Abstract
Prostate cancer treatment is a controversial topic amongst physicians and patients alike. Radical therapies such as prostatectomy and whole gland radiation offer the best outcomes in terms of oncologic efficacy, but the decision to undergo treatment must be weighed against its potential morbidity. Over the past decade, the concept of focal therapy for prostate cancer has been introduced as a potential method of achieving oncologic control with a lesser degree of morbidity. Focal therapy refers to isolated ablation of a tumor focus with sparing of uninvolved, surrounding tissue. While it remains in the early stages of development, considerable research is underway that will help determine the optimal method of achieving this goal. Current areas of investigation include appropriate candidate selection, lesion identification, modality of treatment, and follow-up strategies.
Keywords: cryotherapy, focal therapy, high-intensity focused ultrasound (HIFU), histoscan, prostate cancer
Introduction
Prostate cancer is the most common nondermatologic cancer in American men. Over 200,000 new cases are diagnosed each year affecting approximately one of every six US men [Altekruse et al. 2010]. Since the introduction of prostate-specific antigen (PSA) testing, prostate cancer has undergone a stage migration with a majority of patients now being diagnosed with clinically localized, low-volume, low-grade disease [Stattin et al. 2010]. The clinical significance of these low-risk cases is a source of great debate. Considering the potential morbidity of radical therapy (prostatectomy, radiation) there are an increasing number of critics who argue that prostate cancer is currently being overtreated. This growing sentiment has led to increased interest in alternative strategies and treatment options. Active surveillance is one such approach, yet despite its increasing popularity only 10% of men diagnosed with prostate cancer currently opt to defer therapy [Cooperberg et al. 2007].
Focal therapy has been proposed as a middle ground between active surveillance and radical therapy. The idea behind focal therapy is that by identifying the specific area of the prostate involved by tumor, one might be able to focus treatment and spare uninvolved tissue. While in theory focal therapy is an exciting treatment paradigm, only a limited amount of data is available regarding its efficacy for oncologic control and decreased morbidity. Current areas of investigation include appropriate candidate selection, lesion identification, modality of treatment, and follow-up strategies. In this review we examine the most recent data available regarding focal therapy for prostate cancer and investigate future directions of this evolving treatment strategy.
Treatment goals
Before measuring the efficacy of treatment, it is first important to analyse the criteria by which its success is determined. If success relies on eradication of all disease, the ideal patient population consists of men with unifocal, low- to intermediate-risk disease located within a limited region of the prostate. A recent consensus panel recommended this curative intent approach to focal therapy as important for proof of concept [de la Rosette et al. 2010]. The downside of this approach is that it limits focal therapy to a very select group of men. Our group has shown this to be true finding that of 1400 men undergoing radical prostatectomy, only 11% had unilateral, low-risk disease (PSA <10 ng/ml, Gleason Score (GS) <7, Percent tumor involvement (PTI) <10%) [Tareen et al. 2009]. A similar study by Bott and colleagues showed that unifocal and unilateral disease in men with low to intermediate preoperative risk features (PSA ≤20 ng/ml, GS ≤7, ≤66% cores positive) existed in only 14% and 13% respectively as determined after radical prostatectomy [Bott et al. 2010].
Conversely, there is growing support for the idea that individual foci of cancer within the same gland may be genetically distinct in potential for metastasis and lethality; thus, isolating treatment to a dominant focus of cancer (commonly referred to as the index lesion) may be sufficient to render the disease nonlethal. The concept of isolated treatment of the index lesion is predicated upon several pathologic and biological observations. Pathologic analyses have demonstrated that the index lesion generally comprises the majority of tumor volume, the highest Gleason score, and the site of extracapsular disease, if present [Ohori et al. 2006; Arora et al. 2004; Noguchi et al. 2003; Wise et al. 2002; Haggman et al. 1997; Ruijter et al. 1996]. The index lesion volume itself has been shown to be a predictor of PSA recurrence [Stamey et al. 2001]. Furthermore, the rate of PSA recurrence in cases of unilateral and bilateral disease has been shown to be similar (11.4% versus 14.4%) indicating that secondary tumors do not often metastasize [Mouraviev et al. 2007]. Arora and colleagues analyzed secondary tumors and found that they only contributed to approximately 20% of overall volume with only 3% of them containing elements of high-grade disease [Arora et al. 2004]. Finally, it has been demonstrated that metastases typically arise from a single clonal cell population within the prostate. Liu and colleagues compared the genetic identities of 94 different metastases in 30 men and found a 63–100% match in genomic identity to the primary tumor depending on the type of genomic assay used [Liu et al. 2009].
The implications of targeting the index lesion rather than all tumor foci regardless of clinical features is significant because it would theoretically expand the number of potential candidates for focal therapy. Based on this intent to ablate only clinically significant disease, Bott and colleagues estimated that 59–68% of patients in their cohort would have been suitable for therapy. While the index lesion theory is provocative, validation will require several questions to be answered. How can the index lesion be accurately identified? Is the size of the lesion a reasonable indicator of the likelihood of lethal phenotypes? Is ablation of the index lesion safe and adequate for oncologic control? And, most importantly, can short-term endpoints be utilized to determine oncologic efficacy?
