Abstract
Objective
The development of the ability to process spoken and written language depends upon a network of left hemisphere temporal, parietal, and frontal regions. The present study explored features of brain organization in children with spina bifida meningomyelocele (SBM) and shunted hydrocephalus, who commonly present with precocious development of word reading skills and preservation of vocabulary and grammar skills.
Method
Eight children with SBM were compared with 15 IQ and reading-level matched, typically developing controls on MRI-based morphometric and Magnetic Source Imaging-derived neurophysiological profiles.
Results
Children with SBM showed reduced magnetic activity in left inferior parietal regions during spoken word recognition and pseudoword reading tasks. We also noted reduced surface area/volume in inferior parietal and posterior temporal regions in SBM and increased gray matter volumes in left middle frontal regions and gyral complexity in left posterior temporal and inferior parietal regions.
Conclusions
A complex pattern of changes in cortical morphology and activation may serve as evidence for structural and functional brain reorganization ensuring preservation of language and decoding abilities in children with SBM.
Keywords: phonological decoding, word recognition, hydrocephalus, magnetoencephalography, functional brain imaging
Spina bifida meningomyelocele (SBM) is a congenital CNS disorder characterized by malformations of the spine and brain that begin during formation of the neural tube in the first 30 days of gestation. SBM is the most common congenital birth defect affecting the central nervous system and the most common etiology of congenital hydrocephalus (Detrait et al., 2006). In addition to the defining spinal lesion apparent at birth, children with SBM also experience brain malformations involving the cerebellum/hind-brain (Chiari II; CII) and the corpus callosum. The CII malformation leads to obstruction of the flow of cerebrospinal fluid (CSF), necessitating implantation of a shunt to direct CSF around the obstruction (McCullough, 1990). Hydrocephalus represents a diffuse insult to the brain that stretches and destroys axonal pathways and reduces the thickness of the cerebral mantle (Del Bigio, 2004). In addition, partial dysgenesis and hypoplasia (thinning) of the corpus callosum occur in most children with SBM (Hannay et al., 2009).
Despite significant congenital insults to the brain, intellectual deficiency is not the rule; rather, children with SBM often show unusual patterns of neuropsychological strengths and weaknesses (Dennis, Landry, Barnes, & Fletcher, 2006). Many children with SBM display significantly lower scores on measures that require the assembling and construction of verbal and nonverbal information, such as the evaluation of three dimensional spatial relations, reading and language comprehension, and declarative memory. In contrast, they are stronger on measures that reflect associative learning, such as procedural motor learning, word decoding, vocabulary, and grammar (Dennis et al., 2006).
Little is known about the neural mechanisms that mediate motor, somatosensory, language, and cognitive functions in children with congenital brain defects (Broman & Fletcher, 1999). Such studies are necessary in order to account for the diversity in cognitive abilities and academic achievement profiles among children who present with disrupted neural development. Thus far, studies on SBM, largely by our group, report that the distribution of gray matter, white matter, and CSF is different in children with SBM and age-matched controls in ways that are predictable based on the effects of the congenital malformations and hydrocephalus (Fletcher et al., 1992; Fletcher et al., 1996; Hasan et al., 2008; Juranek et al., 2008; Juranek & Salman, 2010). Thus, the posterior regions of the brain show reduced volumes of gray and white matter with increased CSF volume, while anterior regions are more similar to controls. The cerebellum is smaller in total volume. White matter is reduced more in posterior segments of the corpus callosum and in long connecting white matter pathways (e.g., associative bundles). Not surprisingly, functions that are mediated by the cerebellum and more posterior regions of the brain (parietal and posterior temporal) are impaired in SBM. In addition, tasks that require integration of information, such as text and discourse comprehension, are impaired, which is not surprising given the prominent role of these brain areas in facilitating intersensory and cross-modal integration (e.g., angular gyrus). In contrast, functions mediated by the anterior temporal and frontal regions appear better developed.
A complementary approach to identify potential deviations from the typical organization of the brain circuits that support cognitive functions in SBM is to examine regional brain activation profiles either at rest or during the performance of tasks representative of the cognitive functions listed in the previous paragraphs. Studies of resting-state brain activity measured via electroencephalography have reported both generalized and focal slow wave activity (Al-Sulaiman & Ismail, 1998), as well as focal continuous spike-wave discharges in children with hydrocephalus during sleep (Battaglia et al., 2004; Veggiotti et al., 1998). More recently, a study by our group (Castillo et al., 2009) combining magnetoencephalography (MEG) and diffusion tensor imaging (DTI) found a reduction in spectral power (theta, alpha, and beta bands) in posterior regions of children with SBM, which correlated with decreased surface area of the posterior segments of the corpus callosum. Studies of task-related regional brain activation in children with SBM are virtually nonexistent. Functional brain imaging investigations in typically developing individuals may inform predictions regarding the outline of brain mechanisms involved in spoken word recognition and decoding of printed material: the functions that the present study focuses on. Normally, both functions are mediated by complex networks that include common brain regions such as the superior temporal, middle temporal, and supramarginal gyri, especially in the left hemisphere (e.g., Binder et al., 1996; Booth et al., 2002; Gaillard et al., 2001; Hickok & Poeppel, 2004). The brain circuit responsible for encoding and decoding printed words and pronounceable word-like stimuli comprises, in addition, extrastriate visual cortex (lateral and ventral occipitotemporal areas) and the angular gyrus, primarily in the left hemisphere (Booth et al., 2003; Cornelissen et al., 2009; McCandliss, Cohen, & Dehaene, 2003; Mechelli, Gorno-Tempini, & Price, 2003; Pugh et al., 1996). Activation of prefrontal cortex (mainly inferior and middle frontal regions) is also commonly observed during performance of reading tasks, and is suspected to play a compensatory role among children with reading difficulties (Cao et al., 2006; Richards et al., 2002; Shaywitz et al., 2002; Simos et al., 2007a, b).
Like many populations with congenital neurogenetic disorders, children with SBM often show well-developed spoken word recognition and decoding of printed material despite impairment in posterior temporal and parietal regions (Dennis et al., 2006). Altered organization of the brain circuits that typically support these skills may account for the relative preservation of word reading skills in SBM. Given the extensive individual variability in anatomy and also in phenotypic expression at the cognitive level, it is important to combine measures of regional anatomy with indices of the degree of regional cortical activity in order to explore potential patterns of brain reorganization. Information regarding developmental changes in regional cortical anatomy alone are not sufficient to approach this issue. Thus, while regional estimates of white and gray matter size (cortical surface area, gray and white matter volume, and cortical thickness) are generally assumed to correlate positively with cognitive measures, the relation between measures of regional cortical complexity and cognitive development is less straightforward. For instance, there is preliminary evidence of a gradual decrease in the degree of cortical complexity between childhood and adolescence (White, Su, Schmidt, Kao, & Sapiro, 2010). The latter is commonly assessed through the gyrification index (LGI), which measures the degree of cortical folding (Zilles, Armstrong, Schleicher, & Kretschmann, 1988). Regional estimates of gyrification are often increased in psychiatric and neurological conditions relative to controls (Jou, Minshew, Keshavan, & Hardan, 2010; Kesler et al., 2006; Oyegbile, Magnotta, O’Leary et al., 2004). However, associations between local gyrification indices and cognitive abilities are sometimes reported as positive (e.g., McIntosh, Moorhead, McKirdy et al., 2009) and other times as negative (e.g., Kesler et al., 2006) depending on the brain area(s) measured and the particular disorder. Therefore, the direction of deviations in cortical morphometry alone does not inform on the functional significance of these changes.
The present study adopted a multimodality approach to evaluate anatomical and functional brain organization that support preserved word reading skills in children with SBM. Measures of regional cortical anatomy included gray matter volume, cortical surface area, cortical thickness, and gyrification. Functional brain activation profiles associated with spoken word processing and pseudoword reading were also obtained using magnetic source imaging (MSI). Both tasks represent abilities which typically remain unimpaired in SBM, although normative studies suggest that word reading skills are mediated by posterior language circuits (mainly posterior temporal and inferior parietal cortices). Given the relatively small sample size (eight children with SBM), assessing structural models that include both direct and indirect effects of anatomical and neurophysiological measures on achievement was not feasible. To partially overcome this limitation we adopted a three-step approach. First, we determined differences in anatomical and neurophysiological measures in brain areas known to be important for word recognition and reading, between children with SBM and typically developing controls comparable on word-level reading and general intellectual ability. Group comparisons on anatomical measures also served to assess the compatibility of current results with those from our larger SBM cohort (Juranek et al., 2008; Juranek & Salman, 2010). Second, we established the degree and direction of associations between these measures. Finally, we assessed relations between anatomical and achievement measures and between neurophysiological and achievement measures.
With respect to the first goal of the study, we expected to confirm previous reports from the larger cohort of SBM children (Juranek et al., 2008; Juranek & Salman, 2010) regarding reduced cortical thickness and/or gray matter volume/surface area and increased gyrification in posterior temporal and inferior parietal regions. Further, we assumed that the direction and anatomical outline of regions showing group differences in task-related brain activation would depend upon the direction of associations between degree of regional neurophysiological activity and achievement. Thus, we expected that among anatomical measures, cortical thickness would show the most consistent positive association with the average degree of late neurophysiological activity (i.e., occurring later than 200 ms after stimulus onset) in association areas (Ilg et al., 2008; Schaechter, Moore, Connell, Rosen, & Dijkhuizen, 2006). The nature of associations between gyrification and degree of MSI-derived regional activity is likely more complex given that the later originates primarily from cortex lining the banks of sulci (Murakami & Okada, 2006). Increased gyrification implies increased cortical folding that may lead to increased cortical surface buried within sulci and increased MSI signals compared to signals produced by equal cortical surface located primarily on the crests of gyri. This relation is expected to hold for both typically developing and SBM brains. On the other hand, if increased gyrification reflects disrupted cortical development (Ajayi-Obe, Saeed, Cowan, Rutherford, & Edwards, 2000; Casanova et al., 2004; Demonet & Habib, 2001; Jackson & Plante, 1996), then it may be associated with reduced synchronized neuronal signaling in affected areas in SBM brains, suppressing magnetic signals. The two processes may operate antagonistically, keeping the net MSI-derived cortical current in affected areas constant and reduce or even eliminate differences between SBM and typically developing children.
The relation between degree of regional MEG-derived activation and achievement may also be complex. Thus, positive correlations have been found between degree of activity in left posterior temporal and inferior parietal areas and achievement in nonreading impaired, neurologically intact children (Simos et al., in press). Conversely, among neurologically intact students experiencing reading difficulties studies have either found negative correlations between achievement and activity in areas presumed to play a compensatory role within the circuit for reading (right temporoparietal cortex and inferior frontal gyrus) or failed to find any activity-performance correlations at all.
Method
Participants
Eight children with SBM and eight neurologically intact typically developing (TD) children were group-matched on sex (five boys in each group). The selected children were also right-handed with decoding skills in the average range. Participants with SBM received the four-subtest form of the Stanford–Binet Intelligence test, 4th Edition (Thorndike et al., 1986), from which a composite index of Full-Scale IQ was generated. Controls received the two-subtest version of the Wechsler Abbreviated Scales of Intelligence (Weschler, 1999). Given the use of different measures, we did not compare the two groups directly, although as expected, participants with SBM had slightly lower estimated Full-Scale IQ scores.
Written informed consent was obtained from the parents and assent from children participating in the study according to institutional review boards for the protection of human research participants at the University of Houston and University of Texas Health Science Center, Houston. As Table 1 shows, the group with SBM tended to have slightly higher decoding skills (Woodcock-Johnson III, Word Attack subtest; Woodcock, McGrew, & Mather, 2001) than the TD group. The present group with SBM presented with strong decoding skills and estimated Full-Scale IQ scores which were on average approximately 0.5 SD higher than in our larger cohort of children with SBM (Fletcher et al., 2005). On average, children with SBM had slightly lower sight word reading scores on the Test of Word Reading Efficiency (TOWRE; Torgesen, Wagner, & Raschotte, 1999) than controls (p > .1). Demographic characteristics for the SBM and TD groups in Table 1 show that as a group, children with SBM were slightly older than the TD group, F(1, 15) = 4.78, p = .05, so age was used as a covariate in all subsequent analyses.
Table 1.
Demographic and Psychoeducational Data on the Two Matched Groups of Participants
| Controls (n = 8) | SBM (n = 8) | |
|---|---|---|
| Gender (boys/girls) | 6/2 | 5/3 |
| Age (years) | 14.5 ± 0.8 | 16.1 ± 1.8 |
| Ethnicity (C, H, A-AM) | 1/4/3 | 1/7/0 |
| Handedness (R/L) | 8/0 | 8/0 |
| FSIQ | 99.3 ± 11.2 | 89.8 ±10.0 |
| WJ-III Word Attack | 92.1 ± 2.8 | 99.4 ± 9.6 |
| TOWRE WRE | 94.5 ± 10.4 | 86.0 ± 9.6 |
Note. For ethnicity: C = Caucasian; H = Hispanic; A-AM = African American; Full-Scale IQ (FSIQ) refers to the average of Vocabulary and Pattern Analysis subtests of the Stanford-Binet Fourth Edition for SBM, and the average of two subtests of the Wechsler Abbreviated Scale of Intelligence for Controls; WJ-III Word Attack: Woodcock-Johnson-III Word Attack subtest; Test of Word Reading Efficiency, Sight Word Reading subtest.
Spina bifida was verified by medical review of pathology and neurosurgical operative reports. Table 2 provides the clinical characteristics of the participants with SBM, showing that the eight participants are representative of larger cohorts of these children (Fletcher et al., 2005), with the exception of a low rate of dysgenesis of the corpus callosum. All but one participant (SB4) were born with a meningomyelocele and had shunted hydrocephalus. This child was born with a lipomeningomyelocele and had no concurrent MRI evidence of a Chiari II malformation, but did have imaging studies prior to shunting at 90 days suggestive of a Chiari II malformation. Only one child had more than three shunt revisions and one child had a history of seizures that at the time of testing were controlled without anticonvulsants.
Table 2.
Clinical Characteristics of Participants With SBM
| SBM1 | SBM2 | SBM3 | SBM4 | SBM5 | SBM6 | SBM7 | SBM8 | |
|---|---|---|---|---|---|---|---|---|
| Lesion Level | L | L | L | L | S | L | T | L |
| Chiari | II | II | II | none | II | II | II | II |
| Shunt (days) | 7 | 2 | 15 | 90 | 3 | 15 | 13 | 8 |
| Shunt Side | L | L | R | R | R | R | R | R |
| # Shunt Revisions | 3 | 14 | 0 | 3 | 2 | 3 | 1 | 1 |
| Seizures | no | no | no | no | no | no | no | yes |
| Age (months) | 186 | 217 | 164 | 191 | 217 | 234 | 177 | 241 |
| Corpus Callosum | D | H | H | H | H | H | H | H |
| Ambulatory status | P | U | U | I | P | I | U | I |
Note. Lesion refers to level of spinal lesion (L = lumbar, S = sacral, T = thoracic); Shunt refers to initial insertion in days after birth;
Shunt Revisions: number of shunt revisions; Seizure refers to history of seizures; Corpus callosum: presence of hypoplasticity (H; thinning) or dysgenesis (D) in one or more regions; Ambulatory status (P = partial, U = unable, I = independent).
