’s club vs Controls
p-value:
<0.05,
<0.01,
<0.001, comparing the completers and non-completers in the Runner’s club and Controls
Similar findings were observed when participants were divided into ever-runner and never-runner groups (Table 2).
Table 2.
Cohort Demographics (Ever vs. Never Runners)
| All subjects in 1984 | Non-Completers in 1984 | Completers in 1984 | Completers in 2005 | |||||
|---|---|---|---|---|---|---|---|---|
| Ever Runners | Never Runners | Ever Runners | Never Runners | Ever Runners | Never Runners | Ever Runners | Never Runners | |
| N | 681 | 280 | 324 | 197 | 357 | 83 | ||
| Age, years † | 58.4(5.9)*** | 63.1(7.0) | 60(6.7) +++ | 64(7.0)+++ | 56.9(4.6) *** | 60.0(6.1) | 77.9(4.6) *** | 81.0(6.1) |
| % Male | 83 *** | 45 | 86 | 48 | 80 *** | 40 | ||
| % of White | 97 | 96 | 96 | 95 | 97 | 96 | ||
| Education, years † | 16.8(2.5)*** | 16.2(2.7) | 16.8(2.6) | 16.1(2.8) | 16.8(2.4) * | 16.2(2.3) | 16.8(2.4) * | 16.2(2.3) |
| % Smokers | 2.1 *** | 12.9 | 2.5 | 11.7 | 1.7 *** | 15.7 | 0.8 | 1.2 |
| Body Mass Index (kg/m*2) † | 23.1(2.7)*** | 24.5(3.6) | 23.2(2.7) | 24.7(3.7) | 23.1(2.6) ** | 24.2(3.6) | 23.9(3.4) | 23.9(4.2) |
| Disability Index † | 0.038(0.12)*** | 0.10(0.18) | 0.045(0.12) | 0.120(0.19)+ | 0.032(0.11)* | 0.065(0.13) | 0.23(0.41)*** | 0.51(0.57) |
| Running, minutes/week † | 195(155)*** | 3.34(25) | 188(154.2) | 4(29.0) | 201(156) *** | 1.9(8.1) | 61.4(220.7)*** | 0(0) |
| Vigorous Exercise, min/week † | 269.8(199.1)*** | 72(121) | 267(209.9) | 73.8(134.0) | 272(189) *** | 66.6(82.4) | 269(372) *** | 87(113) |
| Alcoholic drinks/week † | 1.2(1.3) | 1.3(1.4) | 1.2(1.4) | 1.4(1.6) | 1.2(1.2) | 1.0(1.1) | 0.9(1.2) | 0.7(0.9) |
| % with HAQ-DI= 0 | 82.2 *** | 58.6 | 77.5+ | 55.3+ | 86.6 ** | 66.3 | 61.6** | 34.9 |
mean (S.D)
p-value:
<0.05,
<0.01,
<0.001, comparing Ever Runners vs Never Runners
p-value:
<0.05,
<0.01,
<0.001, comparing the completers and non-completers in the Ever Runners and Never Runners
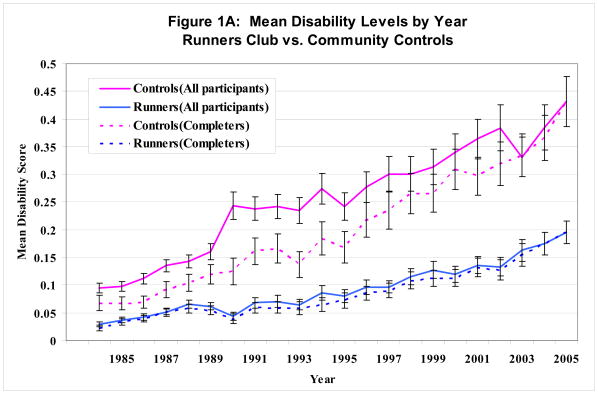
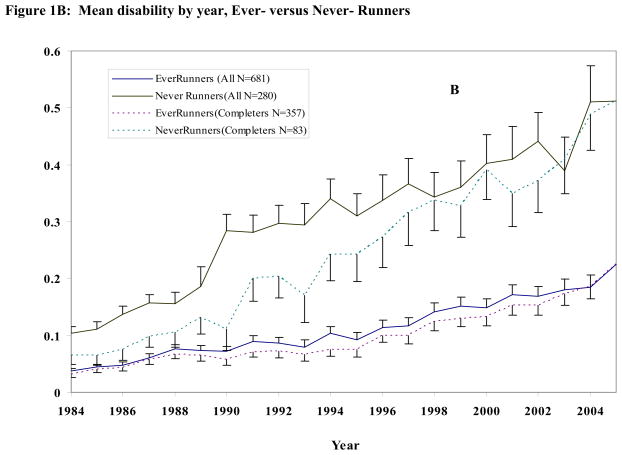
Figure 1 shows the progression of average disability by year.. Mean disability increased with time in all groups. Figure 1A shows mean annual disability levels for runners compared to controls; Figure 1B illustrates disability curves for Ever-Runner versus Never-Runner groups. Separate curves are shown for means computed using data available for all 961 initial study participants and for means computed for only those 440 subjects who completed 21-years of observation.