Candidate selection
To date, the criteria by which men have been selected as candidates for focal therapy has varied widely between studies [de la Rosette et al. 2010; Sartor et al. 2008; Eggener et al. 2007]. As highlighted in the previous section, one would expect different criteria to exist depending on whether the main goals of treatment are oncologic efficacy versus decreased morbidity. Another criteria influencing candidate selection is the optimal means by which tumor focality is determined. To date, inconsistency in accurately mapping tumor foci is the main limitation in terms of patient selection [Taneja and Mason, 2010]. The two areas which are being most heavily investigated for this purpose are transperineal mapping biopsy (TPMB) of the prostate and advanced prostatic imaging. It is likely that one, if not both, of these strategies will ultimately help define optimal characteristics for patient selection.
Prostate biopsy
Traditionally, 6 and 12 core transrectal biopsies have been the preferred method of diagnosing prostate cancer. However, recent studies demonstrate a significant understaging of disease when using these techniques. Concordance rates between unilaterality on pre-operative biopsy versus unilaterality on final pathology after radical prostatectomy are low, ranging from 26% to 35% [Quann et al. 2010; Tareen et al. 2009; Scales et al. 2007; Wise et al; 2002]. Meanwhile, the presence of unifocal disease has been estimated to be even lower, between 11% and 14% [Bott et al. 2010; Tareen et al. 2009]. These findings emphasize the need for a better means of tumor identification prior to treatment.
The discrepancy between biopsy and final pathology findings are most likely a result of limited sampling. To account for this, TPMB has been proposed as a way to more accurately predict tumor focality. The TPMB technique was initially introduced as a method to detect prostate cancer in men with multiple negative biopsies despite high clinical suspicion for cancer, and is generally performed using a brachytherapy grid to take samples at 5 mm intervals. In one large study comparing traditional biopsy to TPMB Onik and colleagues found a large discrepancy in biopsy results [Onik et al. 2009]. In the study, 180 men with unifocal prostate cancer based on initial biopsy underwent TPMB with an average of 50 cores sampled. A majority (61%) were later found to have bilateral disease and a significant number (23%) of cases were upstaged by Gleason score. Based on these findings the authors estimated that the results of the TPMB would have changed management in 69% of their cohort. As a result, TPMB has since been advocated as the gold standard approach to identifying candidates for focal therapy [Eggener, 2010].
Prostate imaging
Critics of TPMB argue that it is an unreasonable option for a screening tool. Saturation biopsies are lengthy procedures that are generally performed in the operating room under general anesthesia or sedation. The associated time demands, cost, and morbidity of taking a large number of biopsies have led many to seek an alternative method for tumor detection and localization. Although a variety of imaging modalities have been explored for this purpose, MRI currently provides the best noninvasive means of depicting tumors within the prostate.
The performance of prostate MRI in tumor identification has improved substantially over the past decade [Villers et al. 2009]. This trend reflects ongoing improvements in MRI hardware and software, combined with increasing experience among radiologists in the interpretation of such images. Perhaps most important has been the emergence of functional MRI techniques, including MR spectroscopy (MRS), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) imaging, for tumor detection. Each of these three techniques has been shown to improve tumor detection compared with standard T2-weighted imaging (T2WI) alone [Turkbey et al. 2010, 2009b; Villers et al. 2009; Haider et al. 2007]. Multiparametric MRI (MpMRI) describes the combination of T2WI with at least two functional sequences and is currently considered the state of the art for prostate imaging.
There is a wide range of reported sensitivities and specificities of MRI in detecting prostate tumors. For instance, in a recent review by Turkbey and colleagues the sensitivities and specificities of T2WI alone for tumor detection, as reported in the literature, were noted to range from 22% to 85% and from 50% to 99%, respectively [Turkbey et al. 2009a]. Reasons for these large ranges include variation in the magnet and coil selection, the interpretation method employed by the radiologist, and the diagnostic criteria used for pathologic correlation across the different studies. One consistently reported observation is that MRI has improved sensitivity for lesions of a greater size [Turkbey et al. 2010; Puech et al. 2009; Girouin et al. 2007]. For instance, Puech and colleagues reported that the sensitivity of combined T2WI and DCE improved from 32% for detection of cancer foci of any volume to 86% for cancer foci >0.5 ml, using prostatectomy as the reference standard [Puech et al. 2009].
MRI findings are proving to have clinical utility not just for tumor detection but also for risk stratification. For instance, Fradet and colleagues demonstrated that even the presence of MRI findings suspicious for tumor in patients on active surveillance was a risk factor for subsequent Gleason score upgrade [Fradet et al. 2010]. Furthermore, MRI has been shown to add incremental value to traditional pre-operative staging nomograms of prostate cancer [Wang et al. 2009, 2006]. In one study, Augustin and colleagues compared the predictive ability of 3-T MRI and Partin nomograms in men who underwent prostatectomy and found that MRI was more accurate in the prediction of extracapsular extension [Augustin et al. 2009]. MpMRI has also demonstrated utility as an adjunct to biopsy in men without a prior cancer diagnosis. Recently, it has become possible to biopsy suspicious MRI findings in such patients directly under MRI guidance. As an example of this technique, Roethke and colleagues reported that among 100 patients with at least one prior negative transrectal ultrasound (TRUS) biopsy who underwent targeted MRI-guided biopsy of suspicious findings, cancer was detected in 52 patients [Roethke et al. 2011].
It is expected that the clinical utility of prostate MRI will continue to improve given ongoing technological advancements combined with efforts to develop structured prostate MRI reporting schemes [Dickinson et al. 2011]. However, at this point in time, the question of whether MpMRI is a reliable alternative to TPMB remains unanswered, particularly in view of the suboptimal depiction of small tumors using MRI. Further prospective and comparative series between MpMRI and TPMB will be necessary to help answer this question.
Histoscan
A novel technology utilizing computer-aided ultrasonography, termed histoscanning (HS), has emerged as another promising new method for mapping tumor foci within the prostate. HS exploits differential backscatter of ultrasound waves produced by altered characteristics present in malignant tissues to distinguish cancer from benign. Braeckman and colleagues reported the initial series comparing HS to whole mount specimens in 29 men undergoing radical prostatectomy [Braeckman et al. 2008, 2007]. The first 15 men comprised a ‘training set’ used to calibrate and refine characterization algorithms which were then applied and tested on the subsequent 14 men included in the study. HS demonstrated 100% sensitivity and Negative predictive value (NPV) for predicting the presence of tumor foci ≥0.5 ml within the test set. HS also demonstrated accurate prediction of individual tumor volume and overall tumor volume.
Further HS studies are limited to presented abstracts. Kumaar and colleagues examined HS’s ability to detect smaller tumors and demonstrated similar success [Kumaar et al. 2009]. For lesions ≥0.2 ml, HS demonstrated sensitivity and NPV of 88% and 92%, respectively. Norgaard and colleagues found that increasing total tumor volume on HS correlated with increasing positivity of biopsies in 42 men with no prior history of prostate cancer [Norgaard and Autier, 2010]. Zatura and colleagues further demonstrated that increasing tumor volume on HS in 50 men with a history of previous negative biopsies predicted men likely to have a positive result on rebiopsy [Zatura et al. 2010]. In all, HS provides a promising new noninvasive mapping strategy in prostate cancer. Further large prospective series are required to validate early trial results and establish its role in patient selection algorithms for focal therapy.
Focal therapies
To date, a number of different ablative techniques have been used for focal therapy of the prostate. There are only a limited number of published results available at this time; thus, consensus on optimal ablative approach, course of treatment and follow-up strategy is still being reached.
Cryotherapy
Of all of the ablative technologies available, cryoablation is the one that has been used and studied for the longest. While initial use of cryotherapy in prostate cancer centered on destruction of the whole gland, it has more recently been investigated as a tool for focal therapy. Advanced computer algorithms have made it possible to strategically plan cryoprobe placement in order to maximize destruction of targeted tissue while sparing uninvolved but adjacent structures [Levy et al. 2010; Mouraviev et al. 2010].
The initial experience in focal therapy using cryoablation came from Onik and colleagues [Onik et al. 2002]. In their study, nine men with unilateral prostate cancer on biopsy underwent cryoablation with preservation of the neurovascular bundle on the side contralateral to known disease. At a mean follow up of 3 years all men had stable PSAs, six of six men with repeat biopsies were negative for pathologic recurrence, and seven of nine men were potent.
Several other clinical studies have investigated the use of focal cryotherapy since Onik’s initial report [Lukka et al. 2011; Truesdale et al. 2010; Dhar et al. 2009; Onik et al. 2009, 2008, 2007; Ellis et al. 2007; Lambert et al. 2007; Bahn et al. 2006]. While a majority have been performed via a hemiablative technique, optimal cryoprobe placement has yet to be determined. As an example of alternative strategies, Ellis and colleagues modified their approach by the use of an additional cryoprobe located in the side contralateral to the known disease [Ellis et al. 2007]. In terms of outcomes, potency rates after treatment range from 65% to 90%. Meanwhile, incontinence is rarely reported, ranging from 0% to 3.6%.
Although biochemical and biopsy-proven recurrence rates are reported in each study, there is a lack of consensus between them on how recurrence was defined and which patients were rebiopsied. It is clear that recurrence rates after focal therapy are higher than those of whole gland treatment; yet, what percentage of recurrence is from untreated disease versus disease missed on preoperative staging is a confounder to follow-up statistics that makes them difficult to interpret. The largest published experience and outcomes with focal cryotherapy comes from the Cryo On-Line Data (COLD) registry [Dhar et al. 2009]. Of 795 patients that had been treated with focal cryoablation, 5-year biochemical disease-free rates ranged from 81% to 83% based on ASTRO criteria and from 35% to 68% based on PHOENIX criteria. Of the patients who underwent rebiopsy, 25% had evidence of cancer (4.5% of the total cohort).
