Abstract
Interferon regulatory factor 6 (IRF6) is a non-canonical member of the interferon regulatory factor family of transcription factors. We recently identified IRF6 as a novel Maspin-interacting protein in mammary epithelial cells. Maspin is a tumor suppressor in the breast and has also been implicated in mammary gland morphogenesis. To explore a possible role for IRF6 in conjunction with Maspin during mammary gland growth and differentiation, we examined the expression of IRF6 and Maspin during post-utero mammary gland development using a combination of in vitro and in vivo approaches. The data revealed that the expression of IRF6 and Maspin is temporally and spatially regulated throughout mammary gland development, with maximal expression of both proteins occurring in fully differentiated, lactating lobuloalveolar cells. We further show that IRF6 adopts a lumenal localization pattern following complete epithelial cell polarization and present new evidence for the secretion of IRF6 into the milk. These results support the hypothesis that IRF6 and Maspin are important for mammary epithelial cell differentiation, and advance our understanding of the Maspin-IRF6 partnership during normal mammary gland development.
Keywords: breast cancer, cell differentiation, interferon regulatory factor 6, mammary gland, Maspin
Introduction
The development of a mature, functional mammary gland is a multi-step process characterized by cycles of cellular proliferation and growth, cell differentiation, and apoptosis. Mammary gland development can be divided into five distinct stages: the embryonic/fetal period distinguished by the development of the mammary anlage; the neonatal/prepubertal period defined by isometric glandular growth; the pubertal period, which is typified by allometric growth, ductal elongation and branching morphogenesis; and functional differentiation, also termed lactogenesis, which only occurs during pregnancy and is characterized by differentiation of the lobuloalveolar units in preparation for lactation (Hovey et al. 2002). The fifth stage of development, termed post-lactational involution, occurs following removal of the suckling stimulus and is accompanied by a complete remodeling of the breast tissue, which returns the breast to a near-virgin-like state in preparation for another pregnancy. Involution can be divided into two distinct stages. Stage I is initiated following milk stasis and consists of apoptosis of the milk-producing lobuloalveolar cells. This first stage is reversible if the suckling stimulus is reintroduced shortly after removal. Stage II, triggered by the systemic loss of lactogenic hormone stimulation, is irreversible and is characterized by proteinase gene activation, basement membrane degradation and tissue remodeling (Li et al. 1997).
Maspin (mammary serine protease inhibitor, SerpinB5) is a non-inhibitory serpin with tumor suppressive properties in the breast and is lost during cancer progression (Zou et al. 1994). More recent studies also demonstrate a role for Maspin during breast development. In a murine model, the targeted overexpression of Maspin during pregnancy resulted in altered differentiation and branching morphogenesis concomitant with an increase in apoptosis (Zhang et al. 1999). However, the pathways through which Maspin functions in development remain poorly understood.
We recently reported an interaction between Maspin and interferon regulatory factor 6 (IRF6) (Bailey et al. 2005). IRF6 belongs to the nine member IRF family of transcription factors, which are primarily studied in the context of innate immunity and the regulation of type I interferons, but have also been implicated in diverse cellular processes ranging from cellular proliferation to apoptosis and tumor suppression (Lin et al. 1998; Barnes et al. 2001; Moriyama et al. 2001; Duguay et al. 2002; Barnes et al. 2003). However, very little is known about the function of IRF6. We previously demonstrated that IRF6 is expressed in normal mammary epithelial cells and that, similar to Maspin, IRF6 expression is reduced or absent in breast carcinoma cells (Bailey et al. 2005). We also demonstrated a role for IRF6 in promoting cellular quiescence, an important step in cellular differentiation (Bailey et al. 2008). Other studies have recently identified a critical role for IRF6 in epidermal keratinocyte differentiation, which is blocked in the absence of functional IRF6 (Ingraham et al. 2006; Richardson et al. 2006). However, a direct role for IRF6 during normal mammary gland development has not been demonstrated. Based on these findings, we hypothesized that IRF6, in cooperation with Maspin, functions to promote differentiation of the mammary epithelial cell.
To enhance our understanding of the roles of both IRF6 and Maspin during normal mammary gland development, we evaluated protein expression patterns at multiple time points throughout postnatal mammary gland morphogenesis and differentiation. We demonstrate that Maspin and IRF6 are temporally regulated during mammary gland development and follow similar expression patterns throughout the differentiation and involution processes. These results support our hypothesis that IRF6 and Maspin play important roles during differentiation of the mammary gland and possibly provide new insights into the novel interactive partnership between these two proteins.
Materials and methods
Cell culture
The MCF-10 A cell line has been characterized as a normal, spontaneously immortalized human mammary epithelial cell line (Tait et al. 1990). MCF-10 A cells were maintained in complete growth medium consisting of HAM F-12 and Dulbecco’s Modified Eagle Medium (DMEM) (1:1, Gibco) supplemented with 5% horse serum (Invitrogen), epidermal growth factor (20 ng/mL, Peprotech), hydrocortisone (0.5 μg/mL, Sigma), insulin (10 μg/mL, Sigma), and cholera toxin (100 ng/mL, Sigma) with penicillin and streptomycin (Debnath et al. 2003). Cell cultures were determined to be free of Mycoplasma contamination using the GenProbe rapid detection system.