Both MEG and MRIs were available for seven additional, neurologically intact participants with a mean age of 161 (SD = 11) months, age range: 142–180 months, with negative history of psychiatric disorder (including ADHD) and typical reading development (as indicated by scores over 90 points on the WJ-III Word Attack and Letter-Word Identification subtests). The entire group of control participants (N = 15) had mean Full-Scale IQ scores of 99 (SD = 8) points (range: 86–114 points). Their data was used to assess the concurrent validity of morphometric data, as explained in more detail below.
Stimuli and Tasks
Brain activation profiles associated with language comprehension and reading were investigated in the context of two MEG language mapping protocols validated in many studies, including direct anatomical stimulation as part of neurosurgical interventions (Papanicolaou et al., 2004). The first experiment involved an auditory word recognition task, whereby participants heard target words before the MEG recording session began, and were instructed to remember them so as to identify them when they appeared with new, distractor words during the MEG recording session. The word lists consisted of words with scores of 3.0 or lower on the Paivio Concreteness scale (Paivio, Yuille, & Madigan, 1968). Word frequency ranged from “very frequent” (AA) to nine occurrences per million for some words. Five words were used as targets randomly mixed with 35 distractors and presented in three blocks. The spoken words were produced by a native English speaker with a flat intonation (duration between 300 and 750 ms, mean 450 ms), digitized with a sampling rate of 22,000 Hz and 16-bit resolution, and delivered via two 5-m-long plastic tubes terminating in ear inserts at an intensity of 80 dB SPL at the participant’s outer ear. There was no significant difference between target and distractor word lists on average concreteness or frequency. Stimulus presentation parameters were identical during the actual MEG recording and the study session. Stimuli were presented with a variable interstimulus interval (2.5–3.5 s).
In the second experiment, MEG recordings were acquired while children performed a pseudoword reading task, involving threeletter pronounceable nonwords (e.g., lan), subtending 2.0° of visual angle, which were adapted versions of those used previously by our group in studies of reading (Simos et al., 2007). The printed stimuli were presented randomly arranged in four blocks of 25 items each. Stimuli were presented for 1500 ms, one at a time (with a randomly varied interstimulus interval of 3–4 s), on a back-projection screen located approximately 60 cm in front of the participant. Task order was counterbalanced across participants. In order to avoid contamination of data with myogenic artifacts, time-locked brain responses were recorded between the presentation of the stimuli and the onset of the child’s naming response.
Functional Brain Imaging
Magnetoencephalography or magnetic source imaging (MSI) recordings were obtained with a whole-head neuromagnetometer array (4-D Neuroimaging, Magnes WH3600), consisting of 248 first-order axial gradiometer coils and housed in a magnetically shielded chamber. The magnetic flux measurements were digitized at 250 Hz, filtered with a bandpass filter between 0.1 and 20 Hz and subjected to a noise reduction algorithm that is part of the 4D-Neuroimaging software. The single-trial event-related field segments (ERFs) in response to 60–80 stimulus presentations, were averaged after excluding those containing eye movement or other myogenic or mechanical artifacts.
To identify the intracranial origin of ERFs the magnetic flux distribution recorded simultaneously over the entire head surface at successive points (4 ms apart) was analyzed using a minimum norm model to obtain estimates of the time-varying strength of intracranial currents (MNE Software, v. 2.5, Hämäläinen, 2006). This method affords greater spatial resolution and allows detection of simultaneous magnetic sources distributed along the entire cortical surface. The model assumes a continuous distribution of current along the cortical surface which has some minimum norm (Hämäläinen & Ilmoniemi, 1994). Estimated current sources were anatomically constrained by an MRI-derived surface model of each participant’s brain. This surface model was generated by a fully automated cortical surface reconstruction procedure using FreeSurfer software (Dale, Fischl & Sereno, 1999) for producing a detailed geometric description (regular tessellation of the cortical surface consisting of equilateral triangles known as vertices) of the gray-white matter boundary of the neocortical mantle and the mesial temporal lobe. Each hemisphere consisted of approximately 150,000 vertices (depending on each subject’s cortical surface area). For estimating current sources, the MNE software requires the FreeSurfer-derived cortical surface reconstruction for defining the boundaries of a solution source space. A grid-spacing of 7 mm was used to construct icosahedrons to decimate the number of vertices from 150,000 to approximately 3,000 per hemisphere. Additionally, the MNE software was used to construct a single compartment boundary element model using triangular tessellations to model each vertex as a potential current dipole perpendicular to the cortical surface during the forward calculations. The inverse solution was subsequently reduced to obtaining an estimate of the scalar distribution of dipole strength across current sources within orientation-specific cortical patches of vertices (Dale et al., 1999). Coregistration of each MEG dataset with its corresponding MRI dataset was performed using an automated coregistration routine within MNE which aligns digitization points in the MEG headshape file with the fiducial points demarcated on the outer skin surface reconstruction of the MRI.
Based on previous reports on the cortical areas that constitute major components of the brain mechanism for word recognition and reading in children, the following eight ROIs were examined (in both hemispheres): superior (BA22; STG) and middle temporal gyri (BA 21; MTG), the supramarginal (BA 40; SMG), and angular gyri (BA 39; ANG), rostral middle frontal gyrus (BA 46; MFG), inferior frontal (BA 44/45—pars opercularis and pars triangularis; IFG), ventral occipito-temporal (BA 37), and lateral occipito-temporal cortex (BA 19). Given that one of the goals of the study was to identify brain regions with a potential compensatory role for these functions among children with SBM four additional ROIs were examined in exploratory analyses (pars orbitalis, mesial temporal region, inferior temporal gyrus, superior parietal lobule). The MNE program outputs a current estimate measure for each voxel and each 4 ms time point. This measure is then used to compute the dependent measure used in the analyses outlined in the following paragraphs, namely the average current across all voxels defining each of the ROIs listed above and across all of the 4 ms time points comprising 14 successive 50 ms time bins (100 –150, 150–200 ms, etc. up to 800 ms).
MRI Acquisition and Processing
High-resolution brain MRIs were acquired on a Philips 3T scanner equipped with an eight channel phase array head coil and SENSE (Sensitivity Encoding) technology. After a conventional scout sequence, a three-dimensional T1-weighted SPGR sequence was performed in the coronal plane to obtain whole brain coverage. Acquisition parameters of the 3D ultrafast gradient echo (turbo factor = 2) were as follows: repetition time/echo time = 6.5–6.7/3.04–3.14; flip angle = 8°; field of view = 240 × 240 mm; matrix = 256 × 256; slice thickness = 1.5 mm, in-plane pixel dimensions (x, y) = 0.94, 0.94 mm; number of excitations (NEX) = 2.
All scans were analyzed blind to diagnosis, age, and gender. T1-weighted images were reviewed for image quality prior to performing morphometric analyses. Using FreeSurfer v4.0.5 software (www.surfer.nmr.mgh.harvard.edu) on a 64bit Linux computer, a fully automated process was executed to skull-strip and segment each brain into three classes of voxels: gray matter, white matter, and cerebrospinal fluid (Dale & Sereno, 1993; Dale et al., 1999). Subsequently, within FreeSurfer, a fully automated routine consisting of surface-based cortical parcellation of individual gyri yielded morphometric measures of cortical thickness, volume, and surface area within each gyrus (Fischl & Dale, 2000). The Desikan-Killiany atlas of gyral-based definitions was used for subdividing the neocortex into 32 different parcellation units per hemisphere (Desikan et al., 2006). Results were visually inspected for accuracy using FreeSurfer’s tkmedit and tksurfer viewers for volume- and surface-based boundary delineations and labeling; manual edits and control points were used to make corrections as needed. Following completion of the FreeSurfer workflow, a secondary fully automated routine included with FreeSurfer, but requiring MATLAB, was executed to compute a surface-based 3D local gyrification index for each parcellation unit of the neocortex in each hemisphere (Schaer et al., 2008).
