Abstract
Objective. To identify opinions about pharmacy graduates’ science of safety (SoS) educational needs.
Methods. Semi-structured interviews were performed with 25 educators and researchers at US pharmacy colleges and schools and 5 individuals from associations engaged in drug safety-related issues.
Results. Themes that emerged from the 30 interviews with key informants included: pharmacists should meet minimum SoS requirements; medication safety education is inconsistent; and barriers exist to improving SoS curricula. Student deficiencies noted included the lack of: student acceptance of a “culture of safety”: ability to effectively communicate verbally about medication safety; knowledge of the drug development process; and quality improvement skills. Key informants did not agree on how to address these gaps.
Conclusions. While educators, researchers, and other leaders in drug safety-related issues thought that US colleges and schools of pharmacy covered portions of SoS well, there were perceived deficiencies. Minimum standards should be set to assist with curricular adoption of SoS.
Keywords: medication safety, patient safety pharmacy education, science of safety, education
INTRODUCTION
Science of safety is a relatively new term that is not well known in the medical literature. The concept and term originated in a 2006 report from the Institute of Medicine (IOM) that made a series of recommendations about ways the Food and Drug Administration (FDA) can improve the US drug safety system.1
The SoS is defined as the systematic study of the negative impact of drugs and devices on humans at all stages of the product life cycle.1,2 Key elements of the definition include a systems approach to dealing with problems of safety as medications and devices advance from discovery, to development, to testing and use. The SoS seeks to help scientists and practitioners understand, explain, and predict physical risk from exposure to medications and devices.
The purpose of the SoS is to improve the identification, understanding, reporting, management, and communication of medication risk in: (1) preclinical studies (animal toxicology and safety pharmacology); (2) clinical trials [phases 1-3]; and (3) postmarketing studies or phase 4 clinical trials. Using the lifecycle approach allows for safety signals generated at any point in the drug development/marketing process to be evaluated along with relevant benefit-risk data to inform treatment choices and regulatory decision making.3 As such, SoS combines a growing understanding of disease and its molecular origins (including understanding of adverse events resulting from treatment) with new scientific methods of signal detection, data mining, and analysis. This knowledge will enable researchers to generate hypotheses about, confirm the existence of, and identify causal factors for drug and device safety problems in patient populations. This broad SoS definition allows for many opportunities for pharmacists to contribute to patient safety during medication use.4
In response to the IOM report, the FDA reiterated its commitment to strengthening drug safety in the United States and listed 3 initiatives for the agency2:
Strengthen the science that supports our medical product safety system at every stage of the product life cycle from premarket testing and development through postmarket surveillance and risk management;
Improve communication and information flow among all stakeholders engaged in promoting the safe use of medical products;
Improve operations and management to ensure implementation of the review, analysis, consultation, and communication processes needed to strengthen the US drug safety system.
Further impetus for the development of SoS came from the Food and Drug Administration Amendments Act of 2007 (FDAA Act), which gave the FDA additional authority over regulation of medication safety.5 These powers included the authority to mandate postmarketing research by pharmaceutical companies to better understand drug risks, require medication labeling changes to improve safety, and create risk evaluation and mitigation strategies (REMS) for high-risk medications. The Act also opened the door for the FDA to work more closely with educators and other allies to better manage the risks versus the benefits of drugs through each stage of the product lifecycle.6 The FDAA Act placed responsibilities on the FDA that would be difficult to achieve without help from key stakeholders. Pharmacy educators and researchers were deemed to be important stakeholders in furthering SoS because of their expertise, interest, and professional roles.
Consequently, the FDA sought a baseline study of the safety curricula in colleges and schools of pharmacy to better understand pharmacists’ ability to participate in important federal initiatives like the REMS and Sentinel Programs. A study was conducted to assess the curricula of accredited US colleges and schools of pharmacy for inclusion of SoS and provide a program report summarizing findings, lessons-learned, and recommendations for integrating a proactive SoS program for students.4,7 This paper describes the exploratory phase of that study.
