Abstract
Objective. To assess US pharmacy students’ knowledge and perceptions of adverse event reporting.
Methods. To gauge pharmacy students' impressions of adverse event reporting, a 10-question survey instrument was administered that addressed student perceptions of the reporting procedures of the Food and Drug Administration (FDA) and pharmaceutical manufacturers, as well as student understanding of the Health Insurance Portability and Accountability Act (HIPAA) and its relationship to adverse event reporting.
Results. Two hundred twenty-eight pharmacy students responded to the survey. The majority of respondents believed that the FDA is more likely than a pharmaceutical company to take action regarding an adverse event. There were misconceptions relating to the way adverse event reports are handled and the influence of HIPAA regulations on reporting.
Conclusions. Communication between the FDA and pharmaceutical manufacturers regarding adverse event reports is not well understood by pharmacy students. Education about adverse event reporting should evolve so that by the time pharmacy students become practitioners, they are well acquainted with the relevance and importance of adverse event reporting.
Keywords: adverse event, adverse drug reaction, pharmacists, pharmacy students, adverse event reporting, FDA, HIPAA
INTRODUCTION
Pharmacists play a key role in the management of drug therapy. They are in an ideal position to contribute to patient care and provide vital information regarding drug therapy, including the safety of medications. An important part of medication therapy management is to ensure that patients are receiving the intended treatment benefits and are not experiencing any undesired effects, commonly known as adverse events.1 An adverse event is any untoward medical occurrence in a patient to whom a medicinal product has been administered. An adverse event does not necessarily have to have a causal relationship with the treatment.2 If a patient experiences an adverse event, the pharmacist is in a position to take action in several ways, one of which includes reporting the adverse event.3 Understandably, the reporting of adverse events might not be a priority when assisting a patient; however, the information gathered by the pharmacist is invaluable. Pharmacists in clinical settings are able to access patient information that allows them to monitor and report adverse events that occur. In the retail setting, pharmacists may become aware of a potential adverse event while counseling or interacting with patients. These pharmacists have access to patient information and drug therapy regimens, which can contribute greatly to the reporting of an adverse event.
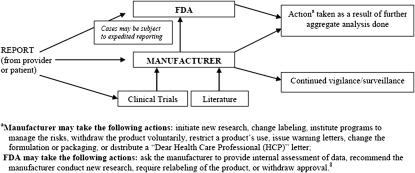
In the United States, adverse events may be voluntarily reported to the Food and Drug Administration's (FDA) MedWatch program and/or directly to the pharmaceutical manufacturer. The minimum required elements of an adverse event report include: (1) a reporter, (2) a patient, (3) an adverse event, and (4) a suspect product. The reporter may remain anonymous. Minimum identifiable information needs to be reported for the patient, which may include the patient's initials. Figure 1 shows an overview of the pathway an adverse event report takes to several destinations, including the manufacturer and the FDA.
Figure 1.
Overview of communication of adverse event reports by a pharmaceutical manufacturer and the Food and Drug Administration (FDA).
Voluntary, post-marketing adverse event reporting has contributed significantly over time to help bring to light adverse drug reactions associated with drug treatment. An adverse drug reaction, in contrast to an adverse event, is characterized by a suspected causal relationship between the drug and the occurrence of the event.2 This reporting has been an important tool used by both the FDA and pharmaceutical manufacturers to detect, investigate, and inform patients and healthcare professionals about previously unknown adverse drug reactions. Improving the quality and quantity of adverse event reports can help regulators and pharmaceutical manufacturers understand, investigate, and communicate actual safety risks.
Any adverse event that is reported to a manufacturer must be communicated to the FDA within specified timelines.4 Reports derived from the FDA's voluntary MedWatch program, as well as adverse event reports that the FDA receives directly from pharmaceutical manufacturers, are stored in the Adverse Event Reporting System (AERS) database. AERS was designed to collect information about adverse events and support postmarketing safety surveillance of approved drugs and biologic products.5 Many pharmaceutical manufacturers also have their own safety databases.
Along with other healthcare professionals, pharmacists are key players in the gathering and reporting of adverse events. In the Accreditation Council for Pharmacy Education (ACPE) Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree (effective July 2007),6 adverse event reporting is indicated as “identifying and reporting medication errors and adverse drug reactions,” and is listed as an activity that students should participate in during required advanced pharmacy practice experiences (APPEs). The objective of this study was to assess the potential for future pharmacist reporting by surveying a sample of US pharmacy students to assess their knowledge and perceptions of adverse event reporting.