Smaller series have found comparable oncologic results. In Onik and colleagues’ follow-up series on 120 men, 7% were found to have biochemical recurrence [Onik et al. 2009, 2008, 2007]. Ellis and colleagues reported a biochemical failure rate of 20% according to the ASTRO definition, with 40% of the patients undergoing follow-up biopsy having evidence of cancer [Ellis et al. 2007]. Lambert and colleagues reported a biochemical recurrence rate of 12% defined as PSA nadir >50% with 43% of patients who underwent repeat biopsy having evidence of cancer. Bahn and colleagues had the lowest rates of biochemical recurrence (7%) with only 1/25 (4%) men having evidence of cancer when undergoing repeat biopsy [Lambert et al. 2007]. Finally, Truesdale and colleagues reported a biochemical failure rate of 27.3% according to the Phoenix definition and a 46% positive rebiopsy rate amongst cases with suspicion for recurrence [Truesdale et al. 2010]. They determined that a higher number of positive cores on preprocedure biopsy was associated with an increased likelihood of biochemical failure and positive postprocedure biopsy.
One common trend amongst these recurrences is that a majority (70–93%) occurred on the untreated side. In general, these would be considered a failure of baseline staging rather than a failure of treatment, although it is difficult to discern whether all of these cancers were pre-existent, prior to therapy. Most of these untreated cancers were able to undergo a second therapy, demonstrating a unique aspect of focal therapy in the opportunity for retreatment.
High-intensity focused ultrasound
High-intensity focused ultrasound (HIFU) works by ablating tissue via the application of mechanical and thermal energy under ultrasound guidance. Unlike cryoablation, HIFU is still considered experimental in the United States thus the majority of published results are limited to clinical trials as well as studies from Canada, Europe, and Japan where the therapy has been approved.
The majority of published results using HIFU have investigated its efficacy as a whole-gland treatment. Based on recent reviews the most commonly encountered morbidities after whole-gland therapy include impotence (44%), urinary incontinence (8%), urinary retention (5.3%), chronic perineal pain (3.4%), and rectourethral fistula (1%) [Lukka et al. 2011]. Meanwhile, biochemical progression-free survival ranges from 45% to 84% at 5 years and 69% at 7 years using either ASTRO or Phoenix criteria [Warmuth et al. 2010].
The initial study demonstrating HIFU success in focal therapy was performed by Madersbacher and colleagues who performed focal HIFU on 10 men with unilateral prostate cancer prior to radical prostatectomy [Madersbacher et al. 1995]. After analyzing the specimens they discovered that HIFU completely eradicated three of 10 tumors and destroyed a mean of 53% of tumor in the other seven cases.
Since then there have been only two published studies evaluating the performance of HIFU when used for focal therapy. Muto and colleagues compared 70 patients with bilateral disease receiving whole-gland HIFU to 29 with unilateral disease who had the transitional zones spared on the unaffected side [Muto et al. 2008]. The key finding of this study was that focal treatment did not appear to compromise cancer control. At 12 months there was a negative biopsy rate of 82% with no statistically significant difference between groups in terms of urinary symptoms based on validated questionnaire. Erectile function was not assessed.
Most recently Ahmed and colleagues performed HIFU hemiablation in 20 patients with unilateral disease [Ahmed et al. 2011]. All but one patient underwent follow-up biopsy at 6 months the results of which were negative for cancer in 89% of cases. It should be noted that biopsies were only taken from the treated side unless a new lesion was noted on the contralateral side during follow-up imaging. Furthermore, at 12 months time 95% of men reported erections sufficient for intercourse and 90% of men were pad-free and leak-free.
Other approaches
While cryoablation and HIFU are currently the two modalities being used most frequently for focal therapy, there are numerous other treatment strategies currently under investigation. Focal laser ablation (FLA) is one such technique that uses laser energy to ablate MRI visible lesions. The advantage of this approach is that it can be done with real-time monitoring via MRI allowing the surgeon to ensure completeness of treatment as well as avoid vital structures in order to minimize morbidity. A phase I trial looking at 12 patients treated with FLA was recently published by Linder and colleagues [Linder et al. 2009] After FLA, 50% of the cohort had No evidence of disease (NED) while 67% of them had NED at the site of ablation. Of the patients who were found to have residual disease at the ablation site, one underwent radical prostatectomy without complication. In addition, there was no significant change in erectile function or voiding after treatment. Further phase II studies are necessary to validate the results of this treatment.
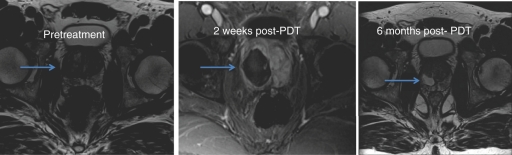
Another new approach currently under investigation is photodynamic therapy (PDT). PDT works via the activation of injected photosensitizers by a specific wavelength of light delivered through a low-power laser optical fiber. This reaction in turn creates toxic reactive oxygen species which cause cell damage and death. PDT can be separated into two subcategories: tissue-activated PDT and vascular-activated PDT. In each case a photosensitizer is injected into the patient and light is then delivered to the target lesion leading to destruction (Figure 1). The consequence of the photosensitizer injection is that it accumulates in other organs (notably skin and eyes) necessitating a period of light protection for the patient until the photosensitizer is no longer present [Moore et al. 2009].
Figure 1.
MRI changes before and after photodynamic therapy of the prostate.