Figure 1. Mean disability levels by year.
(1A) Runners’ Club members versus Community Control subjects. Data from all initial participants (solid lines) and for those who continued participation through 2005 (dashed lines)
(1B) Ever-Runners versus Never-Runners. Data from all initial participants (solid lines) and for those who continued participation through 2005 (Dashed Lines)
Members of the running groups had significantly lower mean disability levels at all time points. Members of both running groups had nearly identical mean disability levels irrespective of completer status, indicating few differences in disability between completers and those who dropped-out or died.
In contrast, there were significant differences in disability levels between the inception cohort and completers among the control groups in both figures. Baseline disability levels in 1984 were statistically lower when computed for completers than when all initial study participants were included. This disability pattern among completers in the control groups continued at almost all time points, indicating differential drop out of the subjects with higher disability among the control groups, creating a potential bias towards lower disability in the observed control groups. However, even when restricting the cohort to completers, runners had significantly lower disability than controls and disability curves continued to diverge at 21 years of follow-up. Analyses by Ever- versus Never-runners showed comparable results.
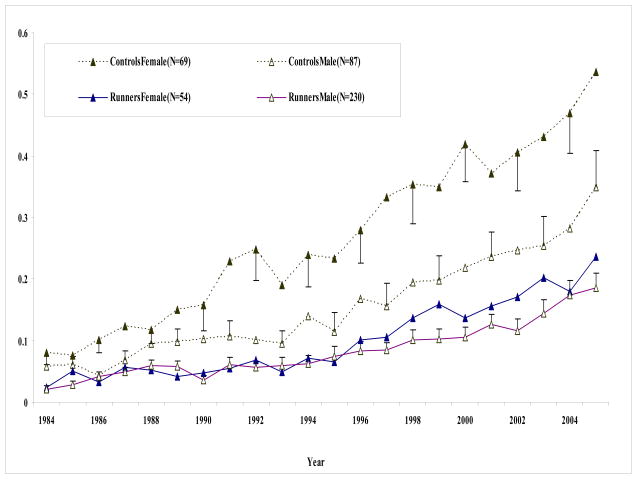
Average disability levels for completers in each group (runners and controls) are separated by gender in Figure 2. Both male and female runners maintained low disability levels at all time points, significantly lower than controls. The difference between runners and controls was most striking for females. Male controls had higher disability levels than male runners at all time points except the initial few years of the study. Few differences existed between male and female runners.
Figure 2.
Mean disability levels by year separated by gender. Mean and standard deviation for runners (solid lines) and controls (dashed lines) by year from 1984 through 2005. Male subjects are depicted with open triangles and females with solid triangles. Only subjects who completed the 21-years of follow-up are included.
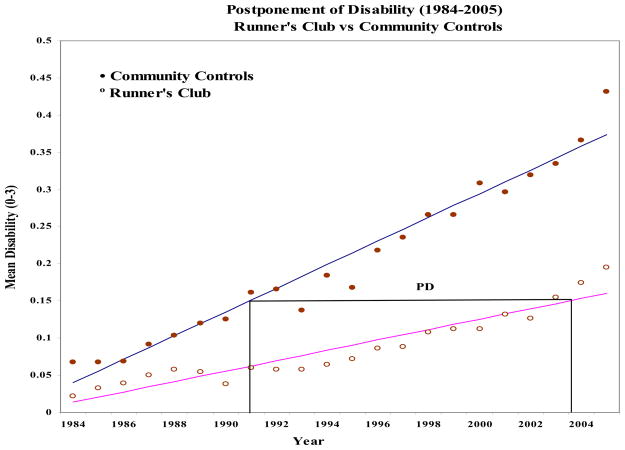
The rate of progression of disability over 21 years of observation using general linear mixed models is shown in Figure 3. The rate of progression of disability was significantly lower for runners (0.007 units/year) compared to controls (0.016 units/year, p<0.001).
