Abstract
Maspin, a unique serine proteinase inhibitor (serpin), plays a key role in mammary gland development and is silenced during breast cancer progression. Maspin has been shown to inhibit tumor cell motility and invasion in cell culture, as well as growth and metastasis in animal models. In this study, we investigated the effect of maspin on the regulation of hypoxia-induced expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR), with respect to invasive potential in metastatic breast cells MDA-MB-231. We hypothesized that maspin can neutralize or mitigate hypoxia- induced expression of uPA/uPAR in metastatic breast cancer cells, resulting in suppression of their invasive potential. To test our hypothesis, we employed the highly invasive MDA-MB-231 breast cancer cells that are devoid of maspin, and transfected them with the maspin gene, and then determined the effect of hypoxia on uPA/uPAR expression. Normal mammary epithelial cells 1436N1 were used as a control. Our findings demonstrate that maspin downregulated the basal and hypoxia-induced uPA/uPAR expression and reduced the stimulatory effect of hypoxia on the in vitro invasive ability of MDA-MB-231-cells. In addition, maspin also inhibited the enzymatic activity of secreted and cell associated uPA in MDA-MB-231 cells. These results indicate that maspin inhibits hypoxia-induced invasion of metastatic breast cancer cells by blocking the uPA system, thus illuminating an important molecular pathway for therapeutic consideration.
Keywords: uPA/uPAR, hypoxia, invasion, maspin, breast cancer
INTRODUCTION
The tumor microenvironment is uniquely different from normal tissues. It exhibits abnormal and chaotic vascular networks, unbalanced blood supply, acidic extracellular pH and variations in perfusion.1 As a consequence, many regions within tumors become chronically hypoxic (0–3% O2). Studies have indicated that cells exposed to a hypoxic environment exhibit reduced sensitivity to radiation and drug therapy;2,3 increased ability to invade the extracellular matrix (ECM) in vitro;4 and greater in vivo metastatic potential.5 However, hypoxia-inducible factor (HIF-1α) which is overexpressed in many cancers including colon, prostate and breast facilitates tumor cells in their interactions with a hostile microenvironment by increasing transcription of many genes.6,7 The protein products of some of these genes promote angiogenesis (to increase oxygen availability), glycolysis (to decrease oxygen consumption), growth-factor signalling, apoptosis, pH-regulation, invasion and metastasis.8,9
In order to invade and metastasize, tumor cells must degrade the ECM, a process facilitated by a combination of proteolytic enzymes, including uPA. UPA is secreted as an enzymatically inactive single chain proenzyme and binds to its cellular receptor uPAR by its amino-terminal fragment (ATF) in an autocrine fashion.10,11 The binding of uPA to uPAR localizes the enzymatic activity of uPA which can trigger a focal and directional proteolysis of the ECM.10,12 The complex of uPA-uPAR then catalyses the conversion of plasminogen to plasmin, a broad spectrum enzyme, which leads to degradation of ECM or activation of other zymogens such as the matrix metalloproteinases (MMP’s).13 UPA/uPAR is overexpressed in many cancers, including breast, prostate, colon and lung carcinoma.14 The increase in uPAR expression has been implicated in the stimulation of invasive potential of tumor cells.13 For example, transfection of human osteosarcoma cells with uPAR cDNA resulted in a four-fold increase in their invasive potential, compared to the control cells.15 Other studies have also indicated that exposure of the MDA-MB-231 breast cancer cells to a hypoxic environment increased the expression of uPAR and significantly enhanced the invasive potential of MDA-MB-231.15
Since activation of uPA-dependent proteolysis and signaling depends on the binding of uPA to uPAR, inhibition of this interaction has been proposed as a potential therapeutic modality to inhibit tumor progression. Studies using blocking antibodies to uPA/uPAR, high molecular weight inhibitors of uPA-uPAR interaction, and targeting uPA/uPAR gene expression have resulted in reduced tumor growth, invasion and metastasis.16–19 Furthermore, the activities of uPA and uPAR are also regulated by members of the serine proteinase inhibitor family (SERPINS), including maspin and plasminogen activator inhibitors-1 and 2 (PAI-1, PAI-2).20
Maspin, a 42-kDa protein, is present in high concentration in normal mammary epithelial and myoepithelial cells, downregulated in primary breast cancer cell lines and totally lost in invasive breast cancer cells.21,22 Loss of maspin expression has been correlated with increased malignancy in breast cancer.22–24 Although experimental studies have demonstrated a tumor suppressive role for maspin at the level of invasion, tumor growth and metastasis,24–25 there is no information on the role of maspin in the regulation of the uPA system under hypoxic conditions on breast cancer cells.
In this study, we hypothesized that maspin could suppress the hypoxia-induced uPA/uPAR expression of metastatic breast cancer cells. To test this hypothesis, we generated stable maspin expressing transfectants of the highly invasive/metastatic MDA-MB-231 breast cancer cells and tested their levels of uPA/uPAR under hypoxia, compared with their control counterpart. The data reveal that maspin downregulated the basal and hypoxia-induced uPA/uPAR expression and reduced the stimulatory effect of hypoxia on the in vitro invasive ability of the metastatic breast cancer cells. Furthermore, we observed that maspin inhibited the enzymatic activity of both secreted and cell associated uPA. These findings offer new insights into the influence of the tumor suppressor gene maspin on the molecular effects of hypoxia on breast cancer.
MATERIALS AND METHODS
Cells and culture conditions
MDA MB-231, MDA-MB-231 transfected with maspin gene, were maintained and propagated in RPMI-1640 supplemented with 10% fetal calf serum (FCS) and gentamicin sulfate (50 mg/l) (Gemini Bioproducts; Calabasas, CA). Immortalized normal mammary epithelial cells 1436N1 (a gift from Dr. Shijie Sheng, Department of Pathology, Wayne State University School of Medicine, Detroit, MI) was maintained in D complete medium (1:1 α-MEM: Ham’s F-12, containing 1% FCS, 1 ng/ml cholera toxin, 10 mM Hepes pH 7.7, 50 μM ascorbic acid and Mito+ serum free supplement). All the cell lines were routinely screened for Mycoplasma species (PCR based and rapid detection system, Roche, Indianapolis, IN) and experiments were performed with 80–90% confluent cultures.
Generation of MDA-MB-231-GFP-maspin
Stable maspin-transfectant of breast carcinoma cells MDA-MB-231 were generated using the Lipofectamine (Invitrogen; Carlsbad, CA) protocol as described previously.26 RT-PCR and Western blot analysis confirmed the expression of maspin in the transfected cells.
Hypoxic culture conditions
For culture under hypoxic conditions, cells were plated on 150-mm culture dishes (Nalgene Nunc International; Rochester, NY) at 80–90% confluency. They were then placed in the airtight hypoxia chamber (Billups-Rothenberg, Modular Incubator Chamber; Del Mar, CA). The chamber was then flushed with a gas mixture containing 1% O2, 5% CO2 and 94% N2 until the oxygen concentration within the chamber reached 1% as measured by a Miniox 1 oxygen analyzer (Catalyst Research Corp, Owings Mills, MD). Under these conditions, the hypoxia chamber equilibrated within 1–2 hours and the O2 level remained at or below 1% throughout the incubation period (up to 24 hrs).
RNA isolation and semiquantitative RT-PCR analysis
Total RNA was isolated from hypoxia-treated breast cancer cells by direct addition of TRIZoL reagent to the culture dishes. Total RNA (1ug) was then reverse transcribed using an oligo (dT) primer and reverse transcriptase using the advantage PCR kit according to manufacturer’s instructions (BD Clontech, Palo Alto, CA). PCR amplification was performed as previously described27 with gene specific primers HIF-1 α, (forward: 5′-CCAGATTCAGGATCAGACACCTAGTCCT-3′: reverse 5-′GCTCCATTCCATTCTGTTCACTAGATTTG-3′) uPA, (forward 5′-TGTGGCCAAAAGACTCTGAGGC-3′ reverse 5′-CTTGGTGTGACTGCGGATCCA-3′) uPAR (forward 5′-GAAGAACAGTGCCTGGATGTGGTGA-3′, reverse 5′-AGGTTTAGGTCCAGAGGAGAGTGCCTC-3′). 18S rRNA primers (forward: 5′-TTGGAGGGCAAGTCTGGTGCCAGCAGC-3′ reverse:5′ TCTGTCAATCCTGTCCGTGTCGGGCC-3′) were used as controls for PCR amplification under hypoxic conditions.
Preparation of cytosolic fractions
To determine whether hypoxia affects sub-cellular distribution of uPA/uPAR, cells which were incubated for (0–24 hrs) under hypoxia were harvested and used for cytosolic fractionation as described previously.28 The protein content of cytosolic fractions were determined using Protein Assay Reagent Kit (Pierce Corp., Rockford, IL).
Western blot analysis
Equal amounts of cytoplasmic protein from various regiments were subjected to 10% SDS-PAGE and then transblotted onto nitrocellulose membrane (Schleicher & Schuell, Keene, NH). The membranes were blocked in TBS-TB buffer (10 mM Tris-HCl (pH 7.4), 150 mM NaCl supplemented with 0.05% Tween-20; 0.05% BSA) containing 5% non-fat dry milk, and incubated with monoclonal antibodies to uPAR 3937 2 μg/ml, uPA, 3689 2 μg/ml, (American Diagnostica Inc, Greenwich, CT) and HIF-1α, 610958, 1:250 dilution, (BD Transduction Laboratories, Lexington, KY) followed by incubation with 1:5000 dilution of HRP-conjugated secondary antibody (Jackson ImmunoResearch Laboratories. Inc, West Grove, PA). The reaction products were visualized using the ECL chemiluminescence detection kit (ECL; Perkin Elmer, Life Sciences Inc, Boston MA). To test for equal loading, the blots were stripped and reprobed with a monoclonal antibody to actin (1:5000 dilution, MAB1501, Chemicon, Temecula, CA).