Statistical Analyses
The MEG data were submitted to an ANCOVA with ROI (8), Time (14), and Hemisphere (2) as the within subjects factors, Group as the between subjects factor, and Age as a covariate. This approach was designed to determine if group differences in the degree of regional activity were time-and hemisphere-dependent (i.e., more systematic for particular time windows and/or restricted to one hemisphere). For the MRI data, the first set of analyses explored group differences between the SBM and TD groups on each one of the four morphometric indices, which were submitted to separate ANCOVAs with ROI (8) and Hemisphere (2) as the within subjects factors, Group as the between subjects factor and Age as a covariate. Group differences were also evaluated on the four additional regions listed above. Pairwise group comparisons were evaluated against corrected alpha level using the Bonferroni method (for 12 or 24 comparisons if the Group by Hemisphere interaction was significant necessitating separate tests for each hemisphere). Next, and in order to aid interpretation of potential group differences, we computed Pearson correlation coefficients between each of these indices and (a) reading performance, and (b) regional degree of neurophysiological activity. Coefficients were first computed separately for each group and when the direction of bivariate association were the same, they were recomputed on the entire sample as partial correlations using age as a covariate.
Results
Magnetoencephalography
The two groups performed comparably on each of the two tasks as indicated by similar percent correct word recognition (auditory task; SBM: M = 62.2, SD = 10.6; TD: M = 57.3, SD = 11.0) and pseudoword reading (SBM: M = 88.7, SD = 10.0; TD: M = 92.6, SD = 4.5, p > .4). As predicted, a significant Group × Hemisphere × Region interaction was found for both auditory word recognition, F(91, 1183) = 2.15, p = .01, and pseudoword reading, F(91, 1183) = 2.05, p = .01, tasks.
Auditory word recognition
Follow up lower-order ANCOVAs revealed a Hemisphere × Group interaction in the SMG, F(1, 13) = 4.90, p = .05. Planned tests indicated that this effect reached the critical level of alpha only during the late (250–800 ms) portion of the waveform (during 50–250 ms: p > .2). These results are summarized in Table 3. Further tests on activity from the late portion of the current source density waveform revealed the expected leftward degree of activity for TD children (Hemisphere simple main effect: F(1, 7) = 8.09, p = .03, η2 = .54) and bilaterally symmetric activity, on average, for the SBM group (p > .4). TD participants showed greater degree of activity than children with SBM in the left hemisphere. Similar levels of SMG activity were found for the two groups in the right hemisphere (p > .3).
Table 3.
Summary of ANOVA Effects Involving Group on Neuropshysiological and Anatomic Measures
| Degree of activity
|
Thickness | Surface | Volume | LGI | ||
|---|---|---|---|---|---|---|
| Word recognition | Pseudoword reading | |||||
| STG | H × G (NI < SBM) F* = 21.39, p = .0001 η2 = .62† |
|||||
| MTG | H × G (NI < SBM) F* = 19.13, p = .0001 η2 = .60† |
|||||
| SMG | H × G (200–800 ms) F* = 8.26, p = .01 η2 = .39 NI > SBM in LH F* = 7.96, p = .01 η2 = .38 |
H × G (50–800 ms) F* = 10.57, p = .006 η2 = .45 NI > SBM in LH F* = 37.33, p = .0001 η2 = .74 |
||||
| ANG | Group (NI > SBM 50–800 ms) F* = 11.05, p = .005 η2 = .46 |
H × G (NI > SBM) (F* = 7.15, p = .02 η2 = .36) |
H × G (NI > SBM) (F* = 7.15, p = .02 η2 = .36) (F* = 4.74, p = .03 η2 = .27) |
H × G (NI > SBM) F* = 71.67, p = .0001 η2 = .85† |
||
| L-OC | Group (NI < SBM) F* = 16.14, p = .001 η2 = .55 |
Group (NI < SBM) F* = 16.89, p = .001 η2 = .57 |
||||
| IFG | Group (NI > SBM) F* = 9.94, p = .008 η2 = .44 |
Group (NI > SBM) F* = 9.97, p = .008 η2 = .43 |
H × G (NI < SBM) F* = 131.18, p = .0001 η2 = .91† |
|||
| MFG | Group (NI < SBM) F* = 10.38, p = .007 η2 = .44 |
Group (NI < SBM) F* = 16.25, p = .001 η2 = .56 |
H × G (NI < SBM) F* = 71.67, p = .0001 η2 = .85† |
|||
| pOrb | Group (NI < SBM) F* = 18.59, p = .001 η2 = .59 |
Group (SBM < NI) F* = 40.09, p = .0001 η2 = .76 |
||||
p < .001 for SBM < NI in the LH; Non-significant Group trends in parentheses; H × G: Hemisphere by Group interaction.
df = 1,13.
Pseudoword reading
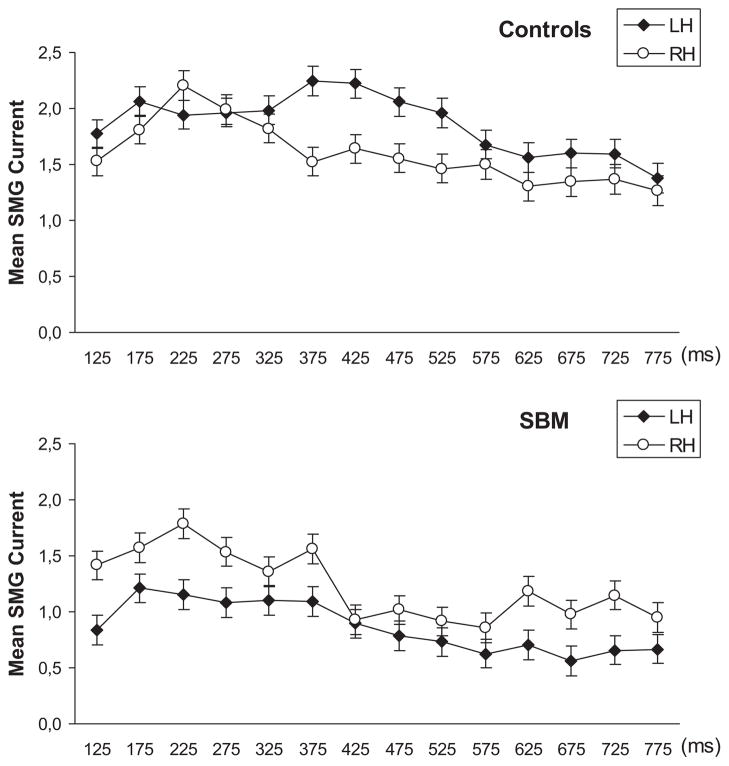
A Hemisphere × Group interaction was also found in the SMG, F(1, 13) = 13.45, p = .003. Similar effect sizes were noted for the late and early segments of the source current density waveform. Significantly greater activity was noted for TD than SBM children in the left SMG (p > .4 for the Group simple main effect in the right SMG; see Table 3). The estimated current source density waveform for SMG is shown in Figure 1 for each group of participants. The expected leftward asymmetry was noted for TD children (Hemisphere simple main effect: F(1, 7) = 12.32, p = .01, η2 = .64), whereas a trend for greater right than left hemisphere activity was found for the children with SBM, F(1, 7) = 6.29, p = .04, η2 = .47. Brain activation image renderings from a representative participant in the TD and SBM groups are shown in Figure 2. Additionally, compared to children with SBM, TD children clearly exhibited greater degree of activity in the ANG, bilaterally during the entire duration of the source current density waveform.
Figure 1.
Temporal progression of estimated current (in nanoAmpere meters) in the SMG for TD (upper panel) and SBM children (lower panel) during performance of the pseudoword reading task. Stimulus onset was at 0 ms.
Figure 2.
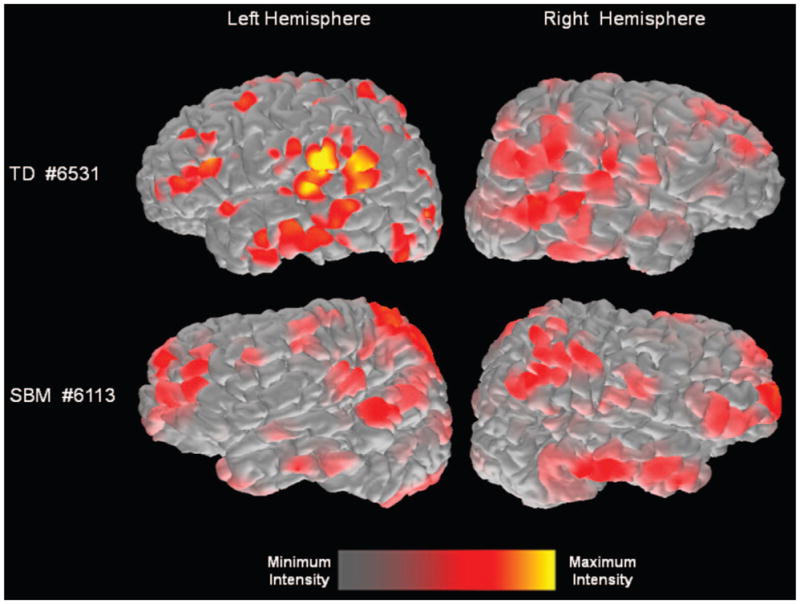
Snapshots of SMG activation during the pseudoword reading task from two representative participants: a TD participant (upper of images) and a child with SBM (lower set of images). The relative intensity of activated voxels is shown at the bottom of the figure. Each set of images presents late activity (averaged between 350 and 500 ms), in the left and right hemispheres.
The degree of hemispheric asymmetry in SMG activity was quantified by means of a laterality quotient [(R−L)/0.5 * (R + L)] confirming the impression of greater leftward asymmetry for TD children (M = −.25, SD = .24) and rightward asymmetry for the group with SBM [M = .11, SD = .35; main effect of Group: F(1, 13) = 7.80, p=.015]. Among participants in the TD group, six showed L>RSMG activity, and two bilaterally symmetric activity, whereas only three children with SBM showed leftward asymmetry (four showed L<R degree of SMG activity, and one bilaterally symmetric activity).
MRI Morphometry Measures
Regional cortical thickness
Bilaterally increased cortical thickness for the children with SBM was found in the lateral occipitotemporal area. Additional exploratory analyses revealed significant elevations in cortical thickness for the children with SBM in pars orbitalis [tests evaluated at α = .05/(8 + 4) = .004 to control for family wise Type I error].
Neocortical surface area
There were no group effects on total surface area (p > .9). The group with SBM displayed reduced surface area in the left ANG, and bilateral IFG. Greater surface area for the group with SBM was found for MFG and lateral occipitotemporal regions in both hemispheres.
Gray matter volume
There were no group differences in total gray matter volume (p > .8). The pattern of regional group differences was identical to that found for surface area, namely reduced gray matter volume for the group with SBM in the left ANG and IFG. Increased volumes for the group with SBM were observed in MFG. Group comparison data are shown quantitatively on the lateral surface of composite MRIs in Figure 3. Group comparisons performed as exploratory tests did not reach the critical level of alpha for surface area and volume measures.
Figure 3.
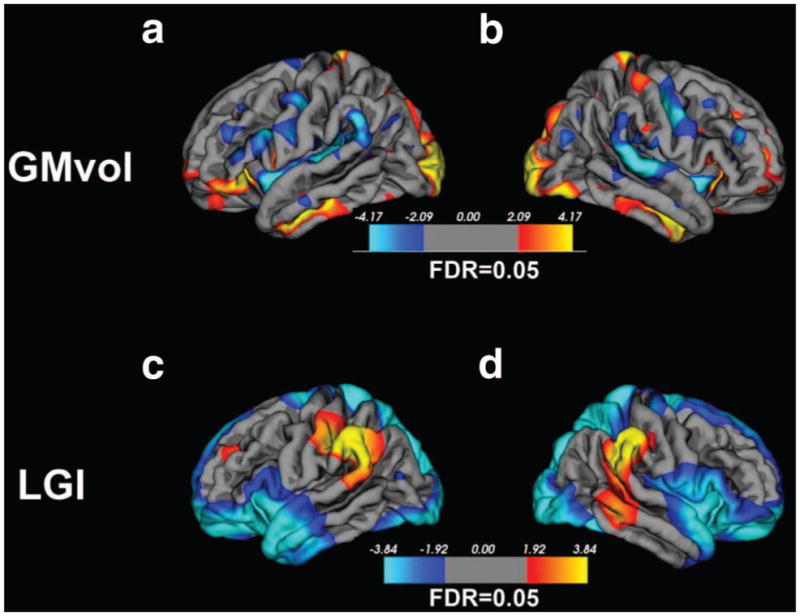
Significant group differences in gray matter volume (upper row) and gyrification index (lower row) displayed on the average, lateral pial surface of all study subjects (n = 23) in the left (a & c) and right hemispheres (b & d). Displayed clusters have been corrected for multiple comparisons using the False Detection Rate method: red and yellow clusters indicate SBM > TD and blue clusters SBM < TD.
Gyrification
Initial planned analyses revealed group differences primarily in left hemisphere regions. Specifically, children with SBM displayed higher gyrification values in left perisylvian and frontal ROIs, as indicated by significant Hemisphere × Group effects in ANG, STG, MTG, IFG, and MFG. In these ROIs, follow-up tests revealed significant Group effects (SBM > TD, evaluated at α = .05/[(8 + 4)*2] = .002) restricted to the left hemisphere (see Table 4). These group differences are projected onto composite MRI renderings in the lower row of images in Figure 3. Moreover, whereas hemisphere asymmetries (L < R) were consistently observed for the TD group in these ROIs, no such hemisphere effects were noted for the group with SBM.
Table 4.
Average Local Gyrification Indices for Typically Developing (TD) and Children With SBM (SD in Parentheses)
| LH
|
RH
|
|||
|---|---|---|---|---|
| Controls‡ | SBM | Controls‡ | SBM | |
| STG† | 3.2 (.05) | 3.8 (.23) | 4.5 (.42) | 3.9 (.50) |
| MTG† | 2.1 (.11) | 3.2 (.23) | 3.6 (.25) | 3.6 (.43) |
| ANG† | 2.6 (.13) | 3.4 (.18) | 3.4 (.16) | 3.4 (.18) |
| L-OC* | 3.0 (.28) | 2.4 (.14) | 2.6 (.44) | 2.4 (.22) |
| IFG† | 2.5 (.13) | 3.9 (.47) | 4.6 (.33) | 4.9 (.30) |
| MFG† | 2.0 (.09) | 2.9 (.17) | 2.9 (.11) | 2.9 (.20) |
| p-Orbitalis** | 4.6 (.39) | 2.6 (.28) | 3.1 (.22) | 2.6 (.24) |
N = 8.
p < .001 for SBM > NI in the LH.
p < .005.
p < .001, for SBM < NI in both hemispheres.
The opposite trend (larger LGI values for the TD than the SBM group) were found in lateral occipitotemporal cortices bilaterally. Exploratory analyses were performed in four additional areas (x 2 hemispheres). Greater LGI values for the TD group were found bilaterally in mesial temporal regions, F(1, 13) = 40.16, p = .0001, η2 = .75 (in addition to pars orbitalis listed in Table 3). A trend in the opposite direction (SBM > TD) was found in the superior parietal lobule, F(1, 13) = 89.84, p = .0001, η2 = .87. Identical results were obtained when SBM data were compared with data from the entire group of neurologically intact participants (N = 15).