Figure 3.
Progression of Disability. Linear mixed models of progression of disability and postponement of disability (PD). Regression lines are derived from linear mixed models and adjusted for the following covariates: age, sex, BMI, smoking, and initial disability level. The PD is defined as the absolute difference between the 2 groups in the time required to cross a given level of disability. Example shown is to reach HAQ-DI of 0.15.
The time required to reach specified levels of disability was significantly longer for runners than for controls. The mean time to reaching a HAQ-DI of 0.075 from study onset was approximately 2.6 years for controls, and 8.7 years for runners yielding a difference of approximately 6.2 years (95%CI: 3.9–8.9). Similarly, the time to reach a HAQ-DI of 0.10 was 8.2 years later (95% CI: 5.1–11.7) and to reach a HAQ-DI of 0.15 was 12.1 years later (95% CI: 8.1–18.3) for runners. These data illustrate that the slower rate of progression of disability continued to increase over time among runners through at least the 21 years of observation.
Results of multivariable Cox regression analyses using time-dependent covariates are shown in Table 3. The final model for disability outcomes (HAQ-DI=0.5 and HAQ-DI=1.0) included the following variables: group membership, age (year), gender, BMI (lagged by 1 year), and weekly vigorous exercise minutes (lagged by 1 year). These analyses were restricted to participants (completers and non-completers) with a baseline HAQ-DI=0. For the outcome of HAQ-DI=0.5, runners had a hazard ratio of 0.62 (95%CI 0.46–0.84) compared to controls. Analysis of covariates showed that greater BMI within 1 year was associated with an increased hazard (HR 1.09, 95%CI 1.05–1.13), as was age (HR 1.07, 95% CI 1.05–1.09), but male sex was associated with decreased risk (HR 0.63, 95% CI 0.47–0.85). Weekly vigorous exercise from all activities was marginally significant (HR 0.96, 95% CI 0.91–1.00, p=0.05). Nearly identical results were obtained for the outcome of HAQ-DI of 1.0.
Table 3.
Multivariable Cox regression analyses for disability and mortality
| Variable | HAQ-DI=0.50* | HAQ-DI=1.0* | Death† |
|---|---|---|---|
| Runner | 0.62 (0.46–0.84) | 0.66 (0.41–1.08) | 0.61 (0.45–0.82) |
| Age (year) | 1.07 (1.05–1.09) | 1.10 (1.06–1.14) | 1.12 (1.10–1.14) |
| Male | 0.63 (0.4700.85) | 0.63 (0.40–1.01) | 1.52 (1.12–2.07) |
| BMI | 1.09 (1.05–1.13) | 1.10 (1.04–1.17) | Not significant |
| Baseline HAQ (0.1 units) | N/A | N/A | 1.16 (1.07–1.25) |
| Exercise (minutes/week) | 0.96 (0.91–1.00) | 0.92 (0.85–1.00) | Not significant |
Analysis restricted to participants with baseline HAQ-DI=0. Variables included in the final model include group (runners vs controls), age (year), gender, BMI (lagged 1 year), and vigorous exercise (lagged 1 year).
Analysis includes all participants at study inception. Final model includes group, age (year), gender, baseline HAQ-DI (0.1 units). BMI, smoking, and exercise did not meet statistical significance to be included in the final model.
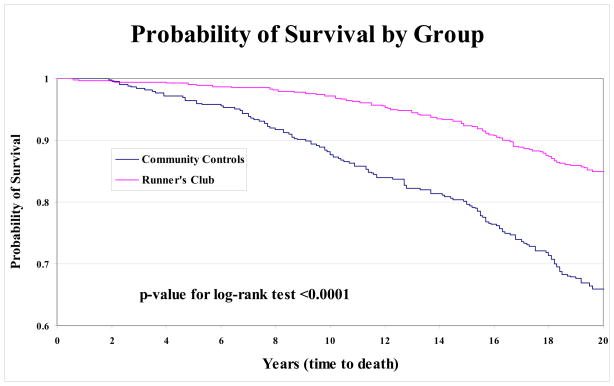
By the end of 2003, 81 (15%) of the runners and 144 (34%) of controls had died. The Kaplan-Meier plot of survival estimates for each group (Figure 4) shows that runners had a significant reduction in early mortality that was maintained, or increased, over the study period (p<0.001).