METHODS
Two ACPE-accredited colleges and schools of pharmacy participated in this study to gauge pharmacy students’ impressions of adverse event reporting. A 10-question survey instrument was mailed to a professor at each institution. The survey instruments were to be completed by students enrolled in the second and third year of their pharmacy education during fall 2008. The students were given 5 minutes to voluntarily complete the survey instrument during one of their classes. To ensure that the most candid answers were provided, the students were asked not to interact with others or use reference materials in answering questions. The survey questions were formulated to determine the pharmacy students’ general knowledge regarding adverse event reporting as well as their perceptions of barriers. These barriers included student perceptions of the reporting procedures of the FDA and pharmaceutical manufacturer as well as their understanding of the Health Insurance Portability and Accountability Act (HIPAA) of 1996 and its relationship to adverse event reporting.
The survey questions regarding the reporting of adverse events to both the FDA and pharmaceutical manufacturers were specifically designed with parallel structure so the respondents could not perceive a bias while answering the survey questions. (A copy of the survey instrument is available from the author upon request.)
RESULTS
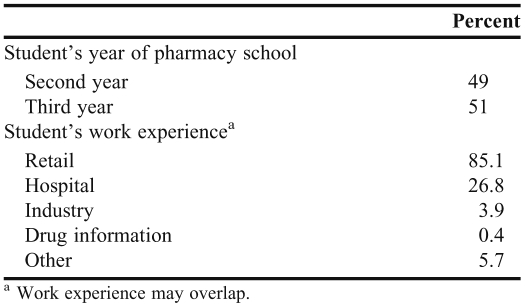
Two hundred twenty-eight of approximately 350 pharmacy students at the 2 participating pharmacy schools responded to the survey. Approximately half of the students were in their second pharmacy year and the other half were in their third year (Table 1). Among the students surveyed, 85% had pharmacy work experience in a retail setting and approximately 27% had experience in a hospital setting.
Table 1.
Demographics of Participants in a Survey of Pharmacy Student Perceptions of Adverse Event Reporting (N = 228)
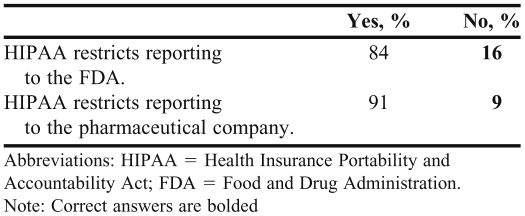
The majority of students (88%) believed that the FDA is more likely than the pharmaceutical company to take action regarding an adverse event. Student perceptions of the communication between the FDA and pharmaceutical manufacturers and the type of information included in an adverse event report are summarized in Tables 2 and 3. Sixty-two percent of students believed that an adverse event reported to the FDA would always be communicated to the pharmaceutical company, while only 25% believed that an adverse event reported to the pharmaceutical company would be communicated to the FDA (Table 2). Of the students surveyed, 84% believed that HIPAA restricts reporting to the FDA and 91% believed that HIPAA restricts reporting to pharmaceutical manufacturers (Table 3).
Table 2.
Pharmacy Students’ Beliefs Regarding How Adverse Event Reports Are Relayed Between the Food and Drug Administration (FDA) and the Pharmaceutical Company (N = 228)a
Table 3.
Pharmacy Students’ Beliefs Regarding Whether the Amount/Type of Information in an Adverse Event/Adverse Drug Reaction Report is Restricted by HIPAA (N = 228)
DISCUSSION
The safety profile of a drug relies on both evidence from clinical trials and postmarketing data. The adverse events derived from clinical trials may not reflect real-world use of the drug. Clinical trials are commonly conducted in controlled environments and are limited to selected groups of patients. Because it is not feasible to anticipate every safety issue prior to drug approval, postmarketing adverse event reporting can make a significant difference in understanding the safety profile of a marketed drug.7 Depending on their quantity and quality, adverse event reports may bring important safety issues to light as well as assist regulators and manufacturers in researching and understanding emerging safety risks. This information can then be communicated to healthcare professionals and patients through labeling changes, “Dear Health Care Professional” letters, FDA warning letters, and other means. Additional action may be taken by regulatory or pharmaceutical manufacturers based on these reports, including formulation and packaging changes and restriction or withdrawal of the product.8
The majority of students surveyed were aware of adverse event reporting from their respective institution's pharmacy curriculum. Additionally, prior work experience and practice environment may have played a role in their overall knowledge of adverse event reporting. The ACPE accreditation standards and guidelines indicate that exposure to adverse event reporting may occur during APPEs but is not required to have a set place in a pharmacy school's curriculum. Introducing and incorporating adverse event reporting early in the curriculum would ensure that pharmacy students become aware and benefit from an understanding of the basics of adverse event reporting.
