Abstract
Introduction. Recent work revealed the development of marked muscle fiber weakness in the diaphragm, but not in the non-respiratory latissimus dorsi, during thoracic surgery. To disentangle the molecular processes that underlie the development of diaphragm muscle fiber weakness during thoracic surgery, we studied changes in the gene expression profile. Methods. Serial biopsies from the diaphragm and the latissimus dorsi muscle were obtained from four patients during thoracotomy for resection of a tumor in the right lung. Biopsies were taken as soon as the diaphragm had been exposed (t0) and again after two hours (t2). Gobal differences in gene expression in diaphragm biopsies were assessed by microarray analysis. Results. 346 differentially expressed gene transcripts were found in the diaphragm at t2 vs. t0. Pathway analysis revealed that genes associated with inflammation (83 genes; p<0.0001) and cell death (118 genes, p<0.0001) pathways were significantly overexpressed at t2. Of the 346 differentially expressed genes in the diaphragm at t2, 258 were also differential in the latissimus dorsi muscle, with the direction of change being identical for all differentially expressed genes. In addition, latissimus dorsi showed exclusive upregula-ton of negative regulators of cell death. Conclusions. Two hours of thoracic surgery result in rapid and profound changes in expression of inflammatory response and apoptotic genes in the diaphragm. The apoptotic response was stronger in the diaphragm than in the latissiums dorsi. These findings suggest that the development of selective diaphragm muscle fiber weakness in these patients might be related to an exaggerated apoptotic response.
Keywords: thoracic surgery, mechanical ventilation, diaphragm, gene expression
Introduction
Postoperative pulmonary complications, such as pneumonia, atelectasis, and respiratory failure, are significant contributors to morbidity in patients who have undergone thoracic, cardiac, or upper abdominal surgery [1,2]. Recent reports [3,4,5,6,7,8,9] indicate that these pulmonary complications after surgery are at least partly caused by postoperative inspiratory muscle weakness. For instance, preoperative inspiratory muscle training has been shown to improve postoperative lung function [7] and to reduce the duration of postoperative mechanical ventilation [8]. Furthermore, recent studies [5] revealed that preoperative inspiratory muscle training in patients undergoing cardiac surgery reduces the incidence of postoperative pulmonary complications by ∼50% and reduces the duration of postoperative hospitalization.
The nature of postoperative inspiratory muscle weakness might include diaphragm muscle fiber weakness. Recent work from our lab revealed the development of marked diaphragm muscle fiber weakness during only two hours of thoracic surgery [4]. This loss of muscle fiber function was not part of a generalized muscle weakness as function was preserved in the non-respiratory latissimus dorsi muscle. Thus, these findings suggested that two hours of thoracic surgery induces selective diaphragm muscle weakness through loss of function of the contractile machinery within diaphragm muscle fibers.
The mechanisms underlying the rapid development of diaphragm muscle weakness during thoracic surgery is unknown, and is likely to be complex. Normal respiratory functions are profoundly altered during general anesthesia, intrathoracic surgery, and mechanical ventilation. It is well known that anesthetics, as well as inflammatory mediators, such as cytokines, can directly affect muscle fiber function [10,11]. In addition, mechanical ventilation-induced inspiratory muscle unloading can result in rapid diaphragm muscle fiber alterations, presumably through activation of proteolytic pathways and oxidative stress [12,13].
In order to disentangle the complex molecular processes that might underlie the development of diaphragm muscle fiber weakness during thoracic surgery, we performed global gene expression profiling. We used a longitudinal approach and determined global gene expression in the diaphragm at the end of short-term thoracic surgery and compared this to that at the start of surgery. To test whether changes in gene expression are part of a generalized muscle response, we also evaluated gene expression in the non-respiratory latissimus dorsi muscle.
Methods
Patients
Four patients between 53 and 72 years old undergoing thoracotomy for resection of a tumor in the right lung were recruited for this prospective, observational, and repeated-measures design study. Biopsies from the diaphragm and the latissimus dorsi muscle were obtained as soon as the diaphragm had been exposed (t0) and again after two hours (t2). The biopsy location at t0 and and t2 were ∼2 cm apart. The exclusion criteria consisted of tumor induction therapy (radio- and/or chemotherapy), history of neuromuscular disease, chronic use of corticosteroids (defined as >7.5 mg/day for at least 3 months, >10% weight loss within last 6 months, COPD (Global Initiative for Obstructive Lung Disease stage II-IV) or CHF (New York Heart Association class III-IV). Informed consent was obtained from each subject, and the study was approved by the local ethical committee. Note that the four patients studied here were also included in a previous study [4].
Biopsy handling
The fresh biopsies were blotted and rinsed to remove visible blood. Biopsies were stabilized using RNAlater solution (Ambion, Austin, TX, USA), and then transferred to liquid nitrogen and stored at -80°C until analysis.
RNA extraction and microarray hybridization
Total RNA was isolated from approximately 0.5 cm3 of tissue sample by homogenization with a polytron bench top homogenizer (T 18 basic ULTRA-TURRAX®, VWR, Amsterdam, the Netherlands) in 3 mL of TRIzol®, and extracted according to the manufacturers protocol (Invitrogen, Breda, the Netherlands). RNA integrity and concentrations were measured on a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) and Nanodrop spