Abstract
BACKGROUND
Locomotor training, including the use of body-weight support in treadmill stepping, is a physical therapy intervention used to improve recovery of the ability to walk after stroke. The effectiveness and appropriate timing of this intervention have not been established.
METHODS
We stratified 408 participants who had had a stroke 2 months earlier according to the extent of walking impairment — moderate (able to walk 0.4 to <0.8 m per second) or severe (able to walk <0.4 m per second) — and randomly assigned them to one of three training groups. One group received training on a treadmill with the use of body-weight support 2 months after the stroke had occurred (early locomotor training), the second group received this training 6 months after the stroke had occurred (late locomotor training), and the third group participated in an exercise program at home managed by a physical therapist 2 months after the stroke (home-exercise program). Each intervention included 36 sessions of 90 minutes each for 12 to 16 weeks. The primary outcome was the proportion of participants in each group who had an improvement in functional walking ability 1 year after the stroke.
RESULTS
At 1 year, 52.0% of all participants had increased functional walking ability. No significant differences in improvement were found between early locomotor training and home exercise (adjusted odds ratio for the primary outcome, 0.83; 95% confidence interval [CI], 0.50 to 1.39) or between late locomotor training and home exercise (adjusted odds ratio, 1.19; 95% CI, 0.72 to 1.99). All groups had similar improvements in walking speed, motor recovery, balance, functional status, and quality of life. Neither the delay in initiating the late locomotor training nor the severity of the initial impairment affected the outcome at 1 year. Ten related serious adverse events were reported (occurring in 2.2% of participants undergoing early locomotor training, 3.5% of those undergoing late locomotor training, and 1.6% of those engaging in home exercise). As compared with the home-exercise group, each of the groups receiving locomotor training had a higher frequency of dizziness or faintness during treatment (P=0.008). Among patients with severe walking impairment, multiple falls were more common in the group receiving early locomotor training than in the other two groups (P = 0.02).
CONCLUSIONS
Locomotor training, including the use of body-weight support in stepping on a treadmill, was not shown to be superior to progressive exercise at home managed by a physical therapist. (Funded by the National Institute of Neurological Disorders and Stroke and the National Center for Medical Rehabilitation Research; LEAPS ClinicalTrials.gov number, NCT00243919.)
More than 790,000 americans have a new or recurrent stroke yearly,1 and two thirds of the 6.4 million survivors may have significant limitations in walking2 and are at high risk for falls,3 fractures,4 and further decline in mobility.5 Walking speed predicts the level of disability. At a walking speed of more than 0.8 m per second, full mobility in the community is likely; at a walking speed of less than 0.4 m per second, mobility is limited to the home; and at speeds of 0.4 to 0.8 m per second, mobility is limited to short walks in the community.6 Improving functional walking capacity is a primary goal of physical therapy interventions.
A report from the National Institutes of Health (NIH) emphasized the need for research to assess the effectiveness and optimal timing, intensity, and duration of poststroke rehabilitation interventions.7 The Locomotor Experience Applied Post-Stroke (LEAPS) Trial was designed to address this need by comparing two different therapeutic exercise programs provided by physical therapists to improve the ability to walk after stroke.8 One intervention was a task-specific walking program that included stepping on a treadmill with partial body-weight support. Pilot studies and small clinical trials9–16 suggested that this program was likely to be effective and led to its rapid adoption, resulting in increased use of commercial lifts and robot-assisted stepping on a treadmill.17,18 A Cochrane review highlighted the urgent need for a well-designed randomized trial to determine the effectiveness of these interventions.19 The LEAPS task-specific walking intervention was compared with an exercise program targeted at the most common gait-relevant impairments after stroke: weakness and poor balance. A prior trial had shown that progressive exercises provided by physical therapists in the home improve walking, endurance, and functional mobility.20
We hypothesized that in addition to usual care (physical therapy provided according to current standards of practice), provision of a specialized locomotor training program that included stepping on a treadmill with body-weight support delivered early (2 months after stroke) or late (6 months after stroke) would be more effective in increasing the proportion of study participants who had higher functional walking levels at 1 year than provision of a control intervention that included progressive strength and balance exercises provided by a physical therapist in the home 2 months after stroke. We also hypothesized that early locomotor training would improve walking speed more than late locomotor training because prior studies suggested that the greatest degree of recovery occurs early2 and is complete by 6 months.21
METHODS
STUDY DESIGN AND OVERSIGHT
The protocol and design for this phase 3, single-blinded, randomized, controlled trial have been described elsewhere.8 The institutional review boards at all participating centers approved the protocol, and all participants provided written informed consent. An independent medical monitor and a data and safety monitoring board were appointed by the NIH. Study biostatisticians had full access to the data and managed quality-control procedures to verify the accuracy and completeness of the data and statistical analyses. All authors contributed to the interpretation of the results and made the decision to submit the manuscript for publication, vouch for the completeness and accuracy of the data, and attest to the fidelity of the report to the study protocol. No commercial support for this study was provided.