Figure 4.
Kaplan-Meier unadjusted survival curves for all cause mortality in Runners Club members and Community Controls from study onset through 19 years of follow-up. All 941 subjects at study inception are included. The difference between groups remained significant (p<0.001 by log rank test).
Multivarible Cox proportional hazard models generated to adjust for other variables at the baseline that were associated with survival (Table 3) found that runners continued to demonstrate a significant survival advantage (HR 0.61, 95% CI 0.45–0.82). As expected, older age (HR 1.12, 95% CI 1.10–1.14), male gender (HR 1.52, 95% CI 1.12–2.07), and initial HAQ-DI level (HR 1.16, 95% CI 1.07–1.25) were associated with increased hazard for mortality. BMI, smoking, and baseline exercise did not meet sufficient significance to be included in the final model.
Causes of death are summarized in Table 4. A total of 225 deaths (23% of all study participants) were seen over 17,201 person-years of observation. Rates of death were increased in controls compared to runners not only for cardiovascular outcomes as anticipated, but also for nearly all identified causes.
Table 4.
Causes of Death since study inception (1984)
| Runners Club Members | Community Controls | Rate Ratio | ||||
|---|---|---|---|---|---|---|
| Cause of Death (N) | # Deaths | Rate* | # Deaths | Rate* | Controls/Runners | p value |
| Total (225) | 81 | 810 | 144 | 1,999 | 2.5 | <0.001 |
| Cardiovascular (72) | 29 | 290 | 43 | 597 | 2.1 | 0.001 |
| Coronary Artery Disease/MI (38) | 14 | 140 | 25 | 347 | 2.5 | 0.003 |
| Stroke (10) | 3 | 30 | 7 | 97 | 3.2 | 0.043 |
| Congestive heart failure (4) | 2 | 20 | 2 | 28 | 1.4 | 0.38 |
| Cancer (n=71) | 30 | 300 | 41 | 569 | 1.9 | 0.004 |
| Prostate (7)† | 4 | 40 | 3 | 42 | 1 | 0.28 |
| Lung (14) | 5 | 50 | 9 | 125 | 2.5 | 0.051 |
| Colon (10) | 4 | 40 | 6 | 83 | 2.1 | 0.13 |
| Breast (4)‡ | 1 | 10 | 3 | 42 | 4.2 | 0.38 |
| Hematologic (11) | 6 | 60 | 5 | 69 | 1.2 | 0.41 |
| Esophageal (3) | 1 | 10 | 2 | 28 | 2.8 | 0.23 |
| Pancreas (3) | 1 | 10 | 2 | 28 | 2.8 | 0.23 |
| Other (19) | 8 | 80 | 11 | 153 | 2 | 0.085 |
| Neurological (20) | 6 | 60 | 14 | 194 | 3.2 | 0.007 |
| Infections (16) | 1 | 10 | 15 | 208 | 20.8 | <0.001 |
| Pneumonia (9) | 0 | 0 | 9 | - | - | - |
| Other (39) | 11 | 110 | 28 | 389 | 3.5 | <0.001 |
| Unknown (7) | 4 | 40 | 3 | 42 | 1 | 0.47 |
Expressed per 100,000 person-years
Male subjects only
Female subjects only
Discussion
This study demonstrates that participation in long-term running and other vigorous exercise among older adults is associated with less disability and lower mortality over two decades of follow-up. We prospectively followed a cohort of healthy adults from a mean age of 59 years in 1984 to 78 in 2005. Not only were average disability levels lower among runners at all time points, but the rate of progression of disability strongly favored runners throughout the study. Results were similar when all participants or completers were analyzed. At the baseline, both groups had negligible disability; however, after 21 years, runners had a mean HAQ-DI score of nearly 0.2, equivalent to having mild functional disability in 1–2 of 8 areas of daily activity. In contrast, mean HAQ-DI of controls at 21 years approached 0.5, equivalent to moderate functional disability in 2of 8 areas or complete inability to perform in at least 1 area of daily functioning. Although these levels are less than what is seen in subjects with chronic musculoskeletal diseases (mean HAQ-DI in rheumatoid arthritis is 0.70 to 1.0 [22,23] and osteoarthritis 0.80 [24]), the higher levels among controls translate into important differences in overall daily functional limitations [25].