In vitro invasion assay
To examine the effect of maspin on hypoxia-induced in vitro invasive potential of breast cancer cells invasion assay was performed using the membrane invasion culture system (MICS), as described previously.29 Briefly, cells were treated under hypoxic and normoxic conditions in the absence or presence of 20 μg/ml neutralizing anti-uPAR antibody (MAB807, R&D systems, Minneapolis, MN), for up to 16 hrs. Subsequently, 5 × 104 cells/wells were seeded into the upper wells of the MICS chamber containing RPMI 1640 Mito+ serum-free media. After 24hrs, the cells that had invaded through the coated matrix into the lower wells were harvested. The percentage of invasion was calculated as the total number of invading cells/total number of cells seeded X 100.
Zymographic analysis of plasminogen activators
The effect of maspin on the enzymatic activity of cell surface associated and secreted uPA in breast cancer cells MDA-MB-231, MDA-MB-231-GFP-maspin and normal mammary epithelial cells1436N1 was examined by gelatin zymography. Cells were cultured in serum-free media in 35 mm tissue culture dishes and incubated under hypoxia for (0–24 hrs). Conditioned media (CM) was then collected and either stored at −80°C or used immediately. Ten microliters of CM and 8 μg of cytosolic proteins were mixed with sample buffer without β-mercaptoethanol and subjected to plasminogen-dependent gelatinolytic zymography as described previously.30
RESULTS
Maspin inhibits hypoxia-induced regulation of uPA/uPAR in invasive/metastatic breast cancer cells
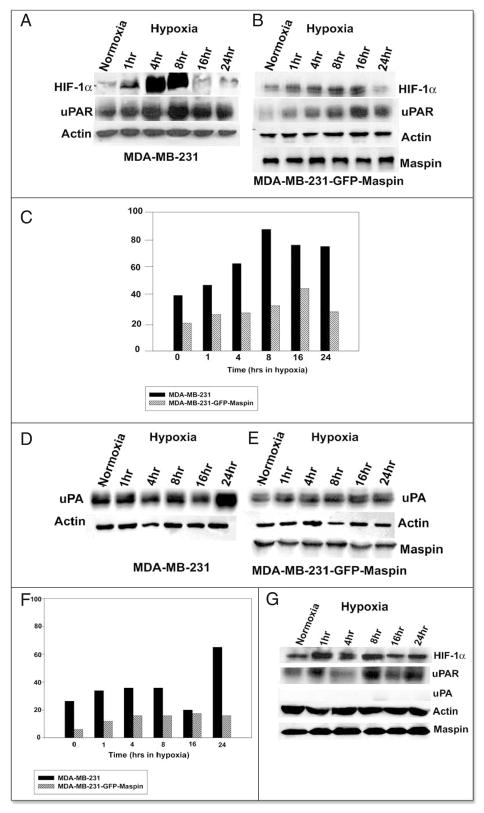
Based on the reported studies, the induced level of transcription factor HIF-1α is a hallmark of hypoxic conditions.9 We first attempted to examine the changes in HIF-1α expression both at the mRNA and protein level in MDA-MB-231 and MDA-MB-231-GFP-maspin cells following exposure to hypoxic conditions, and then tested the consequences on uPA/uPAR expression by RT-PCR and Western blot analysis.
We observed an induction of HIF-1α and uPAR at the mRNA level after 1 hr of exposure to hypoxia, which remained elevated for up to 24 hrs, whereas, no detectable changes in mRNA were observed for uPA (Fig. 1A and C). Interestingly, in the MDA-MB-231-GFP-maspin cells there were no detectable changes under hypoxic conditions in the mRNA levels of either HIF-1α or uPAR and uPA (Fig. 1B and D).
Figure 1.
Effect of maspin on uPA and uPAR expression under hypoxia. MDA-MB-231 (A) and MDA-MB-231-GFP-Maspin (B) were cultured under hypoxia for (0–24 hrs). Total RNA was then isolated and analyzed by RT-PCR using uPAR, uPA and HIF-1α specific primers. 18s rRNA primers were used as control for equal loading. The signals for uPA and uPAR mRNA were quantified by densitometric analysis and represented as the ratio of uPA:18srRNA and uPAR:18s rRNA (C and D). Results shown in the lower panels are representatives of three independent experiments and were performed using Scion image analysis and Sigma plot.
To examine whether mRNA changes were translated at the protein level, Western blot analysis was performed. This analysis indicated that hypoxia induced the protein expression of HIF-1 α and uPAR in the MDA-MB-231 cells reaching its maximum level by 8hrs (Fig. 2A and C). Quantification of uPAR protein bands by densitometry showed a 1–2 fold decrease in uPAR protein levels in cells transfected with maspin (Fig. 2B and C). With respect to uPA we observed that in MDA-MB-231 cells hypoxia induced expression of uPA by 1hr and reached its maximum level by 24 hr (Fig. 2D and F) with no detectable changes in the protein bands from maspin transfected cells (2E). Densitometric analysis showed that maspin was able to inhibit the uPA protein level by 2-fold (Fig. 2F) in MDA-MB-231-GFP-maspin cells under hypoxic conditions. However, the normal mammary epithelial cells 1436N1 revealed no significant increase in uPAR protein expression in hypoxic fractions as compared to controls, and we were unable to detect any expression of uPA protein under hypoxic and normoxic conditions, respectively (Fig. 2G).
Figure 2.
Maspin inhibits the uPAR and uPA expression in MDA-MB- 231-GFP-Maspin cells. Western blot analysis of uPAR and uPA protein in MDA-MB-231 (A) MDA-MB-231-GFP-maspin (B) and normal mammary epithelial cells 1436N1 (C) cultured for (0–24 hrs) under hypoxia. Equal amounts (25 μg) of cytosolic protein was resolved on a 10% sodium dodecyl sulfate-polyacrylamide gel, transblotted onto nitorcellulose membrane, and probed with anti-HIF-1α, uPAR and uPA monoclonal antibodies. Monoclonal antibody to actin was used as a control for equal loading. The data represents the densitometric analysis of results represented as the ratio of uPA: actin and uPAR:actin (C and F). Results shown in the lower panels were performed using Scion image and Sigma plot are representatives of three to five independent experiments.
Maspin affects hypoxia induced tumor cell invasion in vitro
Previous studies have shown that hypoxia increases the invasiveness of MDA-MB-231 cells, which can be abrogated by a function-blocking anti-uPAR antibody.15,31 In the current study, we observed that maspin transfection of MDA-MB-231 significantly reduced the invasive potential of these cells, under both normoxic and hypoxic conditions (Fig. 3).
Figure 3.
Maspin inhibits hypoxia-induced in vitro invasion in human breast cancer cells MDA-MB-231. Equal amounts (50,000) cells/wells were seeded into the upper wells of the MICS chamber containing Mito+ serum-free media, following a 24 hr incubation under either hypoxia or normoxia in the absence or presence of 20 μg/ml anti-uPAR neutralizing antibody. After 24 hrs the cells in the bottom chamber were harvested and counted. The percentage of invasion was determined by counting the number of cells that migrated through a collagen 1V/laminin/gelatin-coated polycarbonate fiter in 24 hr/total number of cells seeded X 100). The Data are expressed as a percentage of normoxic cells and are representatives of 3–4 independent experiments.
Maspin inhibits secreted uPA and cell surface associated uPA-uPAR complexes
UPA is an endogenous activator of the thrombolytic mediator plasminogen, however, in a tumor microenvironment its proteolytic activity could be directed towards the ECM, thus contributing to the degradation of ECM, dissemination of tumor cells, and subsequent tumor progression.13 To assess the effect of maspin on the enhanced proteolytic activity of uPA under hypoxic conditions, we performed zymography in the presence of plasminogen, a specific substrate of uPA.
The zymographic data revealed activity bands at approximately 52–55 kDa in the cytosolic extract (Fig. 4A), representing cell associated uPA/uPAR complexes and in the serum-free conditioned media (Fig. 4C), representing secreted uPA. Our studies indicated that maspin transfection of MB-231 cells resulted in the reduction of both cell associated and secreated uPA activity under normoxic and hypoxic conditions. Interestingly, the results also indicate that the uPA activity in conditioned media under normoxia is about 2-fold higher as compared to cells exposed to hypoxic environment both in wild type MDA-MB-231 and maspin transfected MDA-MB-231-GFP-maspin cells.
Figure 4.
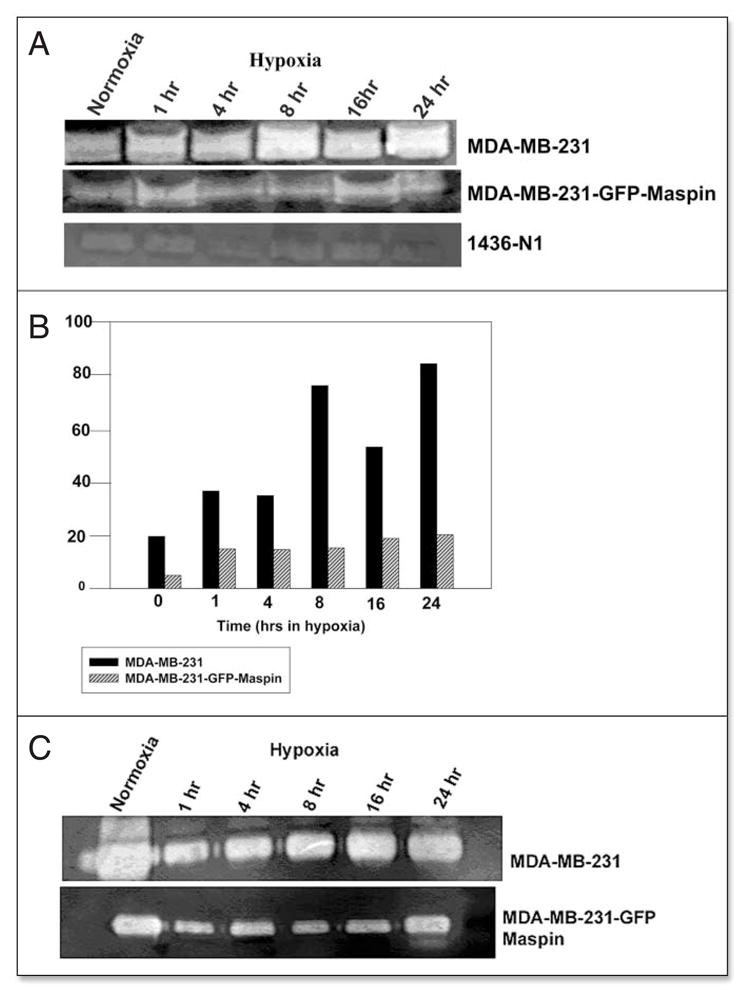
Maspin inhibits the activity of cell associated and secreted uPA in MDA-MB-231 and MDA-MB-231-GFP-Maspin cells. UPA activity of (A) cell-associated and secreted (C) in breast cancer cells MDA-MB-231, MDA-MB-231 GFP-maspin and normal mammary epithelial cells 1436N1 under hypoxia at indicated times as determined by zymography. Clear bands at 50–55 kDa represent enzymatic activity of uPA due to the activation of plasminogen. Levels of uPA activity were quantified by densitometry employing scion image analysis and sigma plot (B and D). Results shown in the lower panels are representatives of three independent experiments.
By comparison analysis of the uPA activity in the cytosolic extract and serum free conditioned media from normal mammary epithelial cells 1436N1 showed extremely low levels of cell associated uPA activity and no detectable levels of secreted uPA (Fig. 4A and B). These data suggest that maspin in normal mammary epithelial cells as compared to metastatic cancer cells could downregulate uPA protein as well as activity levels.
DISCUSSION
Our studies indicate, for the first time, the ability of maspin (a serine proteinase inhibitor) to mitigate the hypoxia-induced uPA/uPAR expression in highly invasive breast cancer cells. Specifically, we have shown that inhibition of uPA complex by the tumor suppressor gene maspin reduced the effect of uPAR dependent activation of uPA and inhibited in vitro invasion of ECM. These observations further extend the reported studies that exposure of trophoblasts, human umbilical vein endothelial (HUVECs), colon and breast carcinoma cells to hypoxic conditions results in the upregulation of uPAR expression and increased in vitro invasive activity15,31,32 In addition, it further complements the reported tumor suppressive effects of maspin on breast cancer cell invasion by identifying its ability to target the uPA system in a hypoxic environment.
The production of pro-uPA and its subsequent activation to uPA by its interaction with uPAR is an important step in cancer cell invasion, as it is required for efficient activation of plasminogen to plasmin and ECM degradation.32,33 Downregulation of both uPA and uPAR would be an efficient way to circumvent the possibility of uPAR-dependent, uPA mediated plasminogen activation.16,35,36 In this regard, various approaches have been utilized to interfere with the expression and/or activity of uPA in tumor cells, including, the use of active site inhibitors, antibodies to uPA, oligonucleotide or RNA directed against uPA.37–38 Other attempts to abrogate the uPA/uPAR interaction include the use of synthetic uPA-derived peptides encompassing the binding region of uPA to uPAR,39 or recombinant souble uPA as scavenger for uPAR.40 The catalytic activity of the uPA system can also be inhibited by its several natural inhibitors, including maspin, PAI-1 and PAI-2.13,19 These inhibitors play distinct roles in tumor progression and PAI-1 has been shown to be specifically involved in the progression of cancer.41 In contrast, maspin has been shown to reduce cell migration in vitro23–24 and angiogenesiss in various cell types.42 Furthermore, studies have shown that recombinant maspin has the ability to inhibit invasion and motility of mammary carcinoma cells in culture.24–25 Additional studies from our laboratory have indicated that maspin suppresses breast cancer cell invasiveness by modulating integrin expression and altering Rac level and activity.26
Our data further substantiate the tumor suppressive role of maspin, by demonstrating that reexpression of maspin in highly invasive and metastatic MDA-MB-231 cells decreased the hypoxia-induced expression of both uPA/uPAR resulting in reduced in vitro invasive ability, thus confirming the significance of the uPA system underlying the invasive behavior of breast cancer cells under hypoxic conditions. We also observed that addition of a neutralizing uPAR antibody to MDA-MB-231 and MDA-MB-231-GFP-maspin cells inhibited the stimulatory effect of hypoxia on cellular invasion, indicating a functional relationship between maspin and the uPA system under hypoxic environment. Analysis of normal mammary epithelial cells 1436N1 (that express high levels of endogenous maspin) revealed little to no amount of uPA protein expression and enzymatic activity, suggesting the possibility that maspin in normal mammary epithelial cells could downregulate uPA protein levels.
Interestingly, the zymography data showed a dramatic decrease in the activity of secreated uPA after hypoxic treatment. Low levels of uPA activity have been reported under hypoxia in CM of MDA-MB-231 breast cancer cells and human microvascular endothelial cells (HMVECs).15,43 Although the precise mechanism for this observation is unknown, it is likely that increased uPAR expression under hypoxia resulted in increased cell-surface bound uPA leading to its rapid internalization and depletion of uPA in the CM. This possibility is further supported by a previous study indicating that cell surface bound uPA induces plasminogen activation several fold when compared with that of unbound uPA.35
Although the tumor suppressive properties of maspin are consistent with those of protease inhibitors, the exact biological mechanism by which maspin exerts its tumor suppressive action is unclear. However, we speculate that maspin regulates the uPA system by inactivating uPA and enhancing the rate of uPA internalization and degradation. It is also likely that maspin inhibits the uPA system by affecting cell adhesion, either directly or indirectly based on maspin’s ability to alter the expression profile of integrins, in particular α5β1, in breast cancer cells.45 Since uPAR is known to interact with α5β1, this interaction may lead to a reduction in uPA binding and a concomitant reduction in cell surface plasminogen activation, eventually inhibiting invasion and metastasis.45
In summary, we have demonstrated that a tumor suppressor gene maspin has the ability to inhibit the hypoxia-induced cell surface plasminogen activation affecting in vitro invasive activity in MDA-MB-231 breast cancer cell (Fig. 5). Since localized plasmin inhibition seems to be an essential route to inhibiting invasion; therefore, maspin is an interesting candidate for therapeutic development in the management of both tumor invasion and metastasis under hypoxic conditions.
Figure 5.

Hypothetical model for the regulation of uPA system by maspin under hypoxia in breast cancer cells. Exposure of cancer cells to hypoxic environment leads to increased secreation of prouPA which then binds to its cellular receptor uPAR. This interaction between uPA-uPAR provides inducible, transient and localized cell surface proteolytic activity, required for tissue invasion, by enhancing the conversion of plasminogen to plasmin. Plasmin can either directly degrade basement ECM or activate other zymogen proteases such as procollagenase, resulting in increase invasive potential of tumor cells. In this model maspin regulates the uPA system by blocking the interaction of uPA with uPAR by binding to uPA and inactivating it. The maspin-uPA complex together with uPAR gets internalized and degraded. Thus, inhibiting migration, invasion and ultimately metastasis by the highly invasive and metastatic breast cancer cells MDA-MB-231.
Acknowledgments
NIH/NCI/CA 75681, the Marilyn Rozeboom Endowment from the Order of the Eastern Star (to M.J.C.H), and Eisenberg Scholarship award (to Z.K-E).
ABBREVIATIONS
- uPA
urokinase plasminogen activator
- uPAR
urokinase plasminogen activator receptor
- HIF-1α
hypoxia-inducible factor-1α
- PAI-1 and 2
Plasminogen activator inhibitor 1 and 2
- ECM
extracellular matrix
- CM
conditioned media
- ATF
amino-terminal fragment
References
- 1.Movsas B, Chapman JD, Greenberg RE, Hanlon AL, Horwitz EM, Pinover WH, Stobbe C, Hanks GE. Increasing levels of hypoxia in prostate carcinoma correlate significantly with increasing clinical stage and patient age. Cancer Res. 2000;89:2018–24. doi: 10.1002/1097-0142(20001101)89:9<2018::aid-cncr19>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Brown JM. The Hypoxic Cell. A target for selective cancer therapy. Cancer Res. 1999;59:5863–70. [PubMed] [Google Scholar]
- 3.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1998;13:139–68. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 4.Cuvier C, Jang A, Hill RP. Exposure to hypoxia, glucose starvation and acidosis: Effect on invasive capacity of murine tumor cells and correlation with cathepsin (L + B) secretion. Clin Exp Metastasis. 1997;15:19–25. doi: 10.1023/a:1018428105463. [DOI] [PubMed] [Google Scholar]
- 5.Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci USA. 1988;85:9533–7. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1 α and HIF-2α in normal human tissues. cancers and tumor-associated macrophages. Am J Pathol. 2000;157:411–21. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Deist PJ, van der Wall E. Levels of hypoxia-inducible factor 1 α during breast carcinogenesis. J Natl Cancer Inst (Bethesda) 2001;93:309–14. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- 8.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1 α in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- 9.Harris AL. Hypoxia-a key regulatory factor in tumour growth. Nature Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 10.Petersen LC, Lund LR, Nielsen LS, Dano K, Skriver L. One-chain urokinase-type plasminogen activator from human sarcoma cells is a proenzyme with little or no intrinsic activity. J Biol Chem. 1988;263:11189–95. [PubMed] [Google Scholar]
- 11.Mondino A, Resnati M, Blasi F. Structure and function of the urokinase receptor. Thromb Haemos. 1999;82:19–22. [PubMed] [Google Scholar]
- 12.Nielsen LS, Hansen JG, Skriver L, Wilson EL, Kaltoft K, Zeuthen J, Dano K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982;21:6410–5. doi: 10.1021/bi00268a014. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: A review. Cancer (Phila) 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Kariko K, Kuo A, Boyd D, Okada SS, Cines DB, Barnathan ES. Overexpression of urokinase receptor increases matrix invasion without altering cell migration in a human osteosarcoma cell line. Cancer Res. 1993;53:3109–17. [PubMed] [Google Scholar]
- 15.Graham CH, Forsdike J, Fitzgerald CF, MacDonald GS. Hypoxia-mediated stimulation of carcinoma cell invasiveness via upregulation of urokinase receptor expression. Int J Cancer. 1999;80:617–23. doi: 10.1002/(sici)1097-0215(19990209)80:4<617::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Lu H, Griscelli F, Opolon P, Sun LQ, Ragot T, Legrand Y, Belin D, Soria J, Soria C, Perricaudet M, Yeh P. Adenovirus-mediated delivery of auPA/uPAR antagonist suppresses angiogenesis-dependent tumor growth and dissemination in mice. Gene Ther. 1998;5:1105–13. doi: 10.1038/sj.gt.3300742. [DOI] [PubMed] [Google Scholar]
- 17.Go Y, Chintala SK, Mahanam S, Gokaslan Z, Venkaiah B, Bjerkvig R, Oka K, Nicolson GL, Sawaua RJS. Inhibition of in vivo tumorigenicity and invasiveness of a human glioblastoma cell line transfected with antisense uPAR vectors. Clin Exp Metastasis. 1997;5:440–6. doi: 10.1023/a:1018410523635. [DOI] [PubMed] [Google Scholar]
- 18.Crowley CW, Cohen RL, Lucas BK, Liu G, Shuman MA, Levinson AD. Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Science. 1993;90:5021–5. doi: 10.1073/pnas.90.11.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis V, Wun TC, Behrendt N, Ronne E, Dano K. inhibition of receptor-bound urokinase by plasminogen-activator inhibitors. J Biol Chem. 1990;65:904–8. [PubMed] [Google Scholar]
- 20.Sheng S, Pemberton P, Sager R. Production, purification, and characterization of recombinant maspin proteins. J Bol Chem. 1994;269:30988–93. [PubMed] [Google Scholar]
- 21.Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–9. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 22.Sager R, Sheng S, Pemberton P, Hendrix MJC. Maspin: A tumor suppressing serpin. In: Buntert U, Birchmeier W, editors. Current Topics in Microbiology and Immunology. Vol. 213. Berlin: Springer-Verlag; 1996. pp. 51–64. [DOI] [PubMed] [Google Scholar]
- 23.Hendrix MJC. De-mystifying the mechanism(s) of maspin. Nat Med. 2000;6:374–6. doi: 10.1038/74624. [DOI] [PubMed] [Google Scholar]
- 24.Sheng S, Carey J, Seftor EA, Dias L, Hendrix MJC, Sager R. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci USA. 1996;93:11669–74. doi: 10.1073/pnas.93.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biliran HJ, Sheng S. Pleiotrophic inhibition of pericellular urokinase-type plasminogen activator system by endogenous tumor suppressive maspin. Cancer Res. 2001;61:8676–82. [PubMed] [Google Scholar]
- 26.Odero MV, Khalkhali-Ellis Z, Chunthapong C, Amir S, Seftor REB, Seftor EA, Hendrix MJC. Maspin regulates different signaling pathways for motility and adhesion in aggressive breast cancer cells. Cancer Biol Ther. 2003:2398–403. doi: 10.4161/cbt.2.4.471. [DOI] [PubMed] [Google Scholar]
- 27.Kirschmann DA, Seftor EA, Fong SF, Nivea DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJC. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–83. [PubMed] [Google Scholar]
- 28.Khalkhali-Ellis Z, Hendrix MJC. Nitric oxide regulation of maspin expression in normal mammary epithelial and breast cancer cells. Am J Pathol. 2003;162:1411–7. doi: 10.1016/S0002-9440(10)64274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrix MJC, Seftor EA, Fidler IJ. A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett. 1987;38:137–47. doi: 10.1016/0304-3835(87)90209-6. [DOI] [PubMed] [Google Scholar]
- 30.Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gel containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 31.Graham CH, Fitzpatrick TE, McCrae KR. Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood. 1998;91:3300–7. [PubMed] [Google Scholar]
- 32.Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghiyev P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–43. [PubMed] [Google Scholar]
- 33.Wang Y. The role and regulation of urokinase-type plasminogen activator receptor gene expression in cancer invasion and metastasis. Med Res Rev. 2001;21:146–170. doi: 10.1002/1098-1128(200103)21:2<146::aid-med1004>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 34.Vassalli JD, Sappino AP, Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991;88:1067–72. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161–95. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt M, Wilhelm OG, Reuning U, Kruger A, Harbeck N, Lengyel E, Graeff H, Gaensbacher B, Kessler H, Buergle M, Stuerzebecher J, Sperl S, Magdolen V. The urokinase plasminogen activator system as a novel target for tumour therapy. Fibrinol Proteol. 2000;14:114–32. [Google Scholar]
- 37.Gondi CS, Lakka SS, Yanamandra N, Siddique K, Dinh DH, Olivero WC, Gujrati M, Rao JS. Expression of antisense uPAR and antisense uPA from a bicistronic adenoviral construct inhibits glioma cell invasion, tumor growth, and angiogenesis. Oncogene. 2003;38:5967–75. doi: 10.1038/sj.onc.1206535. [DOI] [PubMed] [Google Scholar]
- 38.Ellis V, Dano K. Potentiation of plasminogen activation by an anti-urokinase monoclonal antibody due to ternary complex formation. A mechanistic model for receptor-mediated plasminogen activation. J Biol Chem. 1993;268:4806–13. [PubMed] [Google Scholar]
- 39.Ossowski L, Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983;35:611–9. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- 40.Burgle M, Koppitz M, Riemer C, Kessler H, Konig B, Weidle UH, Kellerman J, Lottspeich F, Graeff H, Schmitt M, Goretzki L, Reuning U, Wilhelm O, Magdolen V. Inhibition of the interaction of urokinase-type plasminogen activator (uPA) with its receptor (uPAR) by synthetic peptides. J Biol Chem. 1997;378:231–7. doi: 10.1515/bchm.1997.378.3-4.231. [DOI] [PubMed] [Google Scholar]
- 41.Mohanam S, Chandrasekar N, Yanamandra N, Khawar S, Mirza F, Dinh DH, Olivero WC, Rao JS. Modulation of invasive properties of human glioblastoma cells stably expressing amino-terminal fragment of urokinase-type. Oncogene. 2002;51:7824–30. doi: 10.1038/sj.onc.1205893. [DOI] [PubMed] [Google Scholar]
- 42.Duffy MJ. Urokinase plasminogen activator and its inhibitor, PAI-1, as prognostic markers in breast cancer from pilot to level 1 evidence studies. Clinical Chem. 2000;48:1194–7. [PubMed] [Google Scholar]
- 43.Kroon ME, Koolwijk P, van der Vecht B, van Hinsbergh VW. Urokinase receptor expression on human microvascular endothelial cells is increased by hypoxia: Implications for capillary-like tube formation in a fibrin matrix. Blood. 2000;96:2775–83. [PubMed] [Google Scholar]
- 44.Wojta J, Jones RL, Binder BR, Hoover RL. Reduction in pO2 decreases the fibrinolytic potentialof cultured bovine endothelial cells derived frompulmonary arteries and lung microvasculature. Blood. 1988;71:1703–6. [PubMed] [Google Scholar]
- 45.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–9. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 46.Kruger A, Soeltl R, Lutz V, Wilhelm OG, Magdolen V, Rojo EE, Hantzopoulos PA, Graeff H, Gansbacher B, Schmitt M. Reduction of breast carcinoma tumor growth and lung colonization by overexpression of the soluble urokinase-type plasminogen activator receptor (CD87) Cancer Gene Ther. 2000;7:292–9. doi: 10.1038/sj.cgt.7700144. [DOI] [PubMed] [Google Scholar]
- 47.Mazumdar A, Adam L, Boyd D, Kumar R. Heregulin regulation of urokinase plasminogen activator and its receptor: Human breast epithelial cell invasion. Cancer Res. 2001;61:400–5. [PubMed] [Google Scholar]