Abstract
Objective. To describe the integration of science of safety (SoS) topics in doctor of pharmacy (PharmD) curricula of US colleges and schools of pharmacy.
Methods. A questionnaire that contained items pertaining to what and how SoS topics are taught in PharmD curricula was e-mailed to representatives at 107 US colleges and schools of pharmacy.
Results. The majority of the colleges and schools responding indicated that they had integrated SoS topics into their curriculum, however, some gaps (eg, teaching students about communicating risk, Food and Drug Administration [FDA] Sentinel Initiative, utilizing patient databases) were identified that need to be addressed.
Conclusions. The FDA and the American Association of Colleges of Pharmacy (AACP) should continue to collaborate to develop resources needed to ensure that topics proposed by the FDA in their SoS framework are taught at all colleges and schools of pharmacy.
Keywords: medication safety, pharmacy education, curriculum, science of safety
INTRODUCTION
The Food and Drug Administration Amendments (FDAA) Act of 2007 gave the FDA more authority over the regulation of medication safety, including the authority to require pharmaceutical companies to conduct postmarketing studies and trials, to make safety-related labeling changes, and to develop risk evaluation and mitigation strategies.1 The Act directed the FDA to develop a systematic plan to better manage risks vs. benefits of drugs as they progress through their lifecycles, with an explicit focus on post-approval safety. Thus, advancing the SoS has become an important part of the FDA's role.
The FDA describes the SoS as an emerging discipline that seeks to understand and prevent adverse events.2 It combines the growing understanding of disease and its molecular origins (including understanding of adverse events resulting from treatment) with new scientific methods of signal detection, data mining, and analysis. Using these tools, researchers can generate hypotheses about, confirm the existence of, and identify causal factors for drug and device safety problems in patient populations. The FDA's lifecycle approach to product development and evaluation, where drug and device safety is examined from premarketing to postmarketing, helps to ensure that safety signals generated at any point in the process can be evaluated along with relevant benefit-risk data to inform treatment choices and regulatory decision making.
In 2008, the FDA announced its Safe Use Initiative.3 One of the goals of the initiative is to broaden the FDA's postmarketing mission by placing a new emphasis on partnering with health care providers and medical, pharmacy, and nursing associations to promote the safe use of drugs. The development of a SoS curriculum directed at healthcare professional students and implemented across health professional schools in the United States is expected to better prepare new practitioners to actively contribute to improving the safe use of medical products.4
The expectations that the public and elected government officials have come to have of the FDA over the last decade can be considered both a challenge and an opportunity. The agency has attempted to address these expectations in the FDA Strategic Plan, which consists of 4 main goals.5 The roles pharmacists might play in each of these goals are provided.
Strengthen the FDA for today and tomorrow. Pharmacy professionals and researchers can collaborate with the FDA by participating in programs such as the Sentinel Initiative, Critical Path Initiative, and Adverse Event Reporting System.
Improve patient and consumer safety. Pharmacy faculty members and pharmacists can develop the science of evaluation of best methods for information transmittal and development of tools for communicating risk associated with medications, devices, and biologics.
Increase access to new medical and food products. Pharmacists can facilitate access to new, safe, and effective medical products by improving the monitoring, reporting, and management of high-risk medications.
Improve the quality and safety of manufactured products and the supply chain. Pharmacists can help detect and report sentinel events because of their unique accessibility in the community and their training (eg, knowledge of pharmacoepidemiology, familiarity with adverse event reporting programs, effective communication skills).
Because pharmacists play an important role in safety-related issues, the FDA seeks to more fully comprehend the depth and coverage of SoS topics in pharmacy school curricula. To accomplish this, the FDA joined with the American Association of Colleges of Pharmacy (AACP) and funded a multiphase study to identify ways it could partner with pharmacy professionals in improving medication safety.4,6,7 The purpose of the study phase reported in this paper was to provide baseline information on course content and teaching methods that integrate SoS topics in the educational curricula of professional degree programs at US colleges and schools of pharmacy. A secondary objective was to explore whether there were relationships between college/schools’ characteristics and SoS curricula offerings. This assessment provides a platform for continued collaboration between AACP and the FDA.
METHODS
Development of the questionnaire was informed by an extensive review of the relevant literature and key informant interviews from an earlier phase of the entire project.7 Input also was sought from representatives of AACP, the FDA, and the Health Resources and Services Administration's Pharmacy Services Support Center. AACP leaders and other recognized experts reviewed the questionnaire and provided feedback to enhance content validity.