Most of the students surveyed felt that the FDA was more likely than pharmaceutical manufacturers to “take action” in response to an adverse event report. Furthermore, 62% of students believed that an adverse event reported to the FDA will always be communicated to the pharmaceutical manufacturer, while only a quarter of the students surveyed believed that an adverse event reported to the pharmaceutical manufacturer will be communicated to the FDA (Table 2). In reality, these are misconceptions. The FDA does not regularly relay adverse event reports directly to pharmaceutical manufacturers; however, pharmaceutical manufacturers are required to relay adverse event reports to the FDA based on preset timelines and guidelines.2,4,9 The majority of reports submitted to the FDA are from pharmaceutical manufacturers.10 In fact, up to 90% of the reports in the FDA AERS database have been received from pharmaceutical manufacturers.11
Students responded that they would be more inclined to contact the FDA than the pharmaceutical company to report an adverse event. Diligent and thorough adverse event documentation should be encouraged when reporting to either the FDA or a pharmaceutical manufacturer.
Another important misconception identified in the survey related to HIPAA restrictions. Eighty-four percent of the students surveyed believed that HIPAA restricts reporting to the FDA and 91% believed that HIPAA restricts reporting to pharmaceutical manufacturers (Table 3), despite the fact that HIPAA laws do not restrict the reporting of adverse events or issues relating to the quality, safety, or effectiveness of products to the FDA or pharmaceutical manufacturer. Healthcare professionals are able to report adverse events just as they were prior to the Privacy Rule enactment in April of 2003.12,13
National privacy standards issued by the Department of Health and Human Services (HHS) under HIPAA, called the “Privacy Rule,” help ensure that an individual's health information is properly protected while allowing the flow of health information needed to provide and promote high quality health care.12 This rule protects the privacy of individually identifiable health information, or protected health information (PHI), of both patients and research subjects.13 PHI covers information that is transmitted through electronic, paper, or oral routes and includes the individual's past, present, or future physical or mental health or condition and the provision of health care to the individual. Demographic data as well as data that might lead to the identification of the patient is also included (examples include common identifiers such as name, address, and birthdate).12 The Office for Civil Rights (OCR) statute (Disclosure for Public Health Activities 45 CFR 164.512[b]) states that there is a “legitimate need for public health authorities and others responsible for ensuring public health and safety to have access to protected health information to carry out their public health mission”.14 Additionally, PHI may be disclosed to entities subject to FDA regulation (ie, pharmaceutical manufacturers).12 Thus, the reporting of adverse events and the amount or type of information included in the report is not restricted by HIPAA laws.14
Nurses and physicians are other healthcare professionals who often report adverse events. Traditionally, physicians have reported more adverse events than have either pharmacists or nurses, largely because, in providing care to a patient, physicians are more likely to become aware of adverse events that occur while providing care to a patient in a hospital or clinic setting. However, adverse event reporting by pharmacists is increasingly feasible because of their close work with patients and physicians in the hospital setting where the pharmacist has access to the patient's medical chart and laboratory results. It is important to identify if an organization's protocol (ie, a hospital's reporting system) prevents the reporting of adverse events by certain members of the healthcare team.
Because student surveys were administered at only 2 US colleges and schools of pharmacy, answers may have been biased by specific curriculum, timing, and student populations. The extent to which the selected sample represents other pharmacy curricula and student populations cannot be determined. Because each institution's curriculum might be a student's first introduction to the common concepts in reporting of adverse events, additional exposure and experience in practice would likely help students and future pharmacists gain a better understanding of adverse event reporting.
Additional insight might be obtained by surveying more pharmacy students at various colleges and schools or by surveying students at a later point during their pharmacy education, such as during APPEs. Because adverse event reports commonly originate anywhere in the world and are a global phenomenon, it would also be interesting to solicit pharmacy students’ perceptions of adverse event reports in countries other than the United States. Educational outreaches to pharmacists and pharmacy students may be helpful in fostering awareness of adverse event reporting, leading to improvements in the quality and quantity of these reports. With additional experience in practice, pharmacy students will likely become more knowledgeable about adverse event reporting. It also would be valuable to survey pharmacists in various settings to determine the adverse event reporting rates of practicing pharmacists.
Colleges and schools of pharmacy should evaluate their own curricula to determine whether adverse event reporting information is being discussed sufficiently. The eventual goal of adverse event reporting is to provide meaningful data to regulators and pharmaceutical manufacturers that can have a significant impact on public health and safety. Along with the curricula of pharmacy colleges and schools, pharmacy students have opportunities to be introduced to and learn about adverse event reporting in practice settings, such as hospital programs for adverse event reporting. With the use of modern technology, such as Electronic MedWatch forms on the FDA Web site, adverse event reporting has become easier and more accessible. There are also cell phone applications that put the ability to report adverse events at the fingertips of healthcare professionals.