To date the majority of PDT trials have been performed on an investigative basis with small cohorts. The efficacy of PDT was first demonstrated in 1990 by Windahl and colleagues using vascular targeted photosensitizers (hematoporphyrin for one case and porfimer sodium for the other) in two patients undergoing treatment [Windahl et al. 1990]. Each had negative biopsies at 3 months follow up and one patient who died of lung cancer 6 months after treatment had no evidence of residual cancer on postmortem histological examination. Since then, PDT research has primarily focused on determining the optimal type and dose of photosensitizing agent available as well as ideal duration of light exposure used during treatment. Trachtenberg and colleagues found increasing tissue ablation with increasing light dose on 13 patients undergoing PDT [Trachtenberg et al. 2008]: eight of 13 of these patients had negative biopsies 6 months after treatment. Of note, two patients developed rectourethral fistulas. While there is limited data regarding morbidity of PDT the most common short-term adverse effects included irritative voiding symptoms, stress incontinence, and urinary retention. Further trials are underway to help determine the optimal treatment guidelines for this novel approach [Arumainayagam et al. 2010].
Follow up
One of the main challenges remaining in focal therapy is defining treatment efficacy and follow up. By virtue of the fact that focal therapy preserves prostate tissue, PSA is not expected to become undetectable, but its relative kinetics after therapy may be informative regarding treatment effect. This remains to be determined. Furthermore, traditionally accepted criteria for biochemical recurrence such as the ASTRO and Phoenix criteria are not applicable to focal therapy since they were not designed for use in this setting.
MRI has the potential to be used as a noninvasive alternative to biopsy to confirm treatment success; however, there are limitations to relying on imaging alone as well. For one, not all tumors are necessarily visible on MRI. Furthermore, ablated regions of the prostate tend to have the same hypointense appearance as cancer on T2WI alone [De Visschere et al. 2010]. Advancements in MRI though have made it possible to distinguish between treated areas and cancer. Fibrotic regions post-HIFU have different enhancement patterns than tumors on dynamic contrast-enhanced MRI and thus can be readily distinguished [Rouviere et al. 2010]. In addition, magnetic resonance spectroscopy imaging has been shown to the increase sensitivity of detection of recurrence postcryotherapy [Parivar et al. 1996].
Conclusions
Focal therapy is a promising treatment strategy that has the potential to significantly alter current treatment approaches to prostate cancer. There is accumulating evidence to suggest that it may serve as a middle ground between active surveillance and radical therapy for patients with low- to intermediate-risk disease. However, it is clear that there are critical questions that must be answered prior to its acceptance as a commonly utilized and viable treatment option. With a great deal of enthusiasm amongst patients and clinicians alike, focal therapy is certainly an area prime for further research and investigation.
Table 1.
Summary of pending focal therapy trials.
| Modality | Protocol | n | Biopsy | PSA | GS | |
|---|---|---|---|---|---|---|
| MSKCC | Cryotherapy | Hemiablation | 50 | TRUS | <10 | ≤6 |
| MD Anderson | Cryotherapy | Extended | 100 | TRUS | <10 | <3+4 |
| hemiablation | ||||||
| Milan, Italy | Cryotherapy | Hemiablation | 100 | TRUS | <10 | ≤6 |
| Ahmed/Emberton | HIFU | Focal | 43 | Template | <15 | ≤7 |
| Ahmed/Emberton | H1FU | Index lesion | 26 | Template or | <15 | ≤8 |
| TRUS/MRI | ||||||
| France | HIFU | Hemiablation | 120 | TRUS/MRI | <10 | ≤7 |
| Multicenter | HIFU | Focal, Hemiablation, | 150 | Template or | <15 | ≤7 |
| Europe | Index lesion | TRUS/MRI | ||||
| Multicenter | PDT-WST-11 | Variable | 85 | Not reported | Not | Not |
| Europe | reported | reported |
PSA, prostate-specific antigen; GS, Gleason Score; HIFU, high-frequency ultrasound; TRUS, transrectal ultrasound; MRI, magnetic resonance imaging; PDT, photodynamic therapy.
Acknowledgements
Joseph and Diane Steinberg Charitable Trust.
Funding
This work was supported by the Joseph and Diane Steinberg Charitable Trust.
Conflict of interest statement
The authors declare no conflicts of interest in preparing this article.
References
- Ahmed H.U., Freeman A., Kirkham A., Sahu M., Scott R., Allen C., et al. Focal therapy for localized prostate cancer: A phase I/II trial. J Urol 185: 1246–1254 [DOI] [PubMed] [Google Scholar]
- Altekruse, S.F., Kosary, C.L., Krapcho, M., Neyman, N., Aminou, R., Waldron, W. et al. (2010) SEER Cancer Statistics Review, 1975–2007. http://seer.cancer.gov/csr/1975_2007/ (accessed 13 March 2011)
- Arora R., Koch M.O., Eble J.N., Ulbright T.M., Li L., Cheng L. (2004) Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer 100: 2362–2366 [DOI] [PubMed] [Google Scholar]
- Arumainayagam N., Moore C.M., Ahmed H.U., Emberton M. (2010) Photodynamic therapy for focal ablation of the prostate. World J Urol 28: 571–576 [DOI] [PubMed] [Google Scholar]
- Augustin H., Fritz G.A., Ehammer T., Auprich M., Pummer K. (2009) Accuracy of 3-Tesla magnetic resonance imaging for the staging of prostate cancer in comparison to the Partin tables. Acta Radiol 50: 562–569 [DOI] [PubMed] [Google Scholar]
- Bahn D.K., Silverman P., Lee F., Sr, Badalament R., Bahn E.D., Rewcastle J.C. (2006) Focal prostate cryoablation: Initial results show cancer control and potency preservation. J Endourol 20: 688–692 [DOI] [PubMed] [Google Scholar]
- Bott S.R., Ahmed H.U., Hindley R.G., Abdul-Rahman A., Freeman A., Emberton M. (2010) The index lesion and focal therapy: An analysis of the pathological characteristics of prostate cancer. BJU Int 106: 1607–1611 [DOI] [PubMed] [Google Scholar]
- Braeckman J., Autier P., Garbar C., Marichal M.P., Soviany C., Nir R., et al. (2008) Computer-aided ultrasonography (histoscanning): A novel technology for locating and characterizing prostate cancer. BJU Int 101: 293–298 [DOI] [PubMed] [Google Scholar]
- Braeckman J., Autier P., Soviany C., Nir R., Nir D., Michielsen D., et al. (2008) The accuracy of transrectal ultrasonography supplemented with computer-aided ultrasonography for detecting small prostate cancers. BJU Int 102: 1560–1565 [DOI] [PubMed] [Google Scholar]
- Cooperberg M.R., Broering J.M., Kantoff P.W., Carroll P.R. (2007) Contemporary trends in low risk prostate cancer: Risk assessment and treatment. J Urol 178: S14–S19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Rosette J., Ahmed H., Barentsz J., Johansen T.B., Brausi M., Emberton M., et al. Focal therapy in prostate cancer-report from a consensus panel. J Endourol 24: 775–780 [DOI] [PubMed] [Google Scholar]
- De Visschere P.J., De Meerleer G.O., Futterer J.J., Villeirs G.M. (2010) Role of mri in follow-up after focal therapy for prostate carcinoma. AJR Am J Roentgenol 194: 1427–1433 [DOI] [PubMed] [Google Scholar]
- Dhar N., Cher M., Scionti S., Lugnani F., Jones J.S. (2009) Focal/partial gland prostate cryoablation: results of 795 patients from multiple centers tracked with the COLD registry. Abstracts of the American Urologic Association annual meeting. April 25–30, 2009; Chicago, IL. J Urol 181(Suppl. 4): 715–715 . Abstract 1975 [Google Scholar]
- Dickinson L., Ahmed H.U., Allen C., Barentsz J.O., Carey B., Futterer J.J., et al. (2011) Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: Recommendations from a European consensus meeting. Eur Urol 59: 477–494 [DOI] [PubMed] [Google Scholar]
- Eggener S.E., Scardino P.T., Carroll P.R., Zelefsky M.J., Sartor O., Hricak H., et al. (2007) Focal therapy for localized prostate cancer: A critical appraisal of rationale and modalities. J Urol 178: 2260–2267 [DOI] [PubMed] [Google Scholar]
- Eggener S. (2010) Ablative focal therapy for primary treatment of prostate cancer. AUA Update Series 29(lesson 3): 22–31 [Google Scholar]
- Ellis D.S., Manny T.B., Jr, Rewcastle J.C. (2007) Focal cryosurgery followed by penile rehabilitation as primary treatment for localized prostate cancer: Initial results. Urology 70: 9–15 [DOI] [PubMed] [Google Scholar]
- Fradet V., Kurhanewicz J., Cowan J.E., Karl A., Coakley F.V., Shinohara K., et al. Prostate cancer managed with active surveillance: Role of anatomic MR imaging and MR spectroscopic imaging. Radiology 256: 176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouin N., Mege-Lechevallier F., Tonina Senes A., Bissery A., Rabilloud M., Marechal J.M., et al. (2007) Prostate dynamic contrast-enhanced MRI with simple visual diagnostic criteria: Is it reasonable? Eur Radiol 17: 1498–1509 [DOI] [PubMed] [Google Scholar]
- Haggman M., Nordin B., Mattson S., Busch C. (1997) Morphometric studies of intra-prostatic volume relationships in localized prostatic cancer. Br J Urol 80: 612–617 [DOI] [PubMed] [Google Scholar]
- Haider M.A., Van Der Kwast T.H., Tanguay J., Evans A.J., Hashmi A.T., Lockwood G., et al. (2007) Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol 189: 323–328 [DOI] [PubMed] [Google Scholar]
- Kumaar, S., Ahmed, H.U., Tuernicht, K., Jarmulowicz, M., Bleiberg, H., Braeckman, J. et al. Potential role of prostate Histo Scanning in focal therapy. In Focal Therapy Meeting, 2009.