MSI-MRI correlations
In order to aid interpretation of group differences in morphometric measures and particularly LGI, we performed a series of partial correlations between the two sets of values for the entire group of neurologically intact, typical readers (controlling for age, N = 15). We focused on average cortical thickness and LGI; surface area and gray matter volume—both measures of total size of each area—showed minimal association with the average estimated current produced by each area.
In general, correlation coefficients between cortical thickness and degree of estimated regional current during pseudoword reading were positive for nearly all inferior parietal and posterior temporal areas (left ANG, MTG, SMG: r values between .57 and .72; IFG and MFG: r values between .51 and .62). Negative correlations were noted between LGI and degree of estimated regional current during pseudoword in the left MTG, ANG, SMG, and STG (r values between −.45 and −.67), lateral occipitotemporal cortex (r values between −.54 and −.65), and left pars orbitalis (r values between −.52 and −.54). The pattern of correlations between cortical thickness/LGI and degree of activity was essentially identical among participants with SBM. Moreover, correlation coefficients between cortical thickness or LGI and degree of activity associated with the auditory word recognition task were similar in size and direction for posterior temporal and inferior parietal areas.
MSI-reading ability correlations
As shown in Table 5, the pattern of associations between reading achievement scores and degree of regional activity varied across groups. Moderate positive correlations were found with degree of late activity (350–600 ms) in the left SMG and ANG among TD children. In contrast, the only substantial correlation between activity and achievement among children with SBM was in the right ANG.
Table 5.
Zero Order (for MSI Measures) and Partial Correlation Coefficients (Controlling for Age) Between Degree of Regional Activity or Morphometry and Pseudoword Reading Accuracy†
| MSI
|
NI + SBM (n = 23)
|
|||||
|---|---|---|---|---|---|---|
| NI (n = 15) | SBM (n = 8) | Thickness | Surface | Volume | LGI | |
| LH | ||||||
| SMG | .61 – .63 (400–500 ms) | .73 | .68 | .72 | ||
| ANG | .62 – .69 (350–600 ms) | .65 | .63 | .70 | ||
| STG | .49 | .56 | ||||
| IFG | .62 | |||||
| MFG | .49 | .55 | .64 | |||
| FUS | .52 | .59 | .66 | |||
| RH | ||||||
| ANG | .69 (650–700 ms) | (.68 – .62) (300–650 ms) | ||||
Woodcock-Johnson-III (WJ-III) Word Attack standard scores. In addition, correlations ranging between .49 and .71 were found between cortical thickness, surface area, and gray matter volume in the left MTG and WJ-III Letter-Word Identification standard scores. Critical r values at α = .05 were: .70 (n = 8), .51 (n = 15), and .41 (n = 23).
MRI morphometry-reading ability correlations
Moderate positive correlations were found between reading achievement scores and morphometric measures, for both groups of participants. Correlations were then recomputed across groups (controlling for age; see Table 5). In general, larger surface area, volume and cortical complexity in left posterior temporal and inferior parietal regions were associated with higher pseudoword reading accuracy and word reading efficiency scores. A similar association was also found between achievement and morphometric measures in MFG and ventral occipitotemporal cortex (fusiform gyrus).
Discussion
This is the first report using a functional brain imaging method combined with morphometric MRI to document patterns of regional functional and anatomical gray matter abnormalities in children with SBM. Results pertaining to the first goal of the study concerning the extent and direction of aberrant anatomy and brain function within the brain circuit normally involved in word recognition and reading can be summarized as follows: The group of children with SBM showed reduced neurophysiological activity in the left SMG and ANG during pseudoword reading (this effect was restricted to the left SMG for spoken word recognition). Importantly, we did not find evidence of compensatory increases in activation in regions postulated to play such a role in previous studies of reading, namely the inferior frontal cortex and right temporoparietal cortex (e.g., Cao et al., 2006; Shaywitz et al., 2002). Anatomically, there was a trend for reduced surface area/gray matter volume and significantly increased gyral complexity in the left ANG among children with SBM. Increased gyrification in SBM children was noted in two additional areas, known to be key components of the brain circuits of interest, namely STG and MTG. The pattern of activity-achievement correlations among typically developing children confirms previous reports (Simos et al., in press) of positive associations between degree of activity in left SMG and ANG and reading ability as measured by standardized tests. Such associations were not evident among children with SBM, where instead a trend for positive associations between activity in the right ANG and reading ability was noted. The pattern of morphometry-achievement correlations also confirmed previous reports in neurologically intact individuals (e.g., Hoeft et al., 2007; Leonard, Eckert, Given, Virginia, & Eden, 2006; Silani, Price et al., 2005) for moderate positive associations between standard reading measures and gray matter volume, cortical thickness, and gyrification in ROIs known to be part of the brain circuit for reading (ANG, SMG, STG, and IFG in the left hemisphere). MRI-reading outcome associations were generally in the same direction for both groups of participants. The third set of results reported here concerns the pattern of associations between morphometric and neurophysiological measures. Across groups, higher LGI values were associated with reduced degree of estimated cortical activity in these regions, while cortical thickness was a positive correlate of the degree of cortical activity associated with reading.
The regional profile of group differences in anatomy and activation (reduced surface area/volume and activation in inferior parietal and posterior temporal regions in SBM) combined with positive associations between these measures and reading ability, would place children with SBM at risk for difficulties in wordlevel reading skills (as well as spoken word recognition). Preservation of these abilities in the average range for children in the present SBM group is likely to be the result of reorganization of the brain circuits responsible for these functions. Given the small sample size, preventing the exploration of structural models accounting for the association between cortical function, regional morphometry and achievement, the evidence regarding the nature of the purported reorganization awaits replication with a larger sample. We assumed that regions that may play a compensatory role in the brain circuit for preserved reading ability should show increased magnitude in SBM and a positive association with reading achievement scores among children SBM. Regions that satisfy both conditions included two left hemisphere regions normally involved in the brain mechanism for word-level reading, namely the superior and middle temporal gyri (displaying increased LGI in SBM compared to TD). One additional area, located in the left middle frontal gyrus, also meets both requirements, yet it does not appear to be an indispensable component of the reading mechanism. Moreover, evidence regarding the role of the angular gyrus in the brain circuit for reading in SBM is more complicated. In the left hemisphere this region shows a tendency for reduced surface area and gray matter volume and increased degree of cortical complexity.
Despite their diverse pattern of group effects, each of the three morphometric measures correlates positively with reading ability. One may postulate that this region undergoes a complex pattern of aberrant developmental changes in SBM so that increased cortical complexity compensates for reduced size. Two key findings, however, reduce the likelihood that the left angular gyrus plays a key role in preserving reading ability in SBM: (a) the failure to find a consistent pattern of positive associations between degree of activity in left hemisphere inferior parietal regions and reading achievement in this group, and (b) the significant reduction in the degree of activation during pseudoword reading. While a role of left posterior temporal (and perhaps also middle frontal) regions in reading ability in SBM children cannot be precluded at present, our finding that degree of activity in the right angular gyrus correlated with reading achievement in this group opens the possibility for a role of this region in the reading mechanism.