The increased focus of regulators and pharmaceutical manufacturers on drug safety has led to greater awareness and considerable improvements in adverse event reporting. As pharmacists’ continue to be considered among the most accessible healthcare professionals, their responsibility regarding patient care is growing. Pharmacists have a greater opportunity than ever before to expand their role as advances are made and more is learned about the usefulness of collaborations in medication therapy management. Because of their unique position to identify and relay adverse events to the appropriate sources, pharmacists have an important role in adverse event reporting. Curricula at colleges and schools of pharmacy that actively and accurately promote pharmacists’ role in reporting adverse events need to continue to be implemented and improved to ensure that students entering the professional setting are fully aware of how adverse event reporting impacts the safety profile of drugs and, thus, patient well-being.
CONCLUSION
In the current climate of drug recalls, withdrawals, and increased scrutiny, monitoring the safety of marketed drugs is an essential responsibility for all healthcare professionals. Adverse event reporting assists in the continuous monitoring and surveillance of marketed drugs and allows for an analysis of real-world experiences and outcomes. The responses of pharmacy students in this study show that the communication of adverse event reports between the FDA and pharmaceutical manufacturers is not well understood and that HIPAA is mistakenly perceived to restrict how much and what type of reporting is allowable. There may be an opportunity for pharmacists to increase their knowledge base and role in the reporting of adverse events in the future. Thus, education about adverse event reporting must evolve so that when pharmacy students become practitioners, they have a better understanding of the relevance and importance of reporting adverse events.
REFERENCES
- 1.Pellegrino AN, Martin MT, Tilton JJ, et al. Medication therapy management services: definitions and outcomes. Drugs. 2009;69(4):393–406. doi: 10.2165/00003495-200969040-00001. [DOI] [PubMed] [Google Scholar]
- 2.Post-approval safety data management: definitions and standards for expedited reporting. ICH Harmonized Tripartite Guideline Draft. ICH E2D ver 3.8. July 2003. [Google Scholar]
- 3.Sears EL, Generali JA. Adverse drug reaction and medication error reporting by pharmacy students. Ann Pharmacother. 2005;39(3):452–459. doi: 10.1345/aph.1E369. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. HHS. 21 CFR 314.80. Ch. 1 (4-1-01 Edition) [Google Scholar]
- 5.Food and Drug Administration. Adverse event reporting system (AERS). AERS description. 20 August 2009. http://www.fda.gov/cder/aers/default.htm. Accessed June 27, 2011.
- 6.Accreditation Council for Pharmacy Education. Chicago, IL: 2006. Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. Effective: 01 July 2007. http://www.acpe-accredit.org/pdf/ACPE_Revised_PharmD_Standards_Adopted_Jan152006.pdf. Accesed June 27, 2011. [Google Scholar]
- 7.Talbot JCC, Nilsson BS. Pharmacovigilance in the pharmaceutical industry. Br J Clin Pharmacol. 1998;45(5):427–431. doi: 10.1046/j.1365-2125.1998.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridley DB, Kramer JM, Tilson HH, et al. Marketwatch: spending on postapproval drug safety. Health Affairs. 2006;25(2):429–436. doi: 10.1377/hlthaff.25.2.429. [DOI] [PubMed] [Google Scholar]
- 9.Jones JK. Why should I report an adverse drug event? Medscape. August 2011;16 Available at: http://www.medscape.org/viewarticle/578160. [Google Scholar]
- 10.Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration: your report can make a difference. J Gen Intern Med. 2003;18(1):57–60. doi: 10.1046/j.1525-1497.2003.20130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zielinski SL. FDA attempting to overcome major roadblocks in monitoring drug safety. J Natl Cancer Inst. 2005;97(12):872–873. doi: 10.1093/jnci/97.12.872. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health & Human Services. Summary of the HIPAA privacy rule. OCR Privacy Brief. May 2003. [Google Scholar]
- 13.HIPAA and routine interactions between physicians and pharmaceutical sales representatives. White paper. International Pharmaceutical Privacy Consortium (IPPC) Spring 2003. [Google Scholar]
- 14.OCR HIPAA Privacy Guidance Document. Disclosures for public health activities. [45 CFR 164.512(b)] December 2002. Revised April 2003. http://www.hhs.gov/ocr/privacy/hipaa/understanding/summary/guidanceallsections.pdf.