The specific purpose of this key informant analysis was to identify opinions about significant issues relating to the needs and capabilities of pharmacy graduates. The research attempted to identify themes and ideas that could inform the development of a survey of SoS curricula of accredited US colleges and schools of pharmacy. This exploratory research was conducted because of gaps in the safety and education literature about the integration of SoS topics across the curricula, learning outcomes achieved, level of reinforcement in experiential training, and comprehensiveness of coverage in the curriculum. Results of the subsequent SoS survey are described in a separate publication.8
METHODS
This study used semi-structured qualitative interviews with “key informants” that included: (1) educators and researchers at US pharmacy colleges and schools and (2) individuals from organizations, including the FDA, engaged in promoting medication safety. A qualitative approach was selected because it allows for gathering of rich descriptions that can shed light on the experiences and interpretations of those being questioned.9 Such methods also are helpful when conducting research intended to be used in initial theory development and to allow for greater elaboration beyond the use of survey methodology. The Institutional Review Boards of The University of Arizona, Virginia Commonwealth University, and The University of Mississippi approved study methods and procedures.
Interview questions were developed based on a draft SoS healthcare professional curriculum from the FDA.10 This draft curriculum suggested skills and content areas that would be needed by healthcare professionals to participate in FDA safety initiatives. The curriculum emphasized the molecular origins and progression of disease, adverse consequences of treatments, patient- and population-specific causes and responses, and new methods for identifying, understanding, reporting, managing, and communicating risk. The SoS was defined for informants before the interview to ensure clarity. To allow informants flexibility, other terms such as medication safety were not defined. The interview questions were pilot tested and edits were made before beginning data collection. A description of the proposed curriculum is provided in a previous Journal article.7
Key informants were selected for their expertise and advocacy in various topics delineated in the FDA's draft SoS curriculum. Several criteria were used in selecting key informants. Individuals authoring papers or presentations identified in the literature review were candidates. Specialties of those identified included researchers and educators in the basic sciences (eg, pharmaceutics, pharmacology), clinical practitioners, curriculum committee chairs, educational specialists, clinical researchers, epidemiologists, and social science researchers from public and private colleges and schools of pharmacy, and representatives from institutions and associations that focus on issues related to SoS.
The study plan was to include a sufficient number of expert informants to elicit a mix of views about the needs of pharmacy students regarding safety topics and to reflect current teaching practices. A snowball sampling technique was used for this study. Each of the selected key informants was contacted and invited to participate in an interview. At the end of each interview, the individual was asked to recommend others who might be able to provide insight and expertise into educating about SoS. The number of interviews was capped at 30 because the final 5 interviews did not provide additional information.
An interview protocol was created to collect descriptive information about the key informant's background and experiences in SoS, and to obtain viewpoints on achievements and challenges of teaching the topic. Interviews were conducted by investigators experienced in qualitative research. Each interviewer underwent additional training concerning the conduct of the interview and a key informant interview guide was provided to ensure focus on issues identified in the literature review. Investigators contacted each key informant by phone or e-mail to describe the study, obtain agreement to participate, and schedule an interview time. All interviews were conducted via telephone and lasted 20 to 60 minutes. The authors took notes during interviews. These notes were later verified from interview audio recordings.
Interview notes were de-identified before further analysis. Qualitative data classification techniques were used to identify unifying information.11 Descriptive coding was used to evaluate demographic characteristics. Responses were then grouped according to categories or themes (ie, topic coding). Analytical coding was used to interpret the data and reflect on meanings. Data quality was ensured via recoding of responses by another individual and checking 10% of data for consistency with the original coding.