The investigators defined the SoS as the “systematic study of the negative impact of drugs and devices on humans at all stages of the drug product life cycle.” This definition is based upon source documents from the Institute of Medicine and the FDA.2,8 The SoS refers to knowledge learned at any step of the product development and marketing process, including preclinical animal toxicology and safety studies, clinical studies in humans, safety studies needed for FDA approval, and postmarketing epidemiological research. Science of safety includes translational research that enables health care professionals and other individuals to better identify, understand, report, manage, and communicate the risk of drugs and devices in patient populations.
The questionnaire contained items that covered 3 content domains of the SoS: concepts of safety embedded in preclinical studies (eg, pharmacokinetics, toxicity, signaling of biomarkers for drug-induced problems), concepts of safety studied in different phases of clinical trials (eg, data required for establishing appropriate and safe use, data required for product labeling), and concepts of ongoing safety evaluation after drug approval (eg, risk mitigation strategies, the Sentinel Initiative). With respect to each of these domains, college or school representatives were asked whether the topics were taught in the curriculum and how the topics were taught (eg, didactic/experiential; interprofessional). The questionnaire included items about whether faculty members were experts in these areas, whether postgraduate training (eg, residencies, fellowships, graduate programs) included these topics, and whether students had achieved educational outcomes related to SoS topics. College or school representatives also were asked whether an FDA-developed curriculum for SoS topics was needed. Categorical response options were provided for all questions.
The questionnaire was pretested on 4 pharmacy educators to identify issues related to interpretation, ease of use, and administration time, and was modified based on their comments. The study methods and procedures were reviewed and approved by Institutional Review Boards at the University of Mississippi, Virginia Commonwealth University, and the University of Arizona.
A self-administered survey methodology was used for data collection. The sampling frame consisted of curriculum committee chairs or other designated members who were considered responsible for the curriculum at colleges and schools of pharmacy in the United States. Individuals were identified using the AACP curriculum chair list of full and associate AACP college/school members, which included contact information for a representative at each school. One hundred seven schools were represented on the list. An introductory e-mail that described the project was sent to each college/school representative along with an appended information sheet and questionnaire. The questionnaire was sent as an attachment rather than an online questionnaire in case the school representative needed to show the questions to other faculty members to complete the questionnaire. The college or school representative was encouraged to seek input from department chairs and/or curriculum committee members to help complete the questionnaire. The questionnaire could be returned via fax, e-mail, or regular mail. Nonrespondents were sent 2 additional e-mails as well as called once or twice to remind them to complete the questionnaire. Due to the difficulty in identifying the most appropriate person at each college or school, associate deans and/or other faculty members were contacted when necessary to encourage participation. AACP also made announcements about the questionnaire, such as including messages in the AACP eLert and sending messages to the curriculum chairs listserv and deans listserv. Data collection occurred from August 2009 through July 2010.
A quality control assessment was performed to ensure the data from the paper survey questionnaires were accurately entered into an electronic database, and responses were examined by the investigators for out-of-range values or inconsistencies. Impact of nonresponse bias was estimated by comparing responses of early respondents to that of late respondents. Statistical analyses were performed with SPSS 17.0 for Windows (SPSS, Inc Chicago, IL). Data were summarized using descriptive statistics (frequency counts and percentages). Relationships between SoS curriculum variables and college/school characteristics were evaluated using chi-square tests of association.
RESULTS
Of the 107 colleges and schools of pharmacy invited to participate in the study, 65 returned questionnaires, yielding a useable response rate of 60.7%. There were random missing data points and it was not possible to record some responses because of skipped questions or problems with legibility. Subsequent analyses were performed using available case methods. The average pharmacy student class size for the respondent schools was 120.7 ± 61.4. The percent of students with a bachelor's degree from the respondent colleges and schools was 49.4%, and the percent of students seeking postgraduate (ie, residencies, fellowships, graduate education) training was 20.7%. Of the respondents, 13.8% and 47.7% of the colleges and schools reported an affiliation with a Center for Education and Research on Therapeutics (CERT) and a medical center, respectively. The respondent schools had an average of 2.6 ± 1.2 departments.
No differences were found in the characteristics of the colleges and schools that responded early vs. late, or in their current teaching of SoS topics. No trends were identified in the type of college or school (eg, public/private, new/old) that responded or to what extent they taught SoS topics.