- Lambert E.H., Bolte K., Masson P., Katz A.E. (2007) Focal cryosurgery: Encouraging health outcomes for unifocal prostate cancer. Urology 69: 1117–1120 [DOI] [PubMed] [Google Scholar]
- Levy D., Avallone A., Jones J.S. (2010) Current state of urological cryosurgery: Prostate and kidney. BJU Int 105: 590–600 [DOI] [PubMed] [Google Scholar]
- Lindner U., Weersink R.A., Haider M.A., Gertner M.R., Davidson S.R., Atri M., et al. (2009) Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol 182: 1371–1377 [DOI] [PubMed] [Google Scholar]
- Liu W., Laitinen S., Khan S., Vihinen M., Kowalski J., Yu G., et al. (2009) Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med 15: 559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukka H., Waldron T., Chin J., Mayhew L., Warde P., Winquist E., et al. (2011) High-intensity focused ultrasound for prostate cancer: A systematic review. Clin Oncol (R Coll Radiol) 23: 117–127 [DOI] [PubMed] [Google Scholar]
- Madersbacher S., Pedevilla M., Vingers L., Susani M., Marberger M. (1995) Effect of high-intensity focused ultrasound on human prostate cancer in vivo. Cancer Res 55: 3346–3351 [PubMed] [Google Scholar]
- Moore C.M., Pendse D., Emberton M. (2009) Photodynamic therapy for prostate cancer—a review of current status and future promise. Nat Clin Pract Urol 6: 18–30 [DOI] [PubMed] [Google Scholar]
- Mouraviev V., Johansen T.E., Polascik T.J. (2010) Contemporary results of focal therapy for prostate cancer using cryoablation. J Endourol 24: 827–834 [DOI] [PubMed] [Google Scholar]
- Mouraviev V., Mayes J.M., Sun L., Madden J.F., Moul J.W., Polascik T.J. (2007) Prostate cancer laterality as a rationale of focal ablative therapy for the treatment of clinically localized prostate cancer. Cancer 110: 906–910 [DOI] [PubMed] [Google Scholar]
- Muto S., Yoshii T., Saito K., Kamiyama Y., Ide H., Horie S. (2008) Focal therapy with high-intensity-focused ultrasound in the treatment of localized prostate cancer. Jpn J Clin Oncol 38: 192–199 [DOI] [PubMed] [Google Scholar]
- Noguchi M., Stamey T.A., Mcneal J.E., Nolley R. (2003) Prognostic factors for multifocal prostate cancer in radical prostatectomy specimens: Lack of significance of secondary cancers. J Urol 170: 459–463 [DOI] [PubMed] [Google Scholar]
- Norgaard, N. and Autier, P. (2010) Can HistoScanning help in the assessment of patients with raised PSA level: a pilot study. In EAU Annual Meeting, Barcelona, Spain, April 2010.
- Ohori, M., Eastham, J.A., Koh, H., Kuoiwa, K., Slawin, K. and Wheeler, T. (2006) Is focal therapy reasonable in patients with early stage prostate cancer (CaP) – an analysis of radical prostatectomy (RP) specimens. In AUA Meeting, Abstract #1574.
- Onik G. (2009) Abstract 75: Focal therapy for prostate cancer – 120 patients with up to 12 year follow-up. JVIR 20(Suppl. 1): S30–S30 [Google Scholar]
- Onik G., Miessau M., Bostwick D.G. (2009) Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol 27: 4321–4326 [DOI] [PubMed] [Google Scholar]
- Onik G., Narayan P., Vaughan D., Dineen M., Brunelle R. (2002) Focal “nerve-sparing” cryosurgery for treatment of primary prostate cancer: a new approach to preserving potency. Urology 60: 109–114 [DOI] [PubMed] [Google Scholar]
- Onik G., Vaughan D., Lotenfoe R., Dineen M., Brady J. (2007) “Male lumpectomy”: focal therapy for prostate cancer using cryoablation. Urology 70: 16–21 [DOI] [PubMed] [Google Scholar]
- Onik G., Vaughan D., Lotenfoe R., Dineen M., Brady J. (2008) The “male lumpectomy”: focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol 26: 500–505 [DOI] [PubMed] [Google Scholar]
- Parivar F., Hricak H., Shinohara K., Kurhanewicz J., Vigneron D.B., Nelson S.J., et al. (1996) Detection of locally recurrent prostate cancer after cryosurgery: Evaluation by transrectal ultrasound, magnetic resonance imaging, and three-dimensional proton magnetic resonance spectroscopy. Urology 48: 594–599 [DOI] [PubMed] [Google Scholar]
- Puech P., Potiron E., Lemaitre L., Leroy X., Haber G.P., Crouzet S., et al. (2009) Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: Correlation with radical prostatectomy specimens. Urology 74: 1094–1099 [DOI] [PubMed] [Google Scholar]
- Quann P., Jarrard D.F., Huang W. Current prostate biopsy protocols cannot reliably identify patients for focal therapy: Correlation of low-risk prostate cancer on biopsy with radical prostatectomy findings. Int J Clin Exp Pathol 3: 401–407 [PMC free article] [PubMed] [Google Scholar]
- Roethke, M., Anastasiadis, A.G., Lichy, M., Werner, M., Wagner, P., Kruck, S. et al. (2011) MRI-guided prostate biopsy detects clinically significant cancer: Analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol, in press. [DOI] [PubMed]