A final issue that merits consideration concerns hemispheric asymmetries in the degree of decoding-related activity in inferior parietal regions (mainly the supramarginal gyrus). Such asymmetries were notably absent among children with SBM in sharp contrast with the group of typically developing children. A similar finding has often been reported in developmental reading disability (Pugh et al., 2008; Simos et al., 2000; Simos et al., 2002; Temple et al., 2001), suggesting that a particular physiological phenotype may have distinct developmental antecedents and be associated with different behavioral outcomes. Reduced hemispheric asymmetries in activity could be associated with the callosal hypoplasia that is commonly observed in children with SBM affecting predominantly the rostrum and posterior body and splenium (Barkovich, 2005; Hannay et al., 2009). Reduced interhemispheric signaling between inferior parietal regions was indeed noted in the results of additional exploratory analyses performed on the present data set. Interhemispheric correlations for each time window were consistently higher for the TD group (r > .60 in 9/14 time windows in both ANG and SMG) than the SBM group (r > .6 in only 2/14 time windows in each region). We did not find evidence, however, of increased reliance of the reading mechanism on right inferior parietal regions, consistent with the lack of evidence for significant increases in activity in these regions during reading in the SBM compared to the group of typically developing children. It appears more likely, therefore, that reduced hemispheric asymmetries in inferior parietal activity in SBM result primarily from reduced task-specific activation in the left hemisphere, which is in turn associated, at least in part, with morphological abnormalities in this region (mainly reduced gray matter volume).
Our findings are generally consistent with earlier in vivo anatomical studies of SBM (Fletcher et al., 1992; 1996; 2005; Hasan et al., 2008; Juranek et al., 2008) revealing a posterior-to-anterior gradient in the severity of brain impairment and provide additional data regarding the type of compensatory mechanisms at the neuronal level ensuring adequate development of word-level reading skills. Early disruption of brain development, that begins in utero in children with SBM, is followed later in development by further abnormalities. The latter include posterior gray matter hypoplasia (reduced left inferior parietal volumes) accompanying hypoplasia and dysgenesis of major cerebral commissures (e.g., corpus callosum). A complex pattern of changes in the normal course of regional gyrification was also noted with increases in gyral complexity in traditional perisylvian language areas and reductions in occipital and fronto-polar regions. In addition, hyperplastic features were found in the frontal and temporal lobes, in the form of increased middle frontal gray matter volumes, increased cortical thickness in fronto-polar and anterior mesial frontal regions, and increased gyral complexity in left superior and middle temporal and middle frontal areas. Not all of these changes appear to be directly related to reading outcomes (e.g., reduced gyrification in the left angular gyrus), even when they involve brain regions which are known to serve as critical components of the brain mechanism for reading in typically developing children. Instead, concomitant changes in brain regions which are not indispensable components of the reading mechanisms (especially for subword level skills) may assume a more important role in children with SBM.
The present study has several key features on which conclusions may be drawn with some certainty regarding the type of reorganization that takes place after early brain insult as in the case of SBM. First, functional and anatomical data derived from SBM children were compared to data drawn from carefully selected samples of neurologically intact controls. Both groups showed comparable in-scanner performance, had IQ scores well above the range associated with intellectual deficiency, and reading achievement scores in the average range. Second, both functional (MSI) and anatomical (MRI) measures were computed with reference to the same stereotaxic framework, permitting direct comparisons between the two sets of data. Moreover, these analyses were performed on an expanded set of brain regions permitting detailed exploration of indices of underlying reorganization (rather than more general lobar or hemispheric trends). Third, further analyses examined associations of each set of physiological (i.e., degree of regional neurophysiological activity) and structural measures (i.e., regional gray matter volumes, surface area, and cortical thickness) with language and reading measures in an attempt to establish the functional significance of brain reorganization taking place in SBM. The present sample of children with SBM is small, partly because we selected right-handed children with good decoding ability. However, the sample size is comparable to studies of other samples of neurogenetic disorders that are rarer, such as William’s syndrome (Mobbs et al., 2007), periventricular heterotopia (Walker et al., 2009), and Fragile × syndrome (Watson, Hoeft, Garrett, Hall, & Reiss, 2008). The problem with a small sample is that it may be underpowered for the evaluation of group differences or associations, which may reduce the probability of identifying important associations, although the statistically significant findings in this study met stringent levels of alpha. Because this sample of children with SBM was selected for handedness and decoding ability it may not represent the full spectrum of children with SBM. Selection of the sample was deliberate as handedness differences could obscure the laterality results (many children with SBM are non-right-handed; see Fletcher et al., 2005) and we were specifically interested in how the damaged and reorganized brain of children with SBM mediated the commonly reported findings of stronger development of word-level reading and vocabulary. However, the sample selection strategy may account for the fact that aberrant morphometry was not as extensive in the present sample as in the larger cohort or children with SBM reported by Juranek and Salman (2010; N = 74) who found reduced cortical thickness in STG, MTG, SMG, and ANG and increased LGI in SMG (in addition to the regions reported in the present study). Future studies should examine subgroups of children with SBM to better understand differences in the neural mechanisms because of variations in handedness and word recognition ability. In addition, comparisons with other groups of children with neurogenetic disorders and preserved word reading scores may help elucidate the full range of variability underlying the brain circuit responsible for word recognition.
Acknowledgments
This research was supported in part by Grant P01-HD35946 awarded to JMF from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Contributor Information
Panagiotis G. Simos, Department of Psychology, University of Crete
Andrew C. Papanicolaou, Department of Pediatrics, University of Texas
Eduardo Martinez Castillo, Department of Pediatrics, University of Texas.
Jenifer Juranek, Department of Pediatrics, University of Texas.
Paul T. Cirino, Department of Psychology, University of Houston
Roozbeh Rezaie, Department of Pediatrics, University of Texas.
Jack M. Fletcher, Department of Psychology, University of Houston
References
- Ajayi-Obe M, Saeed N, Cowan FM, Rutherford MA, Edwards AD. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356:1162–1163. doi: 10.1016/s0140-6736(00)02761-6. [DOI] [PubMed] [Google Scholar]
- Al-Sulaiman AA, Ismail HM. Pattern of electroencephalographic abnormalities in children with hydrocephalus: A study of 68 patients. Child’s Nervous System. 1998;14:124–126. doi: 10.1007/s003810050193. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. Pediatric Neuroimaging. 4. New York: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Battaglia D, Acquafondata C, Lettori D, Tartaglione T, Donvito V, Staccioli S, Guzzetta F. Observation of continuous spikewaves during slow sleep in children with myelomeningocele. Child’s Nervous System. 2004;20:462–467. doi: 10.1007/s00381-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, Haughton VM. Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Human Brain Mapping. 2002;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman SH, Fletcher JM, editors. The changing nervous system: Neurobehavioral consequences of early brain disorders. New York: Oxford Press; 1999. [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47:1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Araque J, Giedd J, Rumsey JM. Reduced brain size and gyrification in the brains of dyslexic patients. Journal of Child Neurology. 2004;19:275–281. doi: 10.1177/088307380401900407. [DOI] [PubMed] [Google Scholar]
- Castillo EM, Fletcher JM, Li Z, Hoskison MM, Hasan KM, Passaro A, Papanicolaou AC. Transcallosal connectivity and cortical rhythms: Findings in children with spina bifida. Neuroreport. 2009;20:1188–1192. doi: 10.1097/WNR.0b013e32832f0ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen PL, Kringelbach ML, Ellis AW, Whitney C, Holliday IE, Hansen PC. Activation of the left inferior frontal gyrus in the first 200 ms of reading: Evidence from Magnetoencephalography (MEG) PLoS ONE. 2009;4:e5359. doi: 10.1371/journal.pone.0005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathology. 2004;14:317–324. doi: 10.1111/j.1750-3639.2004.tb00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonet JF, Habib M. Developmental dyslexia: Contribution of modern neuropsychology. Revista de Neurologia (Paris) 2001;157:847–853. [PubMed] [Google Scholar]