Western blot analysis
Whole cell lysate was acquired using standard lysis techniques. Briefly, MCF-10 A cells were washed in cold phosphate-buffered saline (PBS) and lysed in Buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), 0.1% NP-40 with 2 mM Na-ortho-vanadate, 2 mM NaF plus protease inhibitor). Cells were scraped and the slurry was briefly sonicated. Cells were then centrifuged at 12 000 g for 20 min at 4°C to obtain the whole cell lysate. For murine tissue cell lysate, mammary tissue was immediately frozen in liquid nitrogen upon removal. Frozen tissue was homogenized with a mortar and pestle, following which cells were lysed in RIPA buffer (100 mM Tris pH 7.5, 0.15 M NaCl, 1% deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 5 mM ethylenediaminetetraacetic acid [EDTA], 2 mM sodium vanadate and protease inhibitor cocktail). The slurry was briefly sonicated and cells were then centrifuged at 12 000 g for 20 min at 4°C to obtain the whole cell lysate. Equal amounts of cellular protein (25 μg) were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) (10% resolving gel) and Western blot analysis using specific antibody to Maspin (1:5000, BD Biosciences) or IRF6 (1:2000) as described previously (Bailey et al. 2005). Other antibodies and dilutions are as follows: beta-actin (1:10 000; Chemicon International).
3-Dimensional organotypic culture
Protocols were followed as set forth by Debnath et al. (Debnath et al. 2003). Briefly, MCF-10 A cells were maintained in growth medium consisting of DMEM/F12, horse serum (5%), epidermal growth factor (20 ng/mL), hydrocortisone (0.5 μg/mL), cholera toxin (100 ng/mL), insulin (10 μg/mL), and penicillin/streptomycin antibiotics. For the 3-D culture assay, eight well glass chamber slides (Nunc) were overlaid with 40 μL growth factor reduced (GFR) Matrigel (BD Biosciences) which was allowed to solidify at 37°C for 15 min. MCF-10 A cells were harvested and resuspended in 10 mL assay medium (same as growth medium except with 2% serum), counted, and diluted to a final concentration of 25 000 cells/mL. Cells were then mixed 1:1 with 4% GFR Matrigel in an assay medium for a final GFR Matrigel concentration of 2%. 400 μL of this mixture was added to each well of the GFR Matrigel-coated chamber slide for a final overlay cell concentration of 5000 cells/well. Cultures were grown under normal cell culture conditions (5% CO2, 37°C). Cells were fed with assay medium containing 2% GFR Matrigel every 4 days. Cell cultures were fixed in 2% formaldehyde (pH 7.4) for 20 min at room temperature, permeabilized with PBS containing 0.5% Triton X-100 for 10 min at 4°C, then rinsed three times with glycine rinse buffer (130 mM NaCl, 7 mM Na2HPO4, 3.5 mM NaH2PO4, 100 mM glycine) for 15 min at room temperature. Cell cultures were then blocked with 200 μL blocking buffer (130 mM NaCl, 7 mM Na2HPO4, 3.5 mM NaH2PO4, 0.1% bovine serum albumin (BSA), 0.2% Triton X-100, 0.05% Tween-20, 10% goat serum) for 90 min at room temperature. Primary antibody was added to the blocking buffer (anti-IRF6:1:500, anti-Maspin: 1:200) and incubated overnight at 4°C. Following incubation with the primary antibody, cells were washed three times with immunofluorescent (IF) buffer (130 mM NaCl, 7 mM Na2HPO4, 3.5 mM NaH2PO4, 0.1% BSA, 0.2% Triton X-100, 0.05% Tween-20) for 20 min each wash at room temperature. Secondary antibody was added to the blocking buffer at a 1:200 dilution and incubated in the dark for 50 min at room temperature. Samples were then washed three times with IF buffer for 20 min at room temperature. Nuclei were counterstained with DAPI (4′6′-diamidino-2-phenylindole dihydrochloride), 5 ng/mL in for 15 min. Cells were then rinsed once with PBS and mounted with Prolong Gold Anti-fade Reagent (Molecular Probes). Confocal microscopy was carried out using the Zeiss LSM-510 META confocal laser scanning microscope.