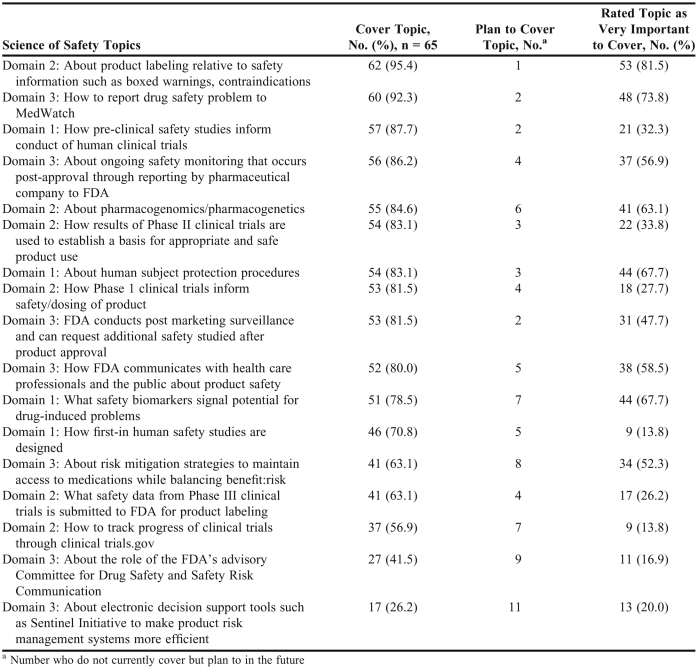
College and school representatives were asked to indicate whether each listed topic in the 3 SoS domains was currently covered in the curriculum. As mentioned before, the 3 domains were defined as domain 1: product safety from preclinical trials; domain 2: product safety from clinical trials; and domain 3: product safety after drug approval and marketing. As shown in Table 1, more than 75% of the responding colleges and schools covered 11 of the 17 topics for all 3 domains. However, less than 75% covered 6 of the topics, most of which were in domains 2 and 3 and related to how drug product safety is monitored and reported. Specifically, this finding suggested that new initiatives of the FDA, such as the Sentinel Initiative, have not been extensively integrated into the pharmacy curricula. Many respondents indicated their college/school is planning to cover these topics in the future.
Table 1.
Science of Safety Topics Covered in US Doctor of Pharmacy Curriculum
College and school representatives’ perceived importance of teaching each of the 17 topics varied substantially (Table 1). Eight topics were rated as “very important” by more than 50% of the respondents. Some topics included in the colleges’/schools’ curriculum were not considered “very important” by the college/school representative who completed the questionnaire.
Respondents also were asked to indicate whether an appropriate amount of time was spent on the topics in each domain at their college or school: 64.6% indicated that adequate time was spent on topics in domain 1; 66.2% indicated that adequate time was spent on topics in domain 2; and 63.1% indicated that adequate time was spent on topics in domain 3. No respondent indicated that too much time was spent on any specific topic. Conversely, 24%-31% of the respondents suggested more time should be spent on various topics. The majority of respondents believed that their college/school was devoting adequate time to SoS topics (Table 1).
The majority of SoS topics were taught in the required curriculum in the lecture-based part or in both the lecture-based/classroom and experiential parts. For domain 1, the majority of colleges and schools teaching the topics had integrated them into their required coursework, mainly though the classroom lecture portion of the curriculum. Some topics were also covered in the experiential part of the curriculum. For domains 2 and 3, most of the topics were covered in the required classroom lecture curriculum. Almost all of the topics in domain 3 are taught in the classroom lecture portion of the curriculum, but many are covered again during experiential training. For example, the majority of colleges and schools that taught “reporting problems to MedWatch” delivered this content through both lecture-based and experiential coursework.
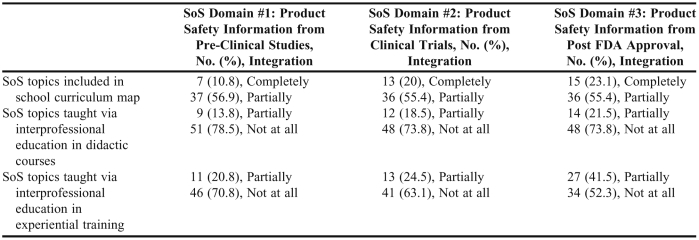
College and school representatives’ responses to whether the SoS topics for each domain were coordinated and integrated into the curriculum and whether they were taught interprofessionally are summarized in Table 2. While colleges and schools of pharmacy were aware of and attempting to incorporate these topics into their PharmD curriculum, few schools reported teaching SoS topics interprofessionally (Table 2).