RESULTS
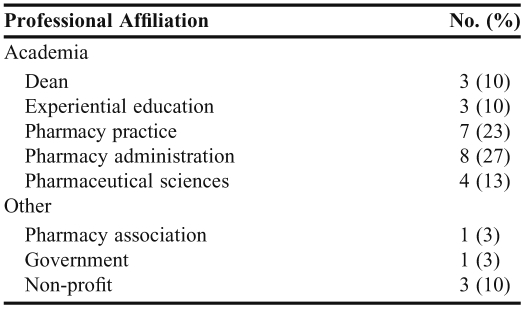
Thirty key informants were contacted and given the opportunity to participate in this study. All who could be contacted agreed to participate, yielding a response rate of 100%. Of those interviewed, 25 (83%) were from 10 different colleges and schools of pharmacy across the nation. Of these, 13 (52%) were from institutions classified as having a research focus and 12 (48%) were from institutions considered to be teaching oriented. A college or school was considered to have a research focus if it was ranked in the top 40 among colleges and schools of pharmacy with National Institutes of Health funding. Other key informants were from the Food and Drug Administration, the American Association of Colleges of Pharmacy, the Critical Path Institute, and the Institute for Safe Medication Practices. Of those interviewed, 22 (73%) were men. The interviewees had diverse backgrounds, although all were leaders in the teaching or implementation of at least one facet of SoS. For example, interviewees represented tenure-track and nontenure-track faculty members; assistant professors, associate professors, full professors, experiential education directors, and deans; clinical faculty members with expertise in ambulatory care, community, and hospital settings; faculty members who specialized in pharmacy administration/outcomes research, medicinal chemistry, toxicology, pharmacology, and law; and members of professional, not-for-profit, and governmental organizations. Additional demographic data are included in Table 1. Common opinion themes that emerged among key informants are detailed below.
Table 1.
Demographics of Key Informants in a Study on Educating Pharmacy Students About the Science of Safety (n = 30)
Newly graduated pharmacists should meet minimum SoS requirements. Respondents agreed that newly graduated pharmacists should possess a minimum set of knowledge, skills, and abilities such as: (1) the ability to identify errors and the causes of those errors; (2) knowledge of how to report concerns regarding medication safety; (3) the skills to measure, build, or change a system to reduce errors and to improve quality; and (4) the ability to effectively communicate verbally with patients and other healthcare providers about medication safety. A representative quote from a key informant was, “…quality includes, not just ensuring the right tablet gets into the bottle, but making sure that the patient takes it correctly, making sure the patient understands the medication, making sure the patient gets well, ultimately.” Key informants agreed that pharmacists are distinctively positioned for a leading SoS role in the US health care team: “[Pharmacists need to] recognize that they have, as a result of where they are…a unique perspective, a unique opportunity, and as a result of these, a unique responsibility.” Key informants advocated that students should be taught to appreciate the history behind the evolution of safety systems in order to prevent history from repeating itself. For example, 1 interviewee mentioned that students should understand the genesis and evolution of unit-dose packaging and systems to fully appreciate what types of errors were occurring at that time and to provide context for future decisions regarding medication risks in practice.
Pharmacy colleges and schools are preparing students well in some areas of medication safety and not well in others. When asked to describe how well pharmacy schools were preparing students in the area of medication safety, only 2 key informants indicated that they felt colleges and schools were doing a “good” or “great” job. A general consensus among key informants was that the teaching of SoS was not systematic (“if they get [certain aspects of SoS], they get it serendipitously”) and that there is not a designated place for it in the curriculum. However, a prevalent theme was the idea that despite certain gaps that need to be addressed, much of the pharmacy curriculum already was devoted to safety. One informant stated “…given that [the FDA's] definition, the entire curriculum of the College of Pharmacy is probably devoted to [SoS]….Probably every discussion we have and every lecture we give has something to do with ensuring or trying to accomplish the safety/effectiveness agenda.” Educators especially believed that students were well prepared as far as basic science and clinical courses such as pharmacology, pharmacotherapeutics, and pharmacokinetics.
When asked to indicate what gaps exist in medication safety education, the following 4 areas were identified: lack of (1) student acceptance of a “culture of safety”; (2) ability to verbally communicate effectively with patients and other healthcare providers about medication safety; (3) knowledge of the research and development process; and (4) skills to measure, build, or change a system to reduce errors and to improve quality. Key informants expressed the concern that students were being taught about the problems that exist (eg, medication errors, insufficient attention to using evidence-based medicine for prescribing, poor post-marketing surveillance), but not how to effectively ameliorate these problems. As one educator phrased it, “I don't think we [as educators] do a good job of teaching students how to implement solutions to those problems.” This theme was the undercurrent of why key informants believed that pharmacy curricula did not adequately teach students to develop problem-solving and decision-making skills. There was no consensus among key informants concerning which schools are doing a particularly good job in preparing their graduates in SoS (ie, no school was mentioned by more than 1 informant).