Animal husbandry and immunohistochemistry
C57/Black6 female mice from Harlan (Indianapolis) were kept according to International Animal Care and Use Committee (IACUC) standards and practices. Mice were killed at the various time points listed following anesthetization with 10% ketamine-xylazine in PBS (ketamine, 80 mg/kg; xylazine, 10 mg/kg) prior to removal of mammary glands. A minimum of three mice were examined at each time point. Pectoral and inguinal groups of mammary glands were evaluated by immunohistochemistry as previously described (Bailey et al. 2005). Briefly, tissue was fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue samples were sectioned at 4 μm, deparaffinized in xylene, and subjected to antigen retrieval using citrate buffer (pH 6.0). Samples were then blocked with hydrogen peroxide, Avidin and Biotin blocks (Vector Laboratories, Inc.) and a serum-free protein block. Primary antibodies were applied for 60 min, followed by incubation in the appropriate biotinylated secondary and streptavidin tertiary antibodies for 20 min each. Antibody to Maspin was used at a 1:200 dilution. Antibody to IRF6 was used at a 1:900 dilution. Maspin and IRF6 expression was determined on a Microm HMS 710i Auto-stainer (TermoFisher/Richard-Allan Scientific) using HRP Detection Systems. Diaminobenzidine (DAB) was used to visualize antigen immunoreactivity. Slides were counterstained with Mayer’s hematoxylin for 5 min, followed by dehydration in ethanol and xylene, and cover-slipped with permanent mounting medium. Slides, including the negative controls, represent serial sections. The negative control represents the appropriate immunoglobulin G (IgG) control at the same dilution as the primary antibody. Relative staining intensity of diaminobenzidine (DAB) was quantified using a CMYK (cyan, magenta, yellow, key) color model adapted from a model by Pham et al. (Pham et al. 2007). Briefly, chromogen intensity, based on a 0–255 scale, was determined from the yellow channel of a CMYK color image. Using Adobe Photoshop CS software, stained cells were selected by inversely selecting highlighted (white) areas. The mean intensity of the selected area was then measured for the yellow channel of the CMYK image. The inverse of the intensity was normalized and graphed using Microsoft Excel software. Images were captured with a Leica DM 4000B microscope mated to a Leica DFC480 5.0 Megapixel CCD camera.
Results
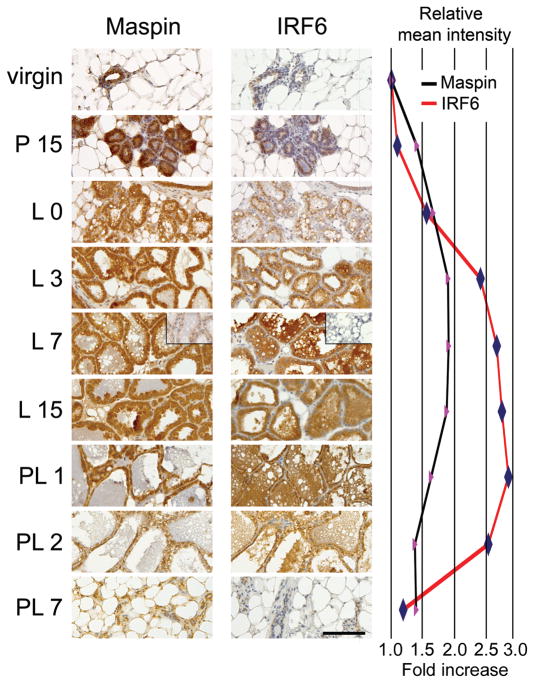
To test the hypothesis that Maspin and IRF6 are involved in the functional differentiation of the mammary gland, we first evaluated the temporal and spatial expression of IRF6 and Maspin during postnatal murine mammary gland development. Mammary tissue was harvested at multiple time points throughout mammary gland development, including pregnancy, lactation, and post-lactation involution, and evaluated for protein expression by immunohistochemistry (Fig. 1). Immunoreactive intensity for both Maspin and IRF6 was weakest during the virgin state. However, following the onset of pregnancy, immunoreactivity for both proteins increased. Maspin appeared to increase steadily, reaching maximum intensity during the early stages of lactation. This maximal level of Maspin was maintained throughout lactation then quickly subsided with the onset of involution, so that by 48 h following pup removal, Maspin immunoreactivity was significantly reduced and by 7 days post-lactation, Maspin expression had returned to near virgin-state levels.
Fig. 1.
The differential expression of Maspin and interferon regulatory factor 6 (IRF6) during postnatal mammary gland development. Immunohistochemistry demonstrating immunoreactivity for Maspin and IRF6 throughout multiple stages of postnatal murine mammary gland development. Wild type C57/Black6 mice were harvested at the following time points: virgin (6–8 weeks old), P 15 (mid-pregnancy, 15 days post-coitum), L 0 (parturition), L 3 (early lactation, 3 days post-parturition); L 7 (mid-lactation), L 15 (late lactation), PL 1 (early stage I involution, 1 day following pup removal), PL 2 (late stage I to early stage II involution), and PL 7 (late stage II involution). Expression is visualized by the staining intensity of 3,3′-diaminobenzidine tetrachloride (DAB), rendering a brown reaction product. Slides are counterstained with Mayer’s hematoxylin. The negative control inset shown for L 7 represents staining with the appropriate immunoglobulin G (IgG) control. Slides, including the negative control, contain serial sections of representative tissues. Pictures were acquired with a Leica DM 4000B microscope mated to a Leica DFC480 CCD camera. Meter bar represents 200 μm. The relative mean intensity of DAB indicating IRF6 (red line) and Maspin (black line) was quantified using a CMYK (cyan-magenta-yellow-key) color model adapted from a model by Pham et al. (Pham et al. 2007), based on the chromogen intensity determined from the yellow channel of a CMYK color image.