Table 2.
Coordination and Integration of Science of Safety (SoS) Topics in US Colleges and Schools of Pharmacy (N = 65)
College and school representatives indicated that some SoS topics were taught in residency and fellowship training programs, graduate programs, and as continuing education (CE) programs (Table 3). Domain 3 topics, which often are covered in the experiential portions of the curriculum, also were covered in practice-related educational experiences such as residency programs, fellowship programs, and CE programs, providing pharmacy graduates with additional knowledge and skills pertaining to the SoS.
Table 3.
Science of Safety Topics Taught Outside Professional Pharmacy Program (n = 65)
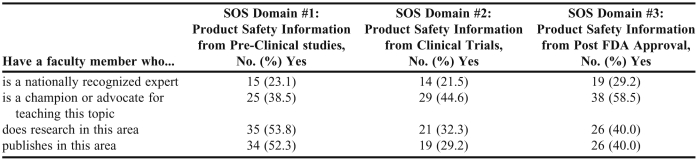
Responses as to whether colleges and schools had faculty members with expertise in the SoS domain areas, and whether there was a champion for teaching these topics at the school are provided in Table 4. While some colleges and schools reported having faculty members who were considered nationally recognized experts on these topics, more indicated that faculty members conduct research in these domains. However, expertise and curricular advocacy for some of the SoS topics may be lacking at some schools.
Table 4.
Science of Safety Expertise Within US Colleges and Schools of Pharmacy (n = 65)
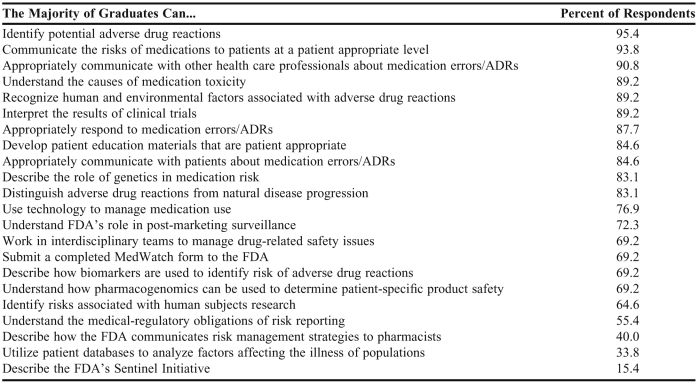
Fifty respondents indicated that their college or school always or often promoted a culture of safety, and 60 reported that their college or school always or often promoted the role of the pharmacist in minimizing risks associated with medication products. The questionnaire provided a list of 22 abilities (Table 5) related to the SoS that pharmacy students should be expected to possess upon graduation. Respondents were asked to indicate whether the majority of their graduates possessed each ability. The majority believed that their pharmacy graduates had acquired the abilities that related to product safety in individual patient care. For example, 95.4% of responding schools indicated that a majority of their graduates could identify potential adverse drug reactions, 93.8% indicated graduates could appropriately communicate the risks of medications to patients, 90.8% indicated graduates could communicate with other health care professionals about adverse drug reactions, 89.2% indicated graduates understood causes of medication toxicity, and 87.7% indicated graduates could appropriately respond to medication errors and adverse drug reactions. Most respondents (64% to 77%) indicated that a majority of their graduates could describe how biomarkers are used to identify risk for adverse drug reactions, use technology to manage medication use, and identify risks associated with human subjects research. However, less than 50% of the responding schools indicated that a majority of their graduates could achieve 3 of the listed abilities, 2 of which were specific to FDA-related tasks.
Table 5.
Colleges and Schools of Pharmacy's Perception of Science of Safety Student Outcomes (n = 65)
Thirteen (20%) respondents indicated that their colleges and schools were “very likely” to adopt an FDA-developed SoS curriculum; 44 were somewhat likely; and 7 were not likely to do so. The likelihood of colleges and schools adopting modules for individual domains differed based on domain, with the greatest interest related to adopting a module for the domain 3 SoS topics pertaining to product safety after FDA approval (57; 87.7%).
Though many cross tabulations were conducted, only a few relationships between colleges’ and schools’ characteristics and their SoS curriculum offerings were significant. Responding colleges and schools with a champion or advocate for domain 1 topics were more likely to rate how pre-clinical safety studies inform the conduct of human clinical trials as “very important” compared to those not having a champion or advocate (p = 0.042). Similar associations were observed for some of the topics listed in domains 2 and 3. For example, having a champion or advocate for domain 3 topics was significantly (p = 0.023) associated with respondents indicating topics such as postmarketing surveillance and reporting drug safety issues to MedWatch as “very important.” Whether the college or school has a champion also was significantly associated with the college's/school's teaching of certain topics. Colleges and schools with a champion or advocate to teach domain 3 topics were more likely to report that they covered how the FDA conducts postmarketing surveillance (p = 0.036), FDA's electronic decision support tools for efficient risk management system (p = 0.005), and how the FDA communicates with health care professionals about product safety (p = 0.004). Similarly, colleges and schools of pharmacy with a graduate program appeared to be more likely to offer certain SoS topics. Other school characteristics did not appear to have significant influence on the SoS topics taught.
DISCUSSION
This study provides important insight into how SoS topics are integrated into the pharmacy curriculum at 65 schools of pharmacy in the United States. Most respondents believed their college or school was devoting adequate time to SoS topics in general, and 11 of the 17 topics appear to be covered in the majority of responding colleges and schools. For the most part, the topics are being covered in the required, lecture/classroom-based portion of the curriculum. More emphasis on these topics, especially those in domain 1, may need to occur during experiential training; and interprofessional education opportunities to cover these topics should be explored.
Most respondents believed their college or school promoted the role of the pharmacist in minimizing risk associated with medication use and that the majority of their graduates are able to accomplish many of the educational abilities related to the SoS. A couple of responding schools had not graduated a class when they responded to the survey, which is a probable explanation for not receiving a 100% response rate on any of the abilities. A few opportunities for improvement in pharmacy education related to SoS topics were identified. There are a few topics (eg, using clinicaltrials.gov, using electronic decision support tools, and understanding the role of the FDA's Advisory Committee for Drug Safety and Safety Risk Communication) that were covered in less than half of the responding colleges and schools. Some of these topics are relatively new, and some pharmacy faculty members may not be aware or knowledgeable about them. Workshops and resources should be available to pharmacy faculty members for them to learn about new initiatives and programs so that the information can be passed on to students.
There were 3 education abilities related to domain 3 that were not being covered in the majority of respondents’ schools that deserve further attention. There is some room for improvement in teaching pharmacy students how to use patient databases, explain the FDA Sentinel Initiative, and describe the FDA's role in risk management and communication. The current Accreditation Council for Pharmacy Education (ACPE) guidelines emphasize the need for population-focused care, informatics, pharmacoepidemiology, and research processes,9 and the practice of pharmaceutical care demands that pharmacists know more about safety issues. Not surprisingly, several respondents indicated that their college or school was planning to begin covering these topics. Science of safety topics will likely be covered better in the future and more pharmacy graduates will be able to describe the FDA's role in product safety and understand postmarketing surveillance, pharmacoepidemiology, and other population-based safety efforts. The FDA should work with AACP to ensure that appropriate resources are available to teach faculty members about the FDA's initiatives and for them then to teach pharmacy students about drug product safety.
There appears to be some interest from pharmacy colleges and schools in adopting a developed curriculum on the SoS. However, many responding colleges and schools already were teaching many of these topics and did not believe they needed an entire curriculum. However, the type of curriculum developed would likely influence adoption rates. A curriculum that was modular in nature might readily allow colleges and schools to adopt modules deemed most appropriate for them. Most respondents (57 of 65) indicated interest in a curriculum for the domain 3 topics pertaining to product safety after FDA approval. Although the questionnaire did not seek reasons for such interests, one can speculate that this domain includes topics not currently covered but plan to be covered in the future. Additionally, this domain includes topics directly related to the FDA and some of the newer trends in postmarketing surveillance. Resources for teaching these domain 3 topics appear to be the most needed at this point in time, and faculty must understand the importance of these topics to the practice of pharmacy and begin to incorporate them into the curriculum.