SoS education should be “hands on” or interactive. Key informants disagreed on the best way to add SoS to pharmacy curricula. Suggestions included: make the SoS a separate required course; use an integrated curricular model that threaded SoS material throughout the curriculum; and expose students to SoS topics during experiential training. Regardless of the method of curricular addition preferred, informants agreed that SoS training should be hands on, interactive, and as “real world” as possible.
When asked about offering tracks that specialize in medication safety, key informant answers varied. Some stated that there should be a core set of standards for all students, supplemented with opportunities to specialize in SoS. Others expressed concern that students have insufficient knowledge about what they want to do professionally and might not be able to make informed decisions regarding specialization.
There Are Barriers to Improving SoS Curricula at Colleges and Schools of Pharmacy. Key informants agreed that one of the greatest barriers to improving SoS education at colleges and schools of pharmacy was finding time in an already packed curriculum. Several also brought up the lack of communication between faculty members as represented by the following quote: “I think the biggest challenge in that [integrating the curriculum] is having faculty work with each other. I think faculty very much work in silence and it's difficult to convince faculty to work together.”
Another barrier noted was lack of faculty expertise in SoS. Interviewees thought it was important for faculty members with expertise in this area to share their expertise by developing curricular materials such as readings, slides, syllabi, as well as a train-the-trainer program.
Some key informants thought that pharmacy employers were part of the problem with some of the issues of medication safety in the United States. Although employers have made steps to improve their dispensing systems, some key informants felt that employers did not emphasize a culture of safety in pharmacy practice. Nevertheless, there also was a belief that pharmacy employers could be part of the solution if they were given the right incentives to focus more on medication safety within their practice.
DISCUSSION
Key informants agreed that new pharmacists should possess a minimum set of SoS knowledge, skills, and abilities. However, the first requirement mentioned by a given informant usually corresponded to their area of expertise. This is to be expected as one typically thinks of issues in the frame of one's own specialty. For example, faculty members who were medicinal chemists were more likely to mention concepts like the importance of the research and development process and basic science knowledge, whereas social scientists were more focused on systems knowledge and the ability to detect errors and causes of those errors as the biggest priority. Many thought their discipline addressed SoS, but few if any perceived SoS as the FDA does, via a product lifecycle approach. SoS is broad and the profession of pharmacy needs to see the bigger picture. To do this, educators should talk to others outside their areas of expertise about SoS topics. Education reform has been recommended in the health sciences to improve safety12 but not enough progress has been made in this area13 since the 1999 IOM report To Err is Human.14 A plan for teaching physicians to provide safer care has been proposed.15 Similarly, the profession of pharmacy needs to decide on the minimum SoS requirements for a new pharmacist. Some in other countries and in other professions have already begun the development of patient safety educational frameworks for education and outcomes16-18 and the US pharmacy educational system should follow suit. Mention of safety and quality is made in the Accreditation Council for Pharmacy Education (ACPE) Accreditation Standards and Guidelines for the Doctor of Pharmacy Degree.19 Elaboration in the ACPE standards on the meaning of the terms safety and quality could benefit colleges and schools of pharmacy. This study provided first steps but a broader consensus is needed to guide colleges and schools.
The key informants thought that pharmacy colleges and schools are not adequately preparing students in all areas of medication safety. A possible reason for this conclusion was the general belief that SoS teaching was not systematic because there was no set place for it in many curricula or any defined topics for SoS that should be taught. While they agreed there were gaps in pharmacy curricula, the key informants did indicate that many facets of SoS already were being taught and integrated. Key informants appeared to be saying that despite gaps that need to be addressed, the entire pharmacy curriculum is devoted to safety. The important issue is how to better define and measure gaps so they can be addressed on a curricular level.