In addition to confirming an overall survival advantage and reduction in cardiovascular related deaths among persons who participate in regular exercise, we also found reduced rate of deaths from other causes including malignancies and neurologic disorders. This is consistent with reports associating regular exercise with reduced incidence of dementia [3], and several cancer types [26–28]. Potential reasons for improved functional status and survival among regular exercisers may include increased cardiovascular fitness and improved aerobic capacity and organ reserve [29–31], increases in skeletal mass and metabolic adaptations of muscle with decreased frailty[29–31], lower levels of circulating inflammatory markers [32], improved response to vaccinations [33], and improved higher-order cognitive functions [34].
This study follows our report of disability and mortality in this cohort after 13 years, showing significantly better outcomes for runners compared to controls [11]. We had anticipated that, with an additional eight years of observation encompassing an additional 132 deaths among participants (93 deaths were reported after 13 years), we would begin to see convergence of disability and survival curves; however this was not the case. Differences between runner and controls for all outcomes continued to diverge after 21 years of follow-up. Interestingly, in our analysis of 21-years of data, aerobic exercise was no longer a statistically significant independent predictor of mortality. Sixty percent of deaths occurred during the eight year period between our last report and the current analysis, and it is possible that with this additional mortality data, that vigorous exercise has become more collinear with running and no longer is identified as an independent predictor of death. Further observation of this cohort, as the remaining 440 participants reach the biologic limits of life expectancy, may be required to further clarify the independent role of non-running, vigorous exercise.
There are limitations in this study that are important to consider. Self-selection bias is always a concern in observational study designs lacking random assignment of external interventions. Unmeasured lifestyle variables, including eating habits and utilization of routine preventive medical care may have influenced results. However, great care was taken to minimize possible selection biases. The control group was selected from a larger group of relatively healthy adults who worked in a university community in 1972 and were socio-economically similar to the Runners’ Club members. Analyses included statistical adjustments made for covariates that differed between the groups at study onset: age, gender, BMI, smoking, weekly exercise, and initial disability.
To control for potential self-selection bias based on group membership rather than running status, we conservatively created groups of Ever- and Never-Runners to account for people who discontinued running prior to study inception due to injury, disability, or other reasons. Results of analyses comparing these groups did not differ appreciably from those obtained by analysis of groups based on club membership.
Drop-out rates are always a concern in longitudinal studies. Overall, 60% of initial study participants who were still alive at the 21 year assessment continued to participate; 62% of surviving runners compared to 54% of surviving controls. Given that observation spanned two decades, the proportion that continued to participate and was not lost to follow-up is quite good and the absolute rate of discontinuation is similar in both groups. However, there were greater differences in baseline characteristics between completers and non-completers among control subjects. Control completers tended to be younger, have lower initial HAQ-DI, and exercise more than did the controls who died or withdrew from study participation: the most severely disabled among controls preferentially discontinued from the study. In contrast, there was little difference between runners who did and did not complete the study. If anything, healthier controls preferentially remained in the study, likely biasing the results conservatively and underestimating differences between runners and controls.
Because we had complete data on mortality for all subjects, analysis of mortality rates and causes of death were not subject to biases raised by selective discontinuation from study participation. Analyses of mortality closely mirrored those seen for disability, with a clear survival advantage associated with physical exercise remaining after adjustment for relevant confounders.
With the exception of death, outcome variables in the study were obtained by self-report. The HAQ-DI has been validated in multiple independent studies using varied populations of healthy subjects as well as those with arthritis and other chronic conditions with excellent reliability [16,17]. Validation of self-report variables against observed performance at cohort inception demonstrated excellent correlation for both groups [13].