The cross-tabulation results suggest that those colleges and schools with a SoS expert or faculty members who teach these topics in a graduate program offer more SoS topics. Other institutional factors such as affiliation with a medical school or class size were not consistently associated with the offering of the safety topics. Thus, faculty members at responding colleges and schools appear to be driving the curricula; and therefore, pharmacy faculty members should be engaged in activities related to the SoS. The FDA should include pharmacy faculty members in discussions and activities related to SoS initiatives. Workshops, continuing education programs, and seminars related to domain 3 topics may be useful and well received by pharmacy faculty members.
The primary limitation of this study is that it was exploratory in nature. In spite of an extensive literature review and key informant consultations, the questionnaire may have left out some important and relevant SoS topics. Other faculty members who did not complete the survey instrument may have had knowledge not reflected in the responses for a given college or school. The relatively small sample size made it difficult to identify trends because the cell sizes became quite small when classifying colleges and schools according to various characteristics. Many tests were conducted to assess whether there were certain college- or school-level characteristics associated with the offering of SoS topics. This approach was consistent with our exploratory data analysis plan, which was intended to provide a mechanism for understanding a large quantity of data. Although only a few relationships were significant, some characteristics were somewhat consistently associated with the perceived importance and offering of topics across the 3 domain topics (ie, the presence of a champion or advocate). Despite some apparent consistencies, multiple tests increase the possibility of chance associations, especially when conducting a nonreplicated study. Thus, caution should be used when interpreting these results.
CONCLUSION
Colleges and schools of pharmacy are devoting significant time and effort toward teaching SoS topics. However, each college and school develops its own curriculum and thus offers the topics in various ways. Also, the topics are not necessarily taught within the FDA's proposed SoS framework. Hopefully, the results of this project will be beneficial for increasing opportunities for collaboration among US colleges and schools of pharmacy, the FDA, and AACP. Improving teaching of the SoS could help to improve the US healthcare delivery system in all practice settings.
ACKNOWLEDGEMENTS
This study was funded in part by grant #8190180 from the US Food and Drug Administration. The authors would like to thank Kyle Null, Pharm D and Namita Joshi for their assistance with the project.
REFERENCES
- 1.Food and Drug Administration Amendments Act (FDAAA) of 2007 Public Law 110-85. Food and Drug Administration, U.S. Department of Health and Human Services. http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FoodandDrugAdministrationAmendmentsActof2007/FullTextofFDAAALaw/default.htm. Accessed July 21, 2011.
- 2.Baciu A, Stratton K, Burke SP. Washington, DC: National Academies Press; 2007. The Future of Drug Safety: Promoting and Protecting the Health of the Public. [Google Scholar]
- 3.Safe Use Initiative, Food and Drug Administration, US Department of Health and Human Services. http://www.fda.gov/DrugSafety/ucm187806.htm. Accessed July 21, 2011.
- 4.Holdford DA, Warholak TL, West-Stru D, Bentley JP, Malone DC, Murphy JE. A Baseline Evaluation of the Integration of the “Science of Safety” into the Curriculum of the Doctor of Pharmacy Degree in US Colleges and Schools of Pharmacy, American Association of Colleges of Pharmacy. http://www.aacp.org/resources/research/Documents/A%20Baseline%20Evaluation%20SOS%20Final.pdf. Accessed July 21, 2011.
- 5.FDA. Strategic Action Plan: Charting Our Course for the Future Fall 2007, Food and Drug Administration, Department of Health and Human Services. http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/StrategicActionPlan/UCM061415.pdf. Accessed July 21, 2011.
- 6.Holdford DA, Warholak T, West DS, et al. Teaching of Science of Safety in U.S. Schools of Pharmacy. American Journal of Pharmaceutical Education. 2011;75(4):Article 77. doi: 10.5688/ajpe75477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warholak T, Holdford D, West D, DeBake D, Bentley JP, Malone D, Murphy JE. Perspectives on educating pharmacy students about the Science of Safety. Am J Pharm Educ. 2011;75(7):Article 142. doi: 10.5688/ajpe757142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Future of Drug Safety-Promoting and Protecting the Health of the Public: FDA's Response to the Institute of Medicine's 2006 Report, Food and Drug Administration, U.S. Department of Health and Human Services. http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM171627.pdf. Accessed July 21, 2011.
- 9.Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree, Accreditation Council for Pharmacy Education. http://www.acpe-accredit.org/pdf/ACPE_Revised_PharmD_Standards_Adopted_Jan152006.pdf. Accessed July 21, 2011.