In contrast to the gradual increase exhibited by Maspin during pregnancy, the intensity of IRF6 immunoreactivity appeared more abrupt and distinct. Immunoreactivity quickly increased following parturition, and continued to increase during lactation, with maximal intensity observed during late lactation and early involution (Fig. 1 P15, L0, L3). However, similar to Maspin, IRF6 immunoreactivity quickly and dramatically decreased to virgin-like levels by 7 days post-lactation. Taken together, these data demonstrate that both Maspin and IRF6 are temporally regulated throughout mammary gland development and functional differentiation of the lobuloalveolar cells.
These data were confirmed by Western blot, which demonstrated that IRF6 protein expression increased throughout lactation, reaching maximal levels by lactation day 15, whereas Maspin expression was high at the time of parturition and remained constant throughout lactation. The expression of both proteins began to decrease during glandular involution (Fig. 2). Furthermore, the data suggest that IRF6 exists in both phosphorylated (lower-mobility [upper] band, [Bailey et al. 2005]) and non-phosphorylated forms and the ratio of phosphorylated and non-phosphorylated IRF6 appeared to remain constant throughout the time points assayed. Previous work has shown that IRF6 phosphorylation affects IRF6 ubiquitination and protein stability (Bailey et al. 2008).
Fig. 2.
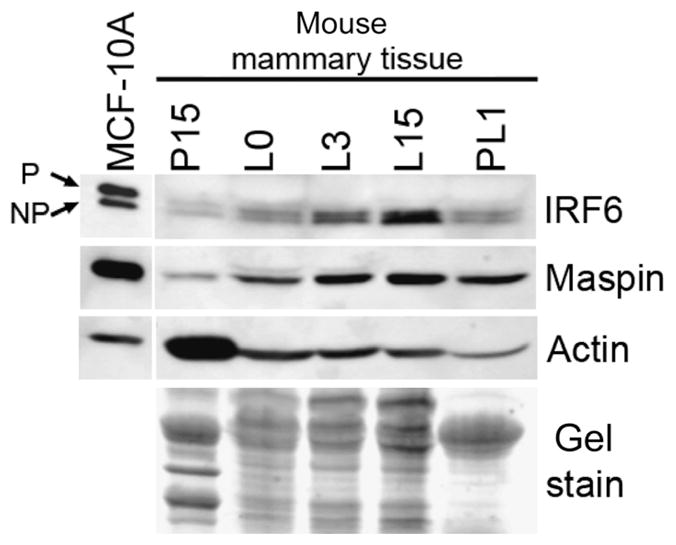
Protein expression of interferon regulatory factor 6 (IRF6) and Maspin during mammary gland development. Western blot analysis depicting the expression of IRF6 and Maspin in murine mammary tissue. Cell extracts were prepared from frozen mammary tissue extracted at the time points shown. Beta-actin and a Coumassie gel stain are provided as loading controls. MCF-10 A whole cell extract was used as a positive control. Phosphorylated (P) and non-phosphorylated (NP) IRF6 isoforms are depicted by arrows.
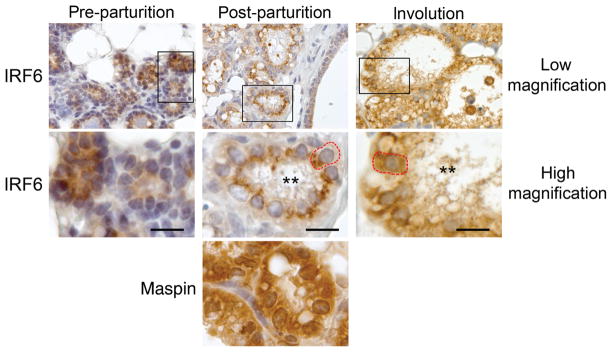
Close examination of the immunohistochemisty data revealed several unique aspects of IRF6 protein localization throughout the various stages of lactogenesis. Parturition induces the activation phase of lactogenesis (lactogenesis stage II) and is characterized by abundant milk protein expression, complete cellular polarization and closure of tight-junction complexes (Lavialle et al. 2000; Nguyen et al. 2001; Mcmanaman and Neville 2003). Prior to parturition, IRF6 appeared diffusely expressed throughout the cell. However, immediately following parturition, we observed that IRF6 appeared to localize to the apical surface of the cell (Fig. 3). This localization pattern was observed throughout lactation but was no longer visible following the onset of involution, indicating that this localization pattern was unique to the lactation stage, when the alveolar epithelial cells were fully polarized. Taken together, these data suggest that IRF6 localization may be associated with cellular polarization.
Fig. 3.
Temporal localization of interferon regulatory factor 6 (IRF6). Immunohistochemistry depicting increased IRF6 immunoreactivity at the lumenal surface of the secretory alveolar cells immediately following parturition (post-parturition) compared with pre-parturition (mid-gestation, E15) and involution (48 h following pup removal). High magnification shows enlargement of boxed area. The secretory alveolar cell is outlined in red. As a comparison, Maspin immunoreactivity is shown for the post-parturition stage and is expressed diffusely throughout the cell. Meter bar represents 25 μm.
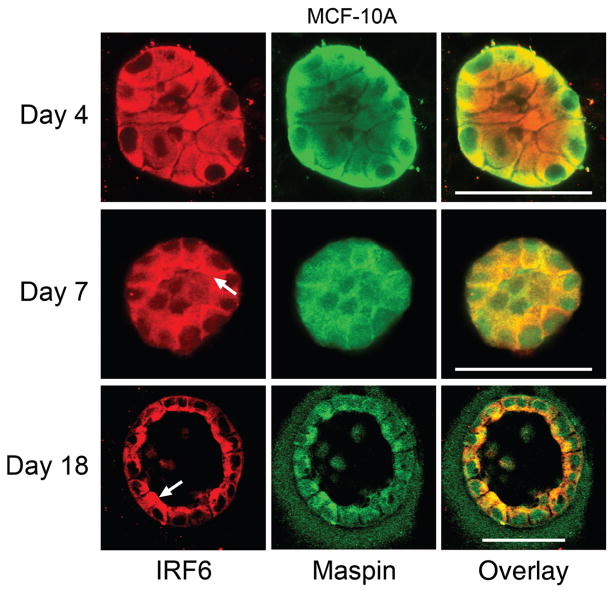
To examine whether the polarized localization of IRF6 could be replicated in vitro, we evaluated IRF6 expression and localization in polarized MCF-10 A human mammary epithelial cells grown in a three-dimensional organotypic culture system. When cultured on a three-dimensional matrix, MCF-10 A cells form polarized, spherical, hollow acinar structures that closely resemble mammary epithelial cell organization in vivo (Debnath et al. 2003). The data show that while the acini are immature and not yet fully polarized, IRF6 was expressed diffusely and evenly throughout the cell (Fig. 4, day 4). As the acinus matured, IRF6 adopted a unique localization pattern at the lumenal surface of the outermost layer of cells, which became prominent by day 7. This polarized localization continued throughout maturation of the acinus and was most pronounced at day 18, when the acinus was mature and completely hollow. Comparatively, Maspin also adopted a polarized localization pattern at the mature stage (albeit less dramatic than IRF6) and co-localized prominently with polarized IRF6. However, at earlier stages of in vitro acinar development, Maspin appeared to predominantly localize to the lateral cell surfaces at points of cell–cell contact. Interestingly, the lumenal localization pattern observed for Maspin during in vitro acinar development was not observed during murine mammary gland development in vivo at the time points assayed and merits further investigation (Fig. 3). These findings support our immunohistochemical observations and together suggest that both the temporal expression and localization of IRF6 and Maspin may be important for proper protein function during mammary gland development and differentiation.
Fig. 4.
In vitro confirmation of interferon regulatory factor 6 (IRF6) lumenal localization. MCF-10 A cells were grown in a 3-D culture system that allows for the growth of hollow, lobular acini in cell culture. Acini were analyzed at three stages of growth: day 4 (immature, unorganized acinus), day 7 (partially organized acinus; hollowing begins via apoptosis of inner cells), and day 18 (acinus is mature and completely hollowed). IRF6 immunofluorescence is shown in red and Maspin immunofluorescence is shown in green. The overlay shows both Maspin and IRF6 with co-localization of these proteins highlighted by yellow. Arrows depict the lumenal localization of IRF6. The assay was carried out according to the protocol by Debnath et al. (Debnath et al. 2003). Imaging was carried out on a Zeiss 510 META confocal laser scanning microscope. Bar represents 50 μm.
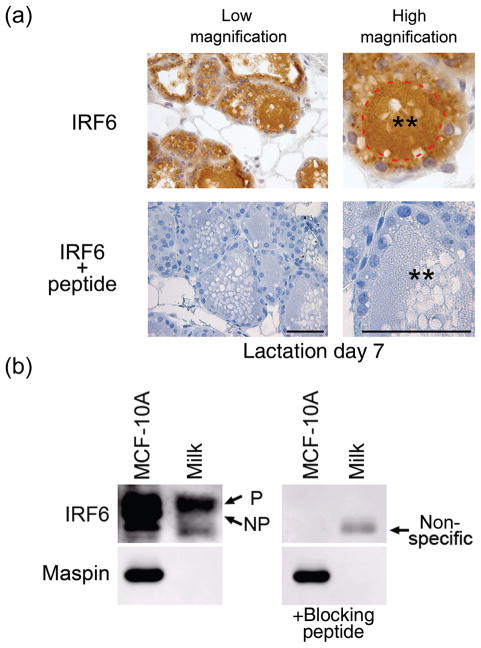
Immunohistochemical analysis of IRF6 expression in murine mammary tissue also revealed an abundant amount of IRF6 immunoreactivity within the lumenal space of mature, lactating lobuloalveolar subunits (Fig. 5A, also Fig. 1: L3, L7, L15, PL1). To verify the specificity of IRF6 immunoreactivity within the milk, immunohistochemistry was carried out with IRF6 antibody that had been pre-incubated with blocking peptide (Fig. 5A). This experimental validation completely abolished IRF6 immunoreactivity in the milk, demonstrating the specificity of the IRF6 antibody (IRF6 + peptide). We then assayed whether IRF6 is also expressed in human breast milk and whether the phosphorylation status of IRF6 affects its lumenal secretion (Fig. 5B). Western blot analysis demonstrated that a single band corresponding with the higher molecular mass isoform (phosphorylated IRF6) was present within human breast milk. This band was eliminated by pre-incubation of the IRF6 antibody with a blocking peptide, indicating the specificity of this band and suggesting that phosphorylated IRF6 is secreted into breast milk. Notably, Maspin was not observed in the secreted milk. We are currently investigating the biological significance of IRF6 secretion in the milk during lactation.