Because our cohorts of runners and controls were relatively homogeneous at baseline (the majority were Caucasian, completed college education, had BMI within normal limits, low alcohol and tobacco consumption) it is possible that these results may not be generalizable to a broader range of persons with different ethnic backgrounds, educational opportunities, access to preventive health care, or lifestyle habits. Alternatively, study of ‘privileged’ persons has the advantage of lessening concern over poverty, insurance status, education, access to heath care, and other social variables which might otherwise be different across groups and constitute a significant bias.
Our findings of decreased disability in addition to prolonged survival among middle-aged and older adults participating in routine physical activities further support recommendations to encourage moderate to vigorous physical activity at all ages. Increasing healthy lifestyle behaviors may not only improve quantity and quality of life, but may hopefully lead to reduced health care expenditures associated with disability and chronic diseases [35].
This study was originally designed as a test of the Compression of Morbidity hypothesis [8,9] with the assumption that runners compared to controls would show a greater compression of disability into the remaining years of life. The present results are suggestive of such an effect. To date, only a quarter of participants have died, and although it remains possible that these trends may be different in the final three-fourths of ultimate decedents, it is unlikely given that mortality rates between groups are expected to converge entirely after age 100, and cumulative lifetime disability seems unlikely to ultimately favor controls given the huge differences favoring runners through age 80 with relatively few years of life remaining. We believe, therefore, that this study contributes to arguments supporting the Compression of Morbidity hypothesis.
Acknowledgments
Grant Support: This study was supported by grant AR43584 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging, National Institutes of Health, Bethesda, MD.
Footnotes
Financial conflicts: none
The authors have no competing conflicts of interest to declare.
References
- 1.Centers for Disease Control and Prevention (CDC) National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions--United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56:4–7. [PubMed] [Google Scholar]
- 2.Burke Gl, Arnold AM, Bild DE, Cushman M, Fried LP, Newman A, Nunn C, Robbins J. Factors associated with healthy aging: the cardiovascular health study. J Am Geriatr Soc. 2001;49:254–62. doi: 10.1046/j.1532-5415.2001.4930254.x. [DOI] [PubMed] [Google Scholar]
- 3.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 4.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 5.Bijnen FC, Feskens EJ, Caspersen CJ, Nagelkerke N, Mostwerd WL, Kromhout D. Baseline and previous physical activity in relation to mortality in elderly men: the Zutphen Elderly Study. Am J Epidemiol. 1999;150:1289–96. doi: 10.1093/oxfordjournals.aje.a009960. [DOI] [PubMed] [Google Scholar]
- 6.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 7.Lee IM, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity. Am J Epidemiol. 2000;151:293–9. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 8.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–5. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 9.Fries JF. Successful aging—an emerging paradigm of gerontology. Clin Geriatr Med. 2002;18:371–82. doi: 10.1016/s0749-0690(02)00021-6. [DOI] [PubMed] [Google Scholar]
- 10.Fries JF, Singh G, Morfeld D, Hubert HB, Lane NE, Brown BW., Jr Running and the development of disability with age. Ann Intern Med. 1994;121:502–9. doi: 10.7326/0003-4819-121-7-199410010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Wang BWE, Ramey DR, Schettler JD, Hubert HB, Fries JF. Postponed development of disability in elderly runners. Arch Intern Med. 2002;162:2285–94. doi: 10.1001/archinte.162.20.2285. [DOI] [PubMed] [Google Scholar]
- 12.The LRC Program Epidemiology Committee. Plasma lipid distributions in selected North American populations: The Lipid Research Clinics Prevalence Study. Circulation. 1979;60:427–39. doi: 10.1161/01.cir.60.2.427. [DOI] [PubMed] [Google Scholar]
- 13.Lane NE, Bloch DA, Wood PD, Fries JF. Aging, long-distance running, and the development of musculoskeletal disability. Am J Med. 1987;82:772–80. doi: 10.1016/0002-9343(87)90014-3. [DOI] [PubMed] [Google Scholar]
- 14.Hubert HB, Fries JF. Predictors of physical disability after age 50: Six year longitudinal study in a runners club and university population. Ann Epidemiol. 1994;4:285–94. doi: 10.1016/1047-2797(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 15.Ward MM, Hubert HB, Shi H, Bloch DA. Physical disability in older runners: prevalence, risk factors, and progression with age. J Gerontol. 1995;50A:M70–7. doi: 10.1093/gerona/50a.2.m70. [DOI] [PubMed] [Google Scholar]
- 16.Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ) Clin Exp Rheumatol. 2005;23 (Suppl 39):S14–8. [PubMed] [Google Scholar]
- 17.Davis HS, MacPherson K, Merry HR, Wentzel C, Rockwood K. Reliability and validity of questions about exercise in the Canadian study of health and aging. Int Psychogeriatr. 2001;13 (Suppl 1):177–82. doi: 10.1017/s1041610202008128. [DOI] [PubMed] [Google Scholar]
- 18.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9:789–93. [PubMed] [Google Scholar]
- 19.Doody MM, Hayes HM, Bilgrad R. Comparability of National Death Index Plus and standard procedures for determining causes of death in epidemiologic studies. Ann Epidemiol. 2001;11:46–50. doi: 10.1016/s1047-2797(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 20.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major U.S. mortality databases. Ann Epidemiol. 2002;12:462–8. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 21.Lindsom ML, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46:673–87. [PubMed] [Google Scholar]
- 22.Krishnan E, Tugwell P, Fries JF. Percentile benchmarks in patients with rheumatoid arthritis: Health Assessment Questionnaire as a quality indicator (QI) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Art Sokka T, Kautiainen H, Hannonen P, Pincus T. Changes in Health Assessment Questionnaire Disability scores over five years in patients with rheumatoid arthritis compared with the general population. Arthritis Rheum. 2006;54:3113–8. doi: 10.1002/art.22130. [DOI] [PubMed] [Google Scholar]
- 24.Bruce B, Fries J. Longitudinal comparison of the Health Assessment Questionnaire (HAQ) and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Arthritis Rheum. 2004;51:730–7. doi: 10.1002/art.20695. [DOI] [PubMed] [Google Scholar]
- 25.Tas U, Verhagen AP, Bierma-Zeinstra SMA, Hofman A, Odding E, Pols HAP, Koes BW. Incidence and risk factors of disability in the elderly: the Rotterdam Study. Preventive Med. 2007;44:272–8. doi: 10.1016/j.ypmed.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Schnohr P, Gronbaek M, Petersen L, Hein HO, Sorensen TI. Physical activity in leisure-time and risk of cancer: a 14-year follow-up of 28,000 Danish men and women. Scand J Public Health. 2005;33:244–9. doi: 10.1080/14034940510005752. [DOI] [PubMed] [Google Scholar]
- 27.Samad AKA, Taylor RS, Marshall T, Chapman AS. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7:204–13. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 28.Tardon A, Lee WJ, Delgado-Rodriguez M, Dosemeci M, Albanes D, Hoover R, Blair A. Leisure-time physical activity and lung cancer: a meta-analysis. Cancer Causes Control. 2005;16:389–97. doi: 10.1007/s10552-004-5026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Krichevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TH. Physical Activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging, and Body Composition study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 30.Beere PA, Russell SD, Morey MC, Kitzman DW, Higgenbothan MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–94. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 31.Talbot LA, Morrell CH, Metter EJ, Fleg JL. Comparison of cardiorespiratory fitness versus leisure time physical activity as predictors of coronary efents in men aged < or = 65 years and >65 years. Am J Cardiol. 2002;89:1187–92. doi: 10.1016/s0002-9149(02)02302-0. [DOI] [PubMed] [Google Scholar]
- 32.Ades PA, Waldmann ML, Meyer WL, Brown KA, Poehlman ET, Pendlebury WW, Leslie KO, Gray PR, Lew RR, LeWinter MM. Skeletal muscle and cardiovascular adaptations to exercise conditions in older coronary patients. Circulation. 1996;94:323–330. doi: 10.1161/01.cir.94.3.323. [DOI] [PubMed] [Google Scholar]
- 33.Kohut ML, Arntson BA, Lee W, Rozeboom K, Yoon KJ, Cunnick JE, McElhaney J. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004;22:2298–2306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Dustman RE, Ruhling RO, Russell EM, Sherer DE, Bonekat HW, Shigeoka JW, Wood JS, Bradford DC. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiol Aging. 1984;5:35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 35.Fries JF, Koop CE, Sokolov J, Beadle CE, Wright D. Beyond heath promotion: reducing need and demand for medical care. Health Aff (Millwood) 1998;17:70–84. doi: 10.1377/hlthaff.17.2.70. [DOI] [PubMed] [Google Scholar]