STUDY POPULATION, SCREENING, AND RANDOMIZATION
Participants were recruited from six inpatient rehabilitation sites in California and Florida. Criteria for inclusion in the study were an age of 18 years or older, a stroke within 45 days before study entry and the ability to undergo randomization within 2 months after the stroke, residual paresis in the leg affected by stroke, the ability to walk 3 m (approximately 10 ft) with assistance from no more than one person and the ability to follow a three-step command, the treating physician’s approval of participation in the study, a self-selected speed for walking 10 m of less than 0.8 m per second, and residence in the community by the time of randomization. The primary criteria for exclusion were dependency on assistance in activities of daily living before the stroke, contraindications to exercise, preexisting neurologic disorders, and inability to travel to the treatment site.8 Patients admitted for inpatient rehabilitation were screened by means of chart review. Eligible patients with a first stroke underwent physical and cognitive screening, and their medical records were subjected to a comprehensive review.8 At 2 months, patients who still met the eligibility criteria and successfully completed an exercise tolerance test22 were enrolled in the intervention phase of the study.
After completion of baseline assessments 2 months after the stroke, participants were randomly assigned to early locomotor training, late locomotor training, or home exercise in a ratio of 7:7:6. Treatment assignments were stratified according to the severity of impairment at baseline and the study site to ensure balance among the three groups.8
INTERVENTIONS
Physical therapists at each site were trained according to a standardized protocol for the locomotor-training and home-exercise interventions.8 The programs were controlled for exercise frequency (90-minute sessions, three times per week) and duration (12 to 16 weeks); participants had to complete between 30 and 36 exercise sessions within this period. Participants also received usual care during the study period.
Locomotor training included stepping on a treadmill with partial body-weight support and manual assistance as needed for 20 to 30 minutes at 3.2 km per hour (0.89 m per second [2.0 mi per hour]), followed by a progressive program of walking over ground for 15 minutes. The bodyweight support system (manufactured by Robomedica) provided dynamic, pneumatic control of the patient’s weight throughout the gait cycle and provided ergonomic seating for trainers assisting with patients’ leg movements. The treadmill (Biodex Medical Systems) speeds ranged from 0 to 1.6 km per hour (0 to 10 mi per hour), increasing by increments of 0.16 km per hour (0.1 mi per hour). The harness (Robertson Harness) could be adjusted for trunk and pelvic support.
The home-exercise program was designed as an active control, not as a high-intensity, task-specific walking program. Progression through the program was managed by a physical therapist in the home, with the goals of enhancing flexibility, range of motion in joints, strength of arms and legs, coordination, and static and dynamic balance. Participants in this program were encouraged to walk daily.
OUTCOMES
Participants were assessed before randomization and 6 and 12 months after the occurrence of stroke by physical therapists who were trained in the use of standardized assessment protocols and were unaware of the participants’ group assignment.8 The primary outcome was the proportion of participants with an improved functional level of walking 1 year after the stroke. Improved functional level was defined as the ability to walk independently at a speed of 0.4 m per second or faster for persons with initially severe gait impairment (ability to walk at <0.4 m per second) or at a speed of 0.8 m per second or faster for persons with initially moderate gait impairment (ability to walk at 0.4 m per second to <0.8 m per second).6,23 These transitions are associated with improvements in home or community ambulation, functional status, and quality of life.6,23 Walking speed was measured as participants were instructed to walk at their usual pace for 10 m over ground.24
Secondary outcomes included changes at 1 year in the speed at which participants walked a distance of 10 m, the distance walked in 6 minutes,25 and the number of steps taken per day as measured by an activity monitor.26 Other outcome measures included scores on the Fugl-Meyer Assessment of Motor Recovery in the legs,27 the Berg Balance Scale,28 the Activities-Specific Balance Confidence Scale,29 the Activities of Daily Living–Instrumental Activities of Daily Living (ADL–IADL) Scale, and the physical mobility and participation domains of the Stroke Impact Scale.30
Participants recorded any falls in a diary, and the number and nature of the falls were monitored in structured telephone interviews conducted by research assistants. Stroke type was assessed by study neurologists in a review of computed tomographic or magnetic resonance imaging studies. Data on coexisting conditions were obtained from chart reviews and from scores on the self-reported Functional Impact Scale,31 the Personal Health Questionnaire 9 (PHQ-9) depression scale,32 and the Mini–Mental State Examination.33 Information on usual care visits was obtained from participants’ monthly logs.
ADVERSE EVENTS
Death, life-threatening events (stroke, myocardial infarction, or fracture), readmission to the hospital, and occurrence of a new disability or incapacity that led to more than 48 hours of limitation in activities of daily living were considered serious adverse events.8 Minor adverse events included a fall with no fracture; dyspnea during treatment; an open sore or blister; cuts; muscle soreness or pain that persisted for more than 48 hours; dizziness or faintness; diaphoresis; hypertension or hypotension during exercise that halted the intervention for the day; and deep-vein thrombosis.8
STATISTICAL ANALYSIS
We used a two-sided significance level of 0.05 to determine whether early or late locomotor training was superior to a home-exercise program in the recovery of ability to walk after stroke. The study-wide error rate was controlled by applying Hochberg’s step-up procedure34 to the two primary comparisons. Working from the assumption that 30% of the participants in the home-exercise program would have an improved functional level of walking, we calculated that we would need a sample of 400 participants to detect a clinically relevant effect size of 20%, with 85% power, adjusting for an estimated loss-to-follow-up rate of 15%. This sample size was also sufficient to detect a mean improvement of 0.1 m per second in walking speed between the early and late locomotor-training groups. The differences in baseline characteristics were compared across the three groups with the use of analysis of variance or a chi-square test. Logistic regression was used to compare the proportions of participants with an improved functional level of walking in the three groups, with adjustment for prespecified covariates (severity of impairment, clinical site, age, stroke type, side of hemiparesis, and presence or absence of depression). The second primary analysis assessed the timing effect and its interaction with the initial severity of gait impairment on the change in walking speed from baseline to 1 year after stroke. Missing data were imputed with the use of the last-observation-carried-forward method in the intention-to-treat analysis and with the use of other procedures in sensitivity analyses (see the Supplementary Appendix, available with the full text of this article at NEJM.org).8
For other outcome variables, paired t-tests were used to compare within-group improvements, and analysis of variance was used to assess differences across the three groups, followed by pairwise comparisons. The number of steps taken in the community was analyzed with the use of the Kruskal–Wallis procedure. SAS software, version 9.1, was used to perform all statistical analyses.
RESULTS
CHARACTERISTICS OF THE STUDY POPULATION
From April 2006 through June 2009, a total of 4909 patients were screened and 3137 were excluded. At the second screening, 1364 patients were excluded. The most common reasons for exclusion were the presence of one or more major coexisting medical conditions, absence of residual paresis in the leg on the side of the body affected by stroke, absence of a primary diagnosis of stroke, no expectation of home discharge, a self-selected walking speed greater than 0.8 m per second, and refusal to provide informed consent. Nineteen persons did not pass the exercise tolerance test before randomization. Of the 408 participants included in the intention-to-treat analysis, 139 were assigned to early locomotor training, 143 to late locomotor training, and 126 to home exercise. (For further details on study screening and randomization, see the Supplementary Appendix.)
No significant differences in baseline characteristics were found across the three groups (Table 1). The mean (±SD) age of the participants was 62.0±12.7 years; 54.9% were men, and 22.1% were black. At randomization, the average number of days since the stroke was 63.8±8.5. A total of 71.1% of participants had had ischemic strokes, 99.5% had modified Rankin scores between 2 and 4 (with 1 indicating no significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, and 5 severe disability), 53.4% walked at a rate of less than 0.4 m per second, and 46.6% walked at a rate between 0.4 m per second and less than 0.8 m per second.
Table 1.
Baseline Characteristics of the Study Population.*
| Characteristic | Early LT (N = 139) | Late LT (N = 143) | HE (N = 126) | P Value |
|---|---|---|---|---|
| Male sex — no. (%) | 85 (61.2) | 74 (51.7) | 65 (51.6) | 0.19 |
| Age at stroke onset — yr | 60.1±12.3 | 63.3±12.5 | 62.6±13.3 | 0.08 |
| Race — no. (%)† | 0.81 | |||
| White | 81 (58.3) | 77 (53.8) | 78 (61.9) | |
| Black | 32 (23.0) | 34 (23.8) | 24 (19.0) | |
| Asian | 19 (13.7) | 20 (14.0) | 15 (11.9) | |
| Other | 7 (5.0) | 12 (8.4) | 9 (7.1) | |
| Ethnic group — no. (%)† | 0.12 | |||
| Hispanic | 26 (18.7) | 15 (10.5) | 22 (17.5) | |
| Time from stroke to randomization — days | 64.1±8.3 | 64.18±9.0 | 62.9±8.0 | 0.40 |
| Stroke characteristics — no. (%) | ||||
| Side of hemiparesis | 0.42 | |||
| Left | 63 (45.3) | 58 (40.6) | 61 (48.4) | |
| Right | 76 (54.7) | 85 (59.4) | 65 (51.6) | |
| Stroke type | 0.94 | |||
| Large vessel‡ | 55 (39.6) | 60 (42.0) | 47 (37.3) | |
| Lacunae§ | 40 (28.8) | 45 (31.5) | 43 (34.1) | |
| Hemorrhage | 27 (19.4) | 22 (15.4) | 21 (16.7) | |
| Undefined | 17 (12.2) | 16 (11.2) | 15 (11.9) | |