Fig. 5.
Interferon regulatory factor 6 (IRF6) is secreted into the milk. (a) Immunohistochemistry of murine mammary tissue at day 7 of lactation (L 7) shown at low and high magnification. The lumenal surface of a mammary acinus is outlined in red. Double asterisks (**) depict the alveolar lumen containing the secreted milk. Blocking peptide to the IRF6 antibody was used to verify antibody specificity. Meter bar represents 50 μm. (b) Western blot analysis demonstrating the presence of IRF6 within human breast milk. The immunoreactive IRF6 band corresponds to the phosphorylated form of IRF6 in MCF-10 A cells. A blocking peptide was used to verify specificity. Maspin was not observed in the milk and serves as a negative control.
Discussion
We recently reported a novel protein interaction between Maspin and IRF6 (Bailey et al. 2005). Although we do not completely understand the function of this protein–protein interaction, we have demonstrated similar protein expression patterns for Maspin and IRF6 in normal mammary epithelial cells and the absence of both proteins in dedifferentiated, invasive breast cancer cells. Further work has also implicated these two proteins in different aspects of cellular differentiation, including cell cycle arrest (Bailey et al. 2008). Thus, in the current study we evaluated the expression of Maspin and IRF6 throughout mammary gland development to test the hypothesis that the expression of Maspin and IRF6 coincides with the differentiation of the mammary epithelial cell. This study demonstrates that protein levels of both Maspin and IRF6 are temporally regulated in a similar fashion, with maximal expression for both proteins achieved during secretory differentiation (lactogenesis stage II) of the lobuloalveolar cells. These new data suggest important roles for IRF6 and Maspin during lactation and support the hypothesis that Maspin and IRF6 cooperatively support a differentiated phenotype. We also demonstrate that IRF6 is secreted into the milk. To our knowledge, this is the first report of the secretion of a member of the IRF transcription factor family.
Mammary gland development is primarily a postnatal process and is characterized by ductal outgrowth, branching morphogenesis and secretory differentiation in preparation for lactation. Following the cessation of lactation, the breast is quickly and efficiently remodeled in a multistep process in preparation for subsequent pregnancies. This process of development and remodeling is complex and requires the precise temporal and spatial regulation of many proteins. Because Maspin has been studied primarily in the context of tumor suppression, its function during breast development is not well understood. Based on findings which implicate Maspin in apoptosis, we initially hypothesized that Maspin would be involved in glandular involution (Zhang et al. 1999; Jiang et al. 2002; Liu et al. 2004). Interestingly, our findings demonstrate maximal protein expression for Maspin during lactation. Although it is possible that the presence of Maspin during lactation may help to prime the cells for apoptosis once the involution signal is given, it is likely that Maspin functions in another capacity during lactation. One likely possibility is that Maspin functions to maintain the differentiated phenotype. Several studies have demonstrated Maspin’s ability to promote an epithelial phenotype in transformed breast cancer cells (Odero-Marah et al. 2003; Bailey et al. 2005). Thus, it is possible that Maspin expression in mammary epithelial cells during lactation promotes the differentiated state of the secretory alveolar cells.
We postulate that Maspin and IRF6 may act in a cooperative manner to promote differentiation. This is supported by the increased expression observed for both Maspin and IRF6 at the time of lobuloalveolar differentiation (lactation). Furthermore, we have previously demonstrated that IRF6 and Maspin synergistically induce cell cycle arrest when simultaneously re-expressed in breast cancer cells (Bailey et al. 2008). Because exit from the cell cycle is an important step in the differentiation process, it is possible that the enhanced expression of these proteins during lactation (specifically the rapid induction of IRF6 following parturition) promotes differentiation of the secretory alveolar cells by inducing exit from the cell cycle and entry into the G(0) phase necessary for differentiation.
The increased expression of IRF6 following parturition suggests the involvement of lactogenic hormones in regulating IRF6 expression. One possible candidate is the dominant lactogenic hormone prolactin. In mice, both prolactin and its receptor are downregulated during pregnancy, and then significantly induced immediately prior to parturition in preparation for lactation (Nishikawa et al. 1994; Mizoguchi et al. 1997). This occurs concomitant with progesterone withdrawal and coincides with our observed increase in IRF6, suggesting a possible relationship between prolactin signaling and IRF6 expression. Furthermore, prolactin is known to stimulate another IRF family member, IRF1, in leukocytes, where prolactin functions to regulate immune cell growth and differentiation (Yu-Lee et al. 1990). It is therefore possible that IRF6 may be upregulated as part of the heightened prolactin signaling associated with lactogenesis.