- Rouviere O., Girouin N., Glas L., Ben Cheikh A., Gelet A., Mege-Lechevallier F., et al. Prostate cancer transrectal HIFU ablation: Detection of local recurrences using T2-weighted and dynamic contrast-enhanced MRI. Eur Radiol 20: 48–55 [DOI] [PubMed] [Google Scholar]
- Ruijter E.T., Van De Kaa C.A., Schalken J.A., Debruyne F.M., Ruiter D.J. (1996) Histological grade heterogeneity in multifocal prostate cancer. Biological and clinical implications. J Pathol 180: 295–299 [DOI] [PubMed] [Google Scholar]
- Sartor A.O., Hricak H., Wheeler T.M., Coleman J., Penson D.F., Carroll P.R., et al. (2008) Evaluating localized prostate cancer and identifying candidates for focal therapy. Urology 72: S12–S24 [DOI] [PubMed] [Google Scholar]
- Scales C.D., Jr, Presti J.C., Jr, Kane C.J., Terris M.K., Aronson W.J., Amling C.L., et al. (2007) Predicting unilateral prostate cancer based on biopsy features: Implications for focal ablative therapy—results from the search database. J Urol 178: 1249–1252 [DOI] [PubMed] [Google Scholar]
- Stamey T.A., Mcneal J.M., Wise A.M., Clayton J.L. (2001) Secondary cancers in the prostate do not determine PSA biochemical failure in untreated men undergoing radical retropubic prostatectomy. Eur Urol 39(Suppl. 4): 22–23 [DOI] [PubMed] [Google Scholar]
- Stattin P., Holmberg E., Johansson J.E., Holmberg L., Adolfsson J., Hugosson J., et al. Outcomes in localized prostate cancer: national prostate cancer register of Sweden follow-up study. J Natl Cancer Inst 102: 950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja S.S., Mason M. (2010) Candidate selection for prostate cancer focal therapy. J Endourol 24: 835–841 [DOI] [PubMed] [Google Scholar]
- Tareen B., Godoy G., Sankin A., Temkin S., Lepor H., Taneja S.S. (2009) Can contemporary transrectal prostate biopsy accurately select candidates for hemi-ablative focal therapy of prostate cancer? BJU Int 104: 195–199 [DOI] [PubMed] [Google Scholar]
- Tareen B., Sankin A., Godoy G., Temkin S., Lepor H., Taneja S.S. (2009) Appropriate candidates for hemiablative focal therapy are infrequently encountered among men selected for radical prostatectomy in contemporary cohort. Urology 73: 351–354; discussion 354–355 [DOI] [PubMed] [Google Scholar]
- Trachtenberg J., Weersink R.A., Davidson S.R., Haider M.A., Bogaards A., Gertner M.R., et al. (2008) Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: A study of escalating light doses. BJU Int 102: 556–562 [DOI] [PubMed] [Google Scholar]
- Truesdale M.D., Cheetham P.J., Hruby G.W., Wenske S., Conforto A.K., Cooper A.B., et al. (2010) An evaluation of patient selection criteria on predicting progression-free survival after primary focal unilateral nerve-sparing cryoablation for prostate cancer: Recommendations for follow up. Cancer J 16: 544–549 [DOI] [PubMed] [Google Scholar]
- Turkbey B., Albert P.S., Kurdziel K., Choyke P.L. (2009a) Imaging localized prostate cancer: Current approaches and new developments. AJR Am J Roentgenol 192: 1471–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkbey B., Pinto P.A., Choyke P.L., Medscape (2009b) Imaging techniques for prostate cancer: Implications for focal therapy. Nat Rev Urol 6: 191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkbey B., Pinto P.A., Mani H., Bernardo M., Pang Y., Mckinney Y.L., et al. (2010) Prostate cancer: value of multiparametric MR imaging at 3 T for detection—histopathologic correlation. Radiology 255: 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villers A., Lemaitre L., Haffner J., Puech P. (2009) Current status of MRI for the diagnosis, staging and prognosis of prostate cancer: Implications for focal therapy and active surveillance. Curr Opin Urol 19: 274–282 [DOI] [PubMed] [Google Scholar]
- Wang L. (2009) Incremental value of magnetic resonance imaging in the advanced management of prostate cancer. World J Radiol 1: 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Hricak H., Kattan M.W., Chen H.N., Scardino P.T., Kuroiwa K. (2006) Prediction of organ-confined prostate cancer: Incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology 238: 597–603 [DOI] [PubMed] [Google Scholar]
- Warmuth M., Johansson T., Mad P. (2010) Systematic review of the efficacy and safety of high-intensity focussed ultrasound for the primary and salvage treatment of prostate cancer. Eur Urol 58: 803–815 [DOI] [PubMed] [Google Scholar]
- Windahl T., Andersson S.O., Lofgren L. (1990) Photodynamic therapy of localised prostatic cancer. Lancet 336: 1139–1139 [DOI] [PubMed] [Google Scholar]
- Wise A.M., Stamey T.A., Mcneal J.E., Clayton J.L. (2002) Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 60: 264–269 [DOI] [PubMed] [Google Scholar]
- Zatura, F., Klezl, P., Barta, J. and Autier, P. (2010) Prostate HistoScanning examination in patients with past negative biopsy sessions: a pilot study. In BAUS Annual Meeting, Manchester, UK, 2010.