| Stroke severity according to modified Rankin scale — no. (%) | 0.19 | |||
| 1 | 0 | 2 (1.4) | 0 | |
| 2 | 12 (8.6) | 21 (14.7) | 21 (16.7) | |
| 3–4 | 127 (91.4) | 120 (83.9) | 105 (83.3) | |
| Coexisting conditions — no. (%) | ||||
| Cardiovascular disease¶ | 36 (25.9) | 36 (25.2) | 37 (29.4) | 0.74 |
| Hypertension¶ | 116 (83.5) | 111 (77.6) | 104 (82.5) | 0.56 |
| Peripheral vascular disease¶ | 12 (8.6) | 12 (8.4) | 13 (10.3) | 0.85 |
| COPD¶ | 5 (3.6) | 14 (9.8) | 7 (5.6) | 0.09 |
| Arthritis or other musculoskeletal condition¶ | 47 (33.8) | 52 (36.4) | 47 (37.3) | 0.81 |
| Diabetes¶ | 47 (33.8) | 51 (35.7) | 43 (34.1) | 0.90 |
| Depression according to PHQ-9|| | 20 (14.4) | 28 (19.6) | 19 (15.1) | 0.44 |
| Cognitive function according to Mini–Mental State Exam** | 26.0±3.2 | 26.2±3.7 | 26.0±3.6 | 0.87 |
| Walking speed — no. (%)†† | 0.96 | |||
| Severe impairment (<0.4 m/sec) | 75 (54.0) | 77 (53.8) | 66 (52.4) | |
| Moderate impairment (0.4–<0.8 m/sec) | 64 (46.0) | 66 (46.2) | 60 (47.6) | |
Plus–minus values are means ± SD. P values were derived from analysis of variance or the chi-square test. For the modified Rankin scale, a score of 0 indicates no symptoms, 1 no significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, and 5 severe disability. For the Mini–Mental State Exam, higher scores indicate higher cognitive function, with a score of 0 to 9 indicating severe impairment, and a score of 25 or more indicating normal function. COPD denotes chronic obstructive pulmonary disease, HE home exercise, LT locomotor training, and PHQ-9 Personal Health Questionnaire 9.
Race and ethnic group were self-reported.
Stroke categorized as affecting a large vessel involved occlusion of a major cerebral artery.
Strokes isolated to the territory of a single, small penetrating artery, most often a lenticulostriate vessel, were classified as lacunae.
Information on these conditions was available for 141 participants in the late LT group.
Diagnosis of depression was based on scores on the PHQ-9 depression scale, which range from 0 to 27, with higher scores indicating greater severity of depression; depression was diagnosed in participants with a score greater than or equal to 10.
The number of participants taking the Mini–Mental State Exam was 121 in the early LT group, 128 in the late LT group, and 119 in the HE group.
The extent of impairment in walking speed was determined as severe if walking speed was less than 0.4 m per second and as moderate if from 0.4 to less than 0.8 m per second.
INTERVENTIONS
The intervention was not completed by 13% of participants in the early locomotor-training group, 17% of those in the late locomotor-training group, and 3% of those in the home-exercise group (P<0.001). All treatment groups progressed as planned in the protocol. The duration of a single home-exercise session (76±10 minutes) was significantly less than that of an early locomotor-training session (83±6 minutes) and a late locomotor-training session (82±5 minutes). The locomotor-training groups had significant increases in the duration of stepping time, decreases in the extent of body-weight support and assistance required, and increases in training speed over 36 sessions (P<0.001 for all three comparisons). During the last three sessions, the average time for stepping on the treadmill was 22±5 minutes, the average maximum treadmill speed was 3.2±0.6 km per hour (2.0±0.4 mi per hour), and the minimum level of body-weight support was 11.9±8.9%. Participants also progressed in the portion of the training involving walking over ground (mean duration, 16.5±4.1 minutes). Participants in the home-exercise group also had improvement in all activities (P<0.001). The mean heart rate during the midpoint of each treadmill training session was 90 beats per minute in both locomotor-training groups and 77 beats per minute in the home-exercise group (P<0.001).
Outside of the study, physical therapy was provided for 81.9% of participants for a period of 2 months to 1 year after the stroke. The majority of the physical therapy sessions occurred in outpatient clinics from 2 to 6 months after the stroke (74.0%). The mean duration of a session was 54±12 minutes, and the mean number of sessions was 25±24. In the early locomotor-training group, 72.7% of participants received usual care physical therapy, as compared with 86.0% of those in the late locomotor-training group and 87.3% of those in the home-exercise group (P = 0.002).
PRIMARY OUTCOME
The primary outcome of transition to a higher functional level of walking 1 year after the stroke was achieved by 52.0% of all participants, with no significant difference in the proportions of participants making this transition among the three groups. The adjusted odds ratio for the comparison of the early locomotor-training group with the home-exercise group was 0.83 (95% confidence interval [CI], 0.50 to 1.39), and that for the comparison of the late locomotor-training group with the home-exercise group was 1.19 (95% CI, 0.72 to 1.99). The adjusted odds ratio for the effect of each 1-year increase in age on the primary outcome was 0.95 (95% CI, 0.93 to 0.97). The effects of other covariates were not significant. Results similar to those from the primary analysis were obtained in sensitivity analyses.