There was not a consensus among the key informants concerning the best way to add SoS to pharmacy curricula (ie, as a standalone course or integrated into each class). However, nationwide consensus on this issue may not be necessary to move forward with development of a SoS curriculum. Once minimum SoS requirements are defined and delineated, it can be left up to each college and school of pharmacy to ensure that outcomes are met. This might provide a vehicle for producing innovative solutions and continuing excellence. Regardless of the method of curricular addition preferred, informants agreed that SoS training should be hands on, interactive, and as “real world” as possible. Published literature in other areas suggests a similar point 20-22 and an effort to include “real-life” SoS education should be incorporated into any SoS educational curriculum.
As with any other change, there is no shortage of barriers to the implementation of SoS in pharmacy education. Curricular time, communication between faculty members, and faculty expertise were seen as the largest barriers. Adding SoS education in an already full curriculum is definitely a valid concern, although there may be ways to add content via homework assignments, early experiential education, and projects. Opening up SoS dialog between disparate departments within colleges and schools of pharmacy may facilitate faculty communication. Each might be pleasantly surprised to find that more SoS education is integrated into the curriculum than was thought. Concerning faculty expertise, if viewed from a life-cycle approach, many colleges/school of pharmacy have a faculty member who has expertise in some of the facets of SoS. For curricular issues left unaddressed, a train-the-trainer program may be helpful to increase knowledge. A turnkey quality improvement education does exist23 and could be used as a starting point to addressing faculty SoS knowledge; however, it addresses only a portion of the SoS. This turnkey program is available to faculty members from the Pharmacy Quality Alliance upon request.24 By definition, key informant interviews involve small, nonrandom situations where participants offer opinions. These are obvious limitations of this study; therefore, future research should include larger, random samples to increase the generalizability of results. In addition, outcome studies evaluating the impact of SoS education on pharmacy practice as well as studies describing how pharmacists use SoS practices. Best SoS educational practices should be identified and made public so maximal benefit can be achieved.
CONCLUSIONS
While key informants believe that US colleges and schools of pharmacy cover some portions of SoS well, perceived deficiencies include the lack of (1) student acceptance of a “culture of safety”; (2) the ability of graduates to effectively verbally communicate with patients and other healthcare providers about medication safety; (3) knowledge of the drug research and development process; and (4) the skills to measure, build, or change a system to reduce errors and to improve quality. The SoS framework should be considered when setting minimum education standards for graduating pharmacists.
ACKNOWLEDGEMENTS
This study was funded in part by a grant #8190180 from the US Food and Drug Administration.
REFERENCES
- 1.Alina Baciu, Kathleen Stratton, Sheila P Burke., editors. Committee on the Assessment of the US Drug Safety System. The Future of Drug Safety: Promoting and Protecting the Health of the Public. Washington, DC: National Academy Press; 2007. [Google Scholar]
- 2. The future of drug safety - promoting and protecting the health of the public. FDA's repsonse to the Institute of Medicine's 2006 report: U.S Department of Health and Human Services, Food and Drug Administration (FDA); January 2007. http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM171627.pdf. Accessed August 11, 2011.
- 3.Food and Drug Administration. U.S Department of Health and Human Services, Food and Drug Administration; 2008. The Sentinel Initiative: National Strategy for Monitoring Medical Product Safety. http://www.fda.gov/downloads/Safety/FDAsSentinelInitiative/UCM124701.pdf. Accessed August 11, 2011. [Google Scholar]
- 4.Holdford DA, Warholak TL, Strum-West D, et al. A baseline evaluation of the integration of the “Science of Safety” into the curriculum of the Doctor of Pharmacy degree in U.S. colleges and schools of pharmacy: American Association of Colleges of Pharmacy. 2010. http://www.aacp.org/resources/research/Documents/A%20Baseline%20Evaluation%20SOS%20Final.pdf. Accessed August 11, 2011.
- 5.Food and Drug Administration Amendments Act (FDAAA) of 2007. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=110_cong_public_laws&docid=f:publ085.110. Accessed August 11, 2011.