Interestingly, maximal IRF6 expression is preserved through the initiation of stage I involution (24 h post-lactation). Stage I involution is characterized by apoptosis of the lobuloalveolar cells, but is reversible if the suckling stimulus is reapplied. Thus, it is possible that IRF6 expression is sustained until the gland is fully committed to the involution process. Alternatively, the high expression of IRF6 at this stage invites the possibility that IRF6 may play an important role in regulating the early events of involution such as lobuloalveolar cell apoptosis. Indeed, the process of involution is driven by many factors commonly used by the innate immune system, thus establishing precedence for the possible involvement of IRFs (Zhao et al. 2002; Clarkson et al. 2004; Stein et al. 2004). In support of this concept, it has recently been demonstrated that IRF1 may promote apoptosis during mammary gland involution in part through cell-extracellular matrix (ECM) signaling (Bowie et al. 2007). However, further work will be needed to address specific functions for IRF6 during involution.
The cellular localization of IRF6 throughout mammary gland differentiation also appears to be important. Our in situ immunohistochemical studies suggest that IRF6 is sequestered to the apical surface of lobuloalveolar cells following parturition. This may be explained in part by the fact that parturition is associated with complete closure of mammary epithelial cell tight junctions, which are leaky prior to parturition (Nguyen and Neville 1998). Tight junction closure may be associated with changes to the tight junction complex involving proteins critical for the regulation of cell polarity (Shin et al. 2006). This is supported by a three-dimensional cell culture system in which MCF-10 A human mammary epithelial cells exhibit vectorial milk protein secretion dependent on proper cell polarization and architecture (Barcellos-Hoff et al. 1989). In this culture system, we show that IRF6 localizes to the apical cell surface only after the outer layer of polarized cells becomes organized. Additionally, the postulate that intact tight junctions are required for the lumenal localization of IRF6 is further supported by immunohistochemical data from the involution stage (stage II, 48 h following pup removal) in which the tight junctions no longer exist and IRF6 immunoreactivity is diffuse throughout the cell. The biological significance of the observed IRF6 localization pattern is not known but may indicate IRF6 involvement in apical membrane-specific cell signaling or may be directly related to its secretion into the lumenal space during lactation. Indeed, the recruitment of IRF6 to the apical surface suggests that IRF6 is specifically secreted via the exocytic pathway, which is the primary mechanism of protein secretion by alveolar cells (McManaman and Neville 2003). The targeted secretion of IRF6 is further supported by Western blot analysis, which indicates that only a single immunoreactive IRF6 population, likely representing phosphorylated IRF6, is detected in human milk. These findings suggest that the phosphorylation of IRF6 may target IRF6 for lumenal secretion. Interestingly, we have previously shown that IRF6 phosphorylation is a signal for IRF6 ubiquitination and proteasomal degradation in cultured MCF-10 A human mammary epithelial cells (Bailey et al. 2008). Further work is needed to address the function(s) of IRF6 phosphorylation and what role secreted IRF6 may play as a constituent of milk.
We have presented the temporal and spatial protein expression patterns for IRF6 and Maspin during mammary gland development and mammary epithelial cell differentiation using both in vitro and in situ approaches. The data indicate that both Maspin and IRF6 are maximally expressed during lactation, which represents the pinnacle of functional differentiation for the mammary epithelial cell. These results support our hypothesis that IRF6 and Maspin function coordinately to promote differentiation of the mammary epithelial cell. This hypothesis is also supported by recent reports linking IRF6 to the terminal differentiation of keratinocytes (Ingraham et al. 2006). Our data further unveil a novel localization pattern for IRF6 in fully polarized mammary epithelial cells, and the first report of the apical secretion of an IRF family member. Advancing our understanding of Maspin and IRF6 may provide important insights into normal mammary gland development and breast cancer tumorigenesis.
Acknowledgments
This work was supported by NIH/CA 75681 awarded to MJCH and R01-DE13513-01 awarded to BCS.