SECONDARY OUTCOMES
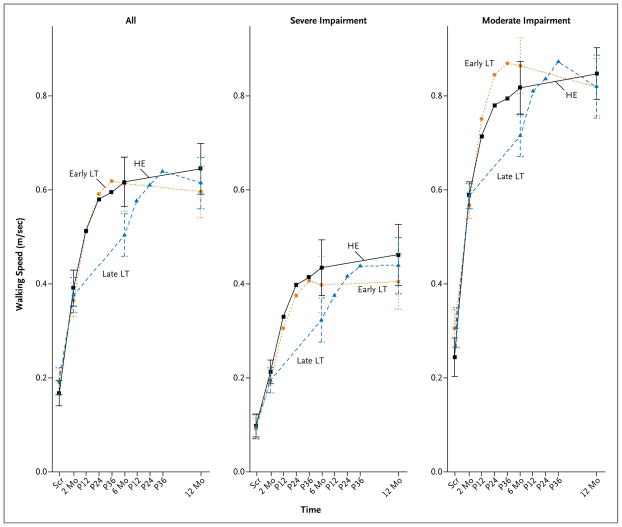
The mean change in comfortable walking speed from baseline to 1 year after stroke was 0.23±0.20 m per second in the early locomotor-training group and 0.24±0.23 m per second in the late locomotor-training group (Table 2). The timing of locomotor training (early vs. late) did not affect changes in walking speed 1 year after stroke (Fig. 1). No significant interaction was found between baseline severity of walking impairment and the timing of locomotor training in their effects on change in walking speed.
Table 2.
Functional Status and Quality of Life at Baseline (2 Months) and Change from Baseline at 6 Months and 12 Months.*
| Variable | Early LT (N = 139) | Late LT (N = 143) | HE (N = 126) | P Value |
|---|---|---|---|---|
| Mean comfortable or usual walking speed — m/sec | ||||
| Baseline | 0.37±0.22 | 0.38±0.23 | 0.39±0.22 | 0.62 |
| Change from baseline | ||||
| 6 mo | 0.25±0.21 | 0.13±0.14 | 0.23±0.20 | <0.001 |
| 12 mo | 0.23±0.20 | 0.24±0.23 | 0.25±0.22 | 0.67 |
| Mean distance walked in 6 min — meters | ||||
| Baseline | 124.1±77.5 | 125.7±81.8 | 126.3±75.0 | 0.97 |
| Change from baseline | ||||
| 6 mo | 81.8±62.8 | 41.0±47.4 | 75.9±69.3 | <0.001 |
| 12 mo | 73.2±69.4 | 79.0±75.1 | 85.2±72.9 | 0.45 |
| Step Activity Monitor — median no. steps per day (25th and 75th percentiles) | ||||
| Baseline | 1468 (601, 3889) | 1664 (647, 3354) | 1882.5 (905, 3384) | 0.75 |
| Change from baseline | ||||
| 6 mo | 1017 (−102, 2209) | 565.5 (−362, 2043) | 1357 (84, 3382) | 0.04 |
| 12 mo | 858 (−253, 2422) | 1022 (−111, 3009) | 1471 (435, 3481) | 0.10 |
| Score on Stroke Impact Scale — range, 0–100† | ||||
| ADL–IADL | ||||
| Baseline | 54.1±20.1 | 55.7±20.4 | 54.4±20.7 | 0.78 |
| Change from baseline | ||||
| 6 mo | 9.8±17.2 | 7.0±17.8 | 13.0±16.9 | 0.03 |
| 12 mo | 9.6±19.5 | 9.4±17.2 | 14.5±19.0 | 0.07 |
| Participation | ||||
| Baseline | 45.0±23.3 | 46.6±23.0 | 44.8±23.5 | 0.77 |
| Change from baseline | ||||
| 6 mo | 11.8±26.8 | 7.7±20.5 | 14.7±22.9 | 0.06 |
| 12 mo | 17.1±25.9 | 13.1±22.0 | 14.4±20.6 | 0.38 |
| Mobility | ||||
| Baseline | 57.0±22.1 | 60.5±20.7 | 59.1±19.5 | 0.366 |
| Change from baseline | ||||
| 6 mo | 15.3±21.4 | 7.0±15.7 | 14.9±20.0 | <0.001 |
| 12 mo | 13.7±21.6 | 12.0±19.1 | 14.2±20.3 | 0.685 |
| Fugl-Meyer score for motor recovery in legs — range, 0–34‡ | ||||
| Baseline | 23.7±6.7 | 24.8±6.4 | 24.7±6.3 | 0.26 |
| Change from baseline | ||||
| 6 mo | 2.2±3.4 | 1.3±3.3 | 2.4±4.1 | 0.04 |
| 12 mo | 1.7±3.9 | 1.5±3.7 | 2.5±4.3 | 0.13 |
| Berg balance score, range, 0–56§ | ||||
| Baseline | 35.0±14.4 | 35.9±14.1 | 36.5±13.6 | 0.71 |
| Change from baseline | ||||
| 6 mo | 8.8±8.1 | 5.3±7.0 | 7.9±8.5 | 0.001 |
| 12 mo | 8.0±7.8 | 5.9±9.1 | 8.3±8.78 | 0.06 |
| Activities–Specific Balance Confidence Scale score, range, 0–100¶ | ||||
| Baseline | 43.6±23.5 | 47.0±25.4 | 44.5±22.6 | 0.48 |
| Change from baseline | ||||
| 6 mo | 13.8±20.8 | 6.2±20.2 | 15.6±19.4 | <0.001 |
| 12 mo | 11.2±22.3 | 11.7±22.1 | 13.9±21.7 | 0.62 |
Plus–minus values are means ±SD. A higher score is better for all outcomes. For cases in which distributions were skewed, medians and 25th and 75th percentiles are provided. P values were derived from an analysis of variance or from Kruskal–Wallis tests of differences across the three groups. All within-group changes from baseline to 12 months were significant (P<0.001) according to the results of paired t-tests. ADL–IADL denotes activities of daily living–instrumental activities of daily living, HE home exercise, and LT locomotor training.
The Stroke Impact Scale is a measure of function (including ADL–IADL and mobility) and quality of life (participation). The ADL–IADL Scale is a single domain of the Stroke Impact Scale in which ADL is defined as the ability to take care of basic needs (e.g., dressing, bathing, and eating) and IADL is defined as the ability to perform activities that make it possible to live independently in the community (e.g., shopping and managing household chores), with 0 indicating complete dependence on others and 100 indicating the ability to live independently without difficulty. The participation domain is defined as the ability to participate in social, work, or leisure activities, with 0 indicating inability to engage in any of these aspects of life and 100 indicating full engagement all the time.
The Fugl-Meyer leg test is a measure of motor function, with 0 indicating the absence of volitional movement and 34 indicating good and selective movements.
The Berg Balance Scale assesses balance in sitting, standing, reaching, shifting weight, and turning, with 0 defined as the inability to balance and 56 defined as the ability to maintain balance independently and without difficulty while performing each task.
The Activities–Specific Balance Confidence Scale is a self-reported measure of confidence that activities such as walking around the house, standing on a chair to reach for something, or getting out of a car can be performed without losing balance or becoming unsteady, with 0 defined as having no confidence that the activities can be performed without losing balance and 100 as having confidence that the activities can be accomplished without losing balance.
Figure 1. Timing of Locomotor Training and Changes in Walking Speed 1 Year after Stroke.
Screening (Scr) was performed at a mean (±SD) of 26.0±11.6 days after stroke. Randomization was performed at baseline, 2 months after stroke. I bars indicate 95% confidence intervals. HE denotes home exercise, LT locomotor training, and P12, P24, and P36 post-training assessments at weeks 12, 24, and 36, respectively.
Six months after the stroke, the early locomotor-training group and the home-exercise group had similar gains in walking speed (0.25±0.21 m per second and 0.23±0.20 m per second, respectively), and these gains were sustained at 1 year. The late locomotor-training group improved walking speed by 0.13±0.14 m per second at 6 months and by 0.24±0.23 m per second at 1 year.
All groups had a similar improvement from baseline to 1 year in the distance walked in 6 minutes, the number of steps taken in the community, activities of daily living, physical mobility and social participation, motor recovery, and balance (Table 2). Participants in the early interventions (early locomotor training and home exercise) had improvements at 6 months that were sustained at 1 year. At 6 months, the late locomotor-training group had significantly less recovery, having received only usual care, but at 1 year had outcomes that were similar to those in the other two groups (Table 2).
Among all participants, 57.6% reported a fall and 5.9% had an injurious fall, but there were no significant differences in falls among the three groups. Multiple falls were recorded for 34.1% of all participants and for 41.0% of those in the early locomotor-training group, 32.9% of those in the late locomotor-training group, and 27.8% of those in the home-exercise group (P=0.07). Among participants with severe walking impairment at baseline (walking speed <0.4 m second), the proportion of patients with multiple falls was higher in the early locomotor-training group (52.0%) than in the late locomotor-training group (36.4%, P=0.05) or the home-exercise group (30.3%, P=0.009).
SAFETY
The frequency of serious adverse events did not differ significantly among the three groups (Table 3). Minor adverse events (mostly falls) were reported by 55.9% of participants, with no significant differences among groups, except that none of the participants in the home-exercise group reported incidents of dizziness or faintness during exercise (0%), as compared with 7.9% of those in the early locomotor-training group (P = 0.001) and 5.6% of those in the late locomotor-training group (P = 0.008).
Table 3.