- Dennis M, Landry SH, Barnes M, Fletcher JM. A model of neurocognitive function in spina bifida over the life span. Journal of the International Neuropsychological Society. 2006;12:285–296. doi: 10.1017/S1355617706060371. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicology and Teratology. 2006;27:515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Science (USA) 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Bohan TP, Brandt ME, Brookshire BL, Beaver SR, Francis, Miner ME. Cerebral white matter and cognition in hydrocephalic children. Archives of Neurology. 1992;49:818–824. doi: 10.1001/archneur.1992.00530320042010. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Brookshire BL, Landry SH, Bohan TP, Davidson KC, Francis DJ, Morris RD. Attentional skills and executive functions in children with early hydrocephalus. Developmental Neuropsychology. 1996;12:53–76. doi: 10.1037//0894-4105.12.4.578. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Copeland K, Frederick J, Blaser S, Kramer L, Northrup H, Dennis M. Spinal lesion level in spina bifida: A source of neural and cognitive heterogeneity. Developmental Neuropsychology. 2005;102:268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, Basso G. Cortical localization of reading in normal children: An fMRI language study. Neurology. 2001;57:47–54. doi: 10.1212/wnl.57.1.47. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M. Martinos Center for Biomedical Imaging. Charlestown: Massachusetts; 2006. MNE Software User’s Guide, v 2.5. MGH/HMS/MIT Athinoula A. [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: Minimum norm estimates. Medical & Biological Engineering & Computing. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Dennis M, Kramer L, Blaser S, Fletcher JM. Partial agenesis of the corpus callosum in spina bifida meningomyelocele and potential compensatory mechanisms. Journal of Clinical and Experimental Neuropsychology. 2009;31:180–194. doi: 10.1080/13803390802209954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Eluvathingal TJ, Kramer LA, Ewing-Cobbs L, Dennis M, Fletcher JM. White matter microstructural abnormalities in children with spina bifida meningomyelocele and hydrocephalus: A diffusion tensor tractography study of the association pathways. Journal of Magnetic Resonance Imaging. 2008;27:700–709. doi: 10.1002/jmri.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, Gabrieli JD. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Science (USA) 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, Woller A, Muhlau M. Gray matter increase induced by practice correlates with task-specific activation: A combined functional and morphometric Magnetic Resonance Imaging Study. The Journal of Neuroscience. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T, Plante E. Gyral morphology in the posterior sylvian region in families affected by developmental language disorder. Neuropsychology Review. 1996;6:81–94. doi: 10.1007/BF01875369. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Minshew NJ, Keshavan MS, Hardan AY. Cortical Gyrification in Autistic and Asperger Disorders: A Preliminary Magnetic Resonance Imaging. Journal of Child Neurology. 2010;25:1462–1467. doi: 10.1177/0883073810368311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Fletcher JM, Hasan KM, Breier JI, Cirino PT, Pazo-Alvarez P, Papanicolaou AC. Neocortical reorganization in spina bifida. Neuroimage. 2008;40:1516–1522. doi: 10.1016/j.neuroimage.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Salman MS. Anomalous development of brain structure and function in spina bifida myelomeningocele. Developmental Disabilities Research Reviews. 2010;16:23–30. doi: 10.1002/ddrr.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, Ment LR. Increased temporal lobe gyrification in preterm children. Neuropsychologia. 2006;44:445–453. doi: 10.1016/j.neuropsychologia.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Leonard C, Eckert M, Given B, VAB, Eden G. Individual differences in anatomy predict reading and oral language impairments in children. Brain. 2006;129:3329–3342. doi: 10.1093/brain/awl262. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McCullough D. Hydrocephalus: Etiology, pathologic effects, diagnosis and natural history. In: Scott RM, editor. Hydrocephalus. Baltimore, MD: Williams & Wilkins; 1990. pp. 180–199. [Google Scholar]
- McIntosh AM, Moorhead TWJ, McKirdy J, Hall J, Sussmann JED, Stanfield AC, Harris JM, Johnstone EC, Lawrie SM. Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiatrica Scandinavica. 2009;119:192–198. doi: 10.1111/j.1600-0447.2008.01286.x. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Eckert MA, Mills D, Korenberg J, Bellugi U, Galaburda AM, Reiss AL. Frontostriatal dysfunction during response inhibition in Williams syndrome. Biological Psychiatry. 2007;62:256–261. doi: 10.1016/j.biopsych.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Murakami S, Okada Y. Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. Journal of Physiology. 2006;575:925–936. doi: 10.1113/jphysiol.2006.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile T, Hansen R, Magnotta V, O’Leary D, Bell B, Seidenberg M, Hermann BP. Quantitative Measurement of Cortical Surface Features in Localization-Related Temporal Lobe Epilepsy. Neuropsychology. 2004;18:729–737. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. Concreteness, imagery, and meaningfulness values for 925 nouns. Journal of Experimental Psychology (Monographs) 1968;76:1–25. doi: 10.1037/h0025327. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Sarkari S, Pataraia E, Maggio WW. Magnetoencephalography: A non-invasive alternative to the Wada procedure. Journal of Neurosurgery. 2004;100:867–876. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Frost SJ, Dandak R, Landi N, Rueckl JG, Constable RT, Mencl WE. Effects of stimulus difficulty and repetition on printed word identification: An fMRI comparison of nonimpaired and reading-disabled adolescent cohorts. Journal of Cognitive Neuroscience. 2008;20:1146–1160. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Richards TL, Berninger VW, Aylward EH, Richards AL, Thomson JB, Nagy WE, Abbott RD. Reproducibility of proton MR spectroscopic imaging (PEPSI): comparison of dyslexic and normal-reading children and effects of treatment on brain lactate levels during language tasks. American Journal of Neuroradiology. 2002;23:1678–1685. [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Transactions of Medical Imaging. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Constable RT, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Paulesu E. Brain abnormalities underlying altered activation in dyslexia: A voxel based morphometry study. Brain. 2005;128:2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, Papanicolaou AC. Dyslexia-specific brain activation profile become normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Denton CA, Papanicolaou AC. Altering the brain circuits for reading through intervention: A magnetic source imaging study. Neuropsychology. 2007;21:485–496. doi: 10.1037/0894-4105.21.4.485. [DOI] [PubMed] [Google Scholar]
- Simos PG, Papanicolaou AC, Breier JI, Fletcher JM, Foorman BR, Bergman E, Papanicolaou AC. Brain activation profiles in dyslexic children during nonword reading: A magnetic source imaging study. Neuroscience Letters. 2000;290:61–65. doi: 10.1016/s0304-3940(00)01322-7. [DOI] [PubMed] [Google Scholar]
- Simos PG, Rezaie R, Fletcher JM, Juranek J, Passaro AD, Li Z, Papanicolaou AC. Functional disruption of the brain mechanism for reading: Effects of comorbidity and task difficulty among children with developmental learning problems. Neuropsychology. doi: 10.1037/a0022550. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JD. Disrupted neural responses to phonological and orthographic processing in dyslexic children: An fMRI study. Neuroreport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Thorndike R, Hagen E, Sattler J. The Stanford–Binet Intel-ligence Scale. 4. Itasca, IL: Riverside; 1986. [Google Scholar]
- Torgesen JK, Wagner R, Rashotte C. Test of Word Reading Efficiency. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Veggiotti P, Beccaria F, Papalia G, Termine C, Piazza F, Lanzi G. Continuous spikes and waves during sleep in children with shunted hydrocephalus. Childs Nervous System. 1998;14:188–194. doi: 10.1007/s003810050209. [DOI] [PubMed] [Google Scholar]
- Walker LM, Katzir T, Liu T, Ly J, Corriveau K, Barzillai M, Chang BS. Gray matter volumes and cognitive ability in the epileptogenic brain malformation of periventricular nodular heterotopia. Epilepsy & Behavior. 2009;15:456–460. doi: 10.1016/j.yebeh.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. Aberrant brain activation during gaze processing in boys with fragile × syndrome. Archives of General Psychiatry. 2008;65:1315–1323. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. Weschler Abbreviated Scales of Intelligence. New York: Psychological Corporation; 1999. [Google Scholar]
- White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in preschool and adolescence. Brain and Cognition. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside; 2001. [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anatomy and Embryology. 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]