- 6.FDA Strategic Action Plan: Charting Our Course for the Future. Washington, DC: Department of Health and Human Services, US Food and Drug Administration; 2007. http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/StrategicActionPlan/UCM061415.pdf. Accessed August 11, 2011. [Google Scholar]
- 7.Holdford DA, Warholak T, West DS, et al. Teaching of Science of Safety in U.S. Schools of Pharmacy. Am J Pharm Educ. 2011;75(4):Article 77. doi: 10.5688/ajpe75477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West DS, Basak R, Bentley J, Holdford D, et al. Baseline assessment of schools of pharmacy science of safety curriculum. Am J Pharm Educ. 2011;75(7):Article 141. doi: 10.5688/ajpe757141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sofaer S. Qualitative methods: what are they and why use them? Health Serv Res. 1999;34(5 Pt 2):1101–1118. [PMC free article] [PubMed] [Google Scholar]
- 10. Marchand H. Proposed curriculum on drug product safety: use and evaluation. July 21, 2008. American Association of Colleges of Pharmacy Annual Meeting. Chicago 2008.
- 11.Richards L. Handling Qualitative Data: A Practical Guide. London: Sage Publications, Ltd; 2005. [Google Scholar]
- 12.Insitute of Medicine. Executive summary. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 13.Leape L, Berwick D, Clancy C, et al. Transforming healthcare: a safety imperative. Qual Saf Health Care. October 2009;18(6):424–428. doi: 10.1136/qshc.2009.036954. [DOI] [PubMed] [Google Scholar]
- 14.Commitee on Quality of Health Care in America. To Err is Human: Building a Safer Health System: Institute of Medicine. Washington DC: National Academy Press; 1999; http://www.nap.edu/openbook.php?record_id=9728&page=1. Accessed August 11, 2011. [Google Scholar]
- 15.Lucian Leape Institute Roundtable On Reforming Medical Education. National Patient Safety Foundation; 2010. Unmet Needs: Teaching Physicians to Provide Safe Patient Care. http://www.npsf.org/download/LLI-Unmet-Needs-Report.pdf. Accessed August 11, 2011. [Google Scholar]
- 16.Walton MM, Shaw T, Barnet S, Ross J. Developing a national patient safety education framework for Australia. Qual Saf Health Care. 2006;15(6):437–442. doi: 10.1136/qshc.2006.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Safety Competencies Steering Committee. The Safety Competencies; Enhancing Patient Safety Across the Health Professions. Ottawa, ON: Canadian Patient Safety Institute; 2008. http://www.patientsafetyinstitute.ca/English/toolsResources/safetyCompetencies/Documents/Safety%20Competencies.pdf. Accessed August 11, 2011. [Google Scholar]
- 18.Emanuel L, Walton M, Hatlie M, et al. The Patient Safety Education Project: An International Collaboration. Agency for Healthcare Research and Quality; 2008. http://www.ahrq.gov/downloads/pub/advances2/vol2/Advances-Emanuel_19.pdf. Accessed August 11, 2011. [PubMed]
- 19.ACPE. Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. Chicago: Accreditation Council for Pharmacy Education; 2007. http://www.acpe-accredit.org/pdf/FinalS2007Guidelines2.0.pdf. Accessed August 11, 2011.
- 20.Henriksen K, Dayton E. Issues in the design of training for quality and safety. Qual Saf Health Care. 2006;15(Supplement 1):i17–i24. doi: 10.1136/qshc.2005.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson TL. Applications of quality assurance principles: teaching medication error reduction skills in a “real world” environment. Am J Pharm Educ. 2003;68(1):Article 17. [Google Scholar]
- 22.Warholak TL. Preceptor perceptions of pharmacy student team quality assurance projects. Am J Pharm Educ. 2009;73(3):Article 47. doi: 10.5688/aj730347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warholak TL, West D, Holdford D. The educating pharmacy students and pharmacists to improve quality (EPIQ) program: a tool for pharmacy practice. J Am Pharm Assoc. 2010;50:534–538. doi: 10.1331/JAPhA.2010.10019. [DOI] [PubMed] [Google Scholar]
- 24.Pharmacy Quality Alliance. Quality Improvement Tools and Resources. http://www.pqaalliance.org/QualityImpTR.htm. Accessed August 11, 2011.