Serious Adverse Events and Multiple Falls.
| Event | All (N = 408) | Early LT (N = 139) | Late LT (N = 143) | HE (N = 126) | P Value |
|---|---|---|---|---|---|
| number (percent) | |||||
| Any serious adverse event | 149 | 51 (36.7) | 59 (41.3) | 39 (31.0) | 0.22 |
|
| |||||
| Recurrent stroke | 10 | 6 (4.3) | 3 (2.1) | 1 (0.8) | 0.17 |
|
| |||||
| Myocardial infarction | 2 | 0 | 1 (0.7) | 1 (0.8) | 0.59 |
|
| |||||
| Fracture | 21 | 8 (5.8) | 8 (5.6) | 5 (4.0) | 0.77 |
|
| |||||
| Hospitalization | 129 | 45 (32.4) | 53 (37.1) | 31 (24.6) | 0.09 |
|
| |||||
| Limitation in activities of daily living for >48 hrs | 7 | 1 (0.7) | 2 (1.4) | 4 (3.2) | 0.29 |
|
| |||||
| Death | 13 | 3 (2.2) | 4 (2.8) | 6 (4.8) | 0.46 |
|
| |||||
| Other | 6 | 1 (0.7) | 1 (0.7) | 4 (3.2) | 0.16 |
|
| |||||
| Multiple falls
| |||||
| Moderate walking impairment at baseline | 52 | 18 (28.1) | 19 (28.8) | 15 (25.0) | 0.88 |
|
| |||||
| Severe walking impairment at baseline† | 87 | 39 (52.0) | 28 (36.4) | 20 (30.3) | 0.02 |
|
| |||||
| All | 139 | 57 (41.0) | 47 (32.9) | 35 (27.8) | 0.07 |
Because a serious adverse event may belong to more than one category (e.g., recurrent stroke and hospitalization), the total number of participants with a serious adverse event does not equal the sum of the individual categories of serious adverse events. HE denotes home exercise, and LT locomotor training.
Among participants with severe walking impairment, a chi-square test showed that the proportion of patients with multiple falls was higher in the early LT group than in the late LT group (P = 0.05) and in the HE group (P = 0.009).
DISCUSSION
In stroke survivors living in the community with marked limitations in walking, task-specific step training that included treadmill training with body-weight support (locomotor training) was not shown to be superior in improving the functional level of walking to home-administered physical therapy focused on less-intensive but progressive strength and balance training. Among all participants in all three training groups, 52% had an improved functional level of walking and clinically meaningful improvements in walking speed,35 distance walked,36 and steps taken in the community. Improvements in balance, activities of daily living, physical mobility, and social participation were also clinically significant.37 Changes in scores on the Fugl-Meyer Assessment of Motor Recovery in the legs were modest.27 Participants with initially moderate impairment and those with initially severe impairment had improvement, and the timing of the locomotor training did not affect outcomes at 1 year. In contrast with the late gains observed in patients assigned to late locomotor training, participants assigned to early locomotor training or home exercise had early gains in walking and functional outcomes (i.e., at 6 months) that were sustained at 1 year. Although 1-year outcomes were similar for participants in the early and late locomotor-training groups, these results suggest that interventions at 2 months may accelerate walking gains after stroke.
Locomotor training was associated with a higher frequency of minor adverse events than was home exercise, and among participants with severe impairment at baseline, those in the early locomotor training group were more likely to have multiple falls than those in either of the other two groups. The locomotor-training interventions stressed stepping and walking and did not include progressive balance-specific training. In previous studies with elderly participants, interventions aimed at improving walking ability that were not accompanied by balance training resulted in increased falls, especially in those with more severe limitations.38 The rate of single falls among all participants (57.6%) was in the range previously reported among persons who had had a stroke (43 to 73%).3 This finding points to the need for increased management of multiple risk factors to prevent falls.39,40
As compared with locomotor training, home exercise requires less expensive equipment, its implementation requires a smaller number of staff members, less training is required for physical therapists, and patients are more likely to comply with the regimen. Collectively, our results suggest that home exercise is a more pragmatic form of therapy with fewer risks.
A limitation of this study is the lack of a group receiving no physical therapy for comparison with the home-exercise and locomotor-training groups at 1 year. Without such a comparison, the effectiveness of home exercise and locomotor training in achieving gains at 1 year that are greater than those attributable to usual care could not be proven. However, in a secondary finding at 6 months after stroke, participants in both early locomotor training and early home exercise had better outcomes than those who had received usual care but had not yet begun locomotor training.
Given the rapid adoption in clinical practice of commercially available lifts and robot-assisted treadmill steppers, it is imperative to compare the effectiveness of these task-specific interventions with that of less complex but structured therapies. At 1 year after stroke, our findings did not establish the superiority of locomotor training on a treadmill that included bodyweight support over home-based physical therapy that emphasized strength and balance, regardless of whether locomotor training was started 2 or 6 months after the stroke. The home-exercise program had fewer risks and may be more feasible. In addition, the rate of multiple falls among the severely impaired participants in the early locomotor-training group suggests that therapy aimed at improving balance should be incorporated into training programs designed to improve walking ability.