References
- Bailey CM, Khalkhali-Ellis Z, Kondo S, Margaryan NV, Seftor RE, Wheaton WW, Amir S, Pins MR, Schutte BC, Hendrix MJ. Mammary serine protease inhibitor (Maspin) binds directly to interferon regulatory factor 6: identification of a novel serpin partnership. J Biol Chem. 2005;280:34210–34217. doi: 10.1074/jbc.M503523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CM, Abbott DE, Margaryan NV, Khalkhali-Ellis Z, Hendrix MJ. Interferon regulatory factor 6 promotes cell cycle arrest and is regulated by the proteasome in a cell cycle-dependent manner. Mol Cell Biol. 2008;28:2235–2243. doi: 10.1128/MCB.01866-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development (Cambridge, England) 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem. 2001;276:23382–23390. doi: 10.1074/jbc.M101216200. [DOI] [PubMed] [Google Scholar]
- Barnes BJ, Kellum MJ, Pinder KE, Frisancho JA, Pitha PM. Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res. 2003;63:6424–6431. [PubMed] [Google Scholar]
- Bowie ML, Troch MM, Delrow J, Dietze EC, Bean GR, Ibarra C, Pandiyan G, Seewaldt VL. Interferon regulatory factor-1 regulates reconstituted extracellular matrix (rECM)-mediated apoptosis in human mammary epithelial cells. Oncogene. 2007;26:2017–2026. doi: 10.1038/sj.onc.1210013. [DOI] [PubMed] [Google Scholar]
- Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6:R92–R109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Duguay D, Mercier F, Stagg J, Martineau D, Bramson J, Servant M, Lin R, Galipeau J, Hiscott J. In vivo interferon regulatory factor 3 tumor suppressor activity in B16 melanoma tumors. Cancer Res. 2002;62:5148–5152. [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Vonderhaar BK. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia. 2002;7:17–38. doi: 10.1023/a:1015766322258. [DOI] [PubMed] [Google Scholar]
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, Murray JC, Schutte BC. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38:1335–1340. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Meng Y, Zhang S, Mensah-Osman E, Sheng S. Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene. 2002;21:4089–4098. doi: 10.1038/sj.onc.1205507. [DOI] [PubMed] [Google Scholar]
- Lavialle F, Rainteau D, Massey-Harroche D, Metz F. Establishment of plasma membrane polarity in mammary epithelial cells correlates with changes in prolactin trafficking and in annexin VI recruitment to membranes. Biochim Biophys Acta. 2000;1464:83–94. doi: 10.1016/s0005-2736(99)00251-5. [DOI] [PubMed] [Google Scholar]
- Li M, Liu X, Robinson G, Bar-Peled U, Wagner KU, Young WS, Hennighausen L, Furth PA. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc Natl Acad Sci USA. 1997;94:3425–3430. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Heylbroeck C, Pitha PM, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol Cell Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yin S, Reddy N, Spencer C, Sheng S. Bax mediates the apoptosis-sensitizing effect of maspin. Cancer Res. 2004;64:1703–1711. doi: 10.1158/0008-5472.can-03-2568. [DOI] [PubMed] [Google Scholar]
- Mcmanaman JL, Neville MC. Mammary physiology and milk secretion. Adv Drug Deliv Rev. 2003;55:629–641. doi: 10.1016/s0169-409x(03)00033-4. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Yamaguchi H, Aoki F, Enami J, Sakai S. Corticosterone is required for the prolactin receptor gene expression in the late pregnant mouse mammary gland. Mol Cell Endocrinol. 1997;132:177–183. doi: 10.1016/s0303-7207(97)00134-2. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Nishiguchi S, Tamori A, Koh N, Yano Y, Kubo S, Hirohashi K, Otani S. Tumor-suppressor effect of interferon regulatory factor-1 in human hepatocellular carcinoma. Clin Cancer Res. 2001;7:1293–1298. [PubMed] [Google Scholar]
- Nguyen DA, Neville MC. Tight junction regulation in the mammary gland. J Mammary Gland Biol Neoplasia. 1998;3:233–246. doi: 10.1023/a:1018707309361. [DOI] [PubMed] [Google Scholar]
- Nguyen DA, Parlow AF, Neville MC. Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J Endocrinol. 2001;170:347–356. doi: 10.1677/joe.0.1700347. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Moore RC, Nonomura N, Oka T. Progesterone and EGF inhibit mouse mammary gland prolactin receptor and beta-casein gene expression. Am J Physiol. 1994;267:C1467–C1472. doi: 10.1152/ajpcell.1994.267.5.C1467. [DOI] [PubMed] [Google Scholar]
- Odero-Marah VA, Khalkhali-Ellis Z, Chunthapong J, Amir S, Seftor RE, Seftor EA, Hendrix MJ. Maspin regulates different signaling pathways for motility and adhesion in aggressive breast cancer cells. Cancer Biol Ther. 2003;2:398–403. doi: 10.4161/cbt.2.4.471. [DOI] [PubMed] [Google Scholar]
- Pham NA, Morrison A, Schwock J, Aviel-Ronen S, Iakovlev V, Tsao MS, Ho J, Hedley DW. Quantitative image analysis of immunohistochemical stains using a CMYK color model. Diagn Pathol. 2007;2:8. doi: 10.1186/1746-1596-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, Shore P, Whitmarsh A, Dixon MJ. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38:1329–1334. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, Bell AK, Ferrier RK, Sandilands GP, Gusterson BA. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–R91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait L, Soule HD, Russo J. Ultrastructural and immunocytochemical characterization of an immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6087–6094. [PubMed] [Google Scholar]
- Yu-Lee LY, Hrachovy JA, Stevens AM, Schwarz LA. Interferon-regulatory factor 1 is an immediate-early gene under transcriptional regulation by prolactin in Nb2 T cells. Mol Cell Biol. 1990;10:3087–3094. doi: 10.1128/mcb.10.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Magit D, Botteri F, Shi HY, He K, Li M, Furth P, Sager R. Maspin plays an important role in mammary gland development. Dev Biol. 1999;215:278–287. doi: 10.1006/dbio.1999.9442. [DOI] [PubMed] [Google Scholar]
- Zhao L, Melenhorst JJ, Hennighausen L. Loss of interleukin 6 results in delayed mammary gland involution: a possible role for mitogen-activated protein kinase and not signal transducer and activator of transcription 3. Mol Endocrinol. 2002;16:2902–2912. doi: 10.1210/me.2001-0330. [DOI] [PubMed] [Google Scholar]
- Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]