Supplementary Material
Acknowledgments
Supported by the National Institute of Neurological Disorders and Stroke (RO1 NS050506) and the National Center for Medical Rehabilitation Research. The body-weight support treadmill systems and the cost of all study screenings and interventions were supported by funding from the National Institutes of Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Heart disease and stroke statistics — 2010 update. Dallas: American Heart Association; 2010. [Google Scholar]
- 2.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 3.Weerdesteyn V, de Niet M, van Duijnhoven HJR, Geurts ACH. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45:1195–213. [PubMed] [Google Scholar]
- 4.Pouwels S, Lalmohamed A, Leufkens B, et al. Risk of hip/femur fracture after stroke: a population-based case-control study. Stroke. 2009;40:3281–5. doi: 10.1161/STROKEAHA.109.554055. [DOI] [PubMed] [Google Scholar]
- 5.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86:1552–6. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 7.Weinrich M, Good DC, Reding M, et al. Timing, intensity, and duration of rehabilitation for hip fracture and stroke: report of a workshop at the National Center for Medical Rehabilitation Research. Neurorehabil Neural Repair. 2004;18:12–28. doi: 10.1177/0888439003262041. [DOI] [PubMed] [Google Scholar]
- 8.Duncan PW, Sullivan KJ, Behrman AL, et al. Protocol for the Locomotor Experience Applied Post-stroke (LEAPS) trial: a randomized controlled trial. BMC Neurol. 2007;7:39. doi: 10.1186/1471-2377-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan KJ, Brown DA, Klassen T, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys Ther. 2007;87:1580–602. doi: 10.2522/ptj.20060310. [DOI] [PubMed] [Google Scholar]
- 10.Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29:1122–8. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- 11.Barbeau H, Visintin M. Optimal outcomes obtained with body-weight support combined with treadmill training in stroke subjects. Arch Phys Med Rehabil. 2003;84:1458–65. doi: 10.1016/s0003-9993(03)00361-7. [DOI] [PubMed] [Google Scholar]
- 12.Hesse S, Bertelt C, Jahnke MT, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke. 1995;26:976–81. doi: 10.1161/01.str.26.6.976. [DOI] [PubMed] [Google Scholar]
- 13.Pohl M, Mehrholz J, Ritschel C, Rückriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke. 2002;33:553–8. doi: 10.1161/hs0202.102365. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–91. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 15.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005;36:2206–11. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 16.Eich HJ, Mach H, Werner C, Hesse S. Aerobic treadmill plus Bobath walking training improves walking in subacute stroke: a randomized controlled trial. Clin Rehabil. 2004;18:640–51. doi: 10.1191/0269215504cr779oa. [DOI] [PubMed] [Google Scholar]
- 17.Hidler J, Nichols D, Pelliccio M, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23:5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 18.Hesse S. Treadmill training with partial body weight support after stroke: a review. NeuroRehabilitation. 2008;23:55–65. [PubMed] [Google Scholar]
- 19.Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2005;4:CD002840. doi: 10.1002/14651858.CD002840.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–80. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 21.Duncan PW, Goldstein LB, Horner RD, Landsman PB, Samsa GP, Matchar DB. Similar motor recovery of upper and lower extremities after stroke. Stroke. 1994;25:1181–8. doi: 10.1161/01.str.25.6.1181. [DOI] [PubMed] [Google Scholar]
- 22.Yates JS, Studenski S, Gollub S, et al. Bicycle ergometry in subacute-stroke survivors: feasibility, safety, and exercise performance. J Aging Phys Act. 2004;12:64–74. doi: 10.1123/japa.12.1.64. [DOI] [PubMed] [Google Scholar]
- 23.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 24.Eng JJ, Chu KS, Dawson AS, Kim CM, Hepburn KE. Functional walk tests in individuals with stroke: relation to perceived exertion and myocardial exertion. Stroke. 2002;33:756–61. doi: 10.1161/hs0302.104195. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Resnick B, Nahm ES, Orwig D, Zimmerman SS, Magaziner J. Measurement of activity in older adults: reliability and validity of the Step Activity Monitor. J Nurs Meas. 2001;9:275–90. [PubMed] [Google Scholar]
- 27.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–40. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 28.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27:27–36. [PubMed] [Google Scholar]
- 29.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Bode RK, Min Lai S, Perera S Glycine Antagonist in Neuroprotection Americans Investigators. Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil. 2003;84:950–63. doi: 10.1016/s0003-9993(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 31.Rigler SK, Studenski S, Wallace D, Reker DM, Duncan PW. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16:420–8. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 32.Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke. 2005;36:635–8. doi: 10.1161/01.STR.0000155688.18207.33. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 34.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–2. [Google Scholar]
- 35.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90:196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulk GD, Echternach JL, Nof L, O’Sullivan S. Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke. Physiother Theory Pract. 2008;24:195–204. doi: 10.1080/09593980701588284. [DOI] [PubMed] [Google Scholar]
- 37.Lin K-C, Fu T, Wu C-Y, et al. Minimal detectable change and clinically important difference of the Stroke Impact Scale in stroke patients. Neurorehabil Neural Repair. 2010;24:486–92. doi: 10.1177/1545968309356295. [DOI] [PubMed] [Google Scholar]
- 38.Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JCT. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc. 2008;56:2234–43. doi: 10.1111/j.1532-5415.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 39.Guideline for prevention of falls in older persons. J Am Geriatr Soc. 2001;49:664–72. [PubMed] [Google Scholar]
- 40.Batchelor F, Hill K, Mackintosh S, Said C. What works in falls prevention after stroke? A systematic review and meta-analysis. Stroke. 2010;41:1715–22. doi: 10.1161/STROKEAHA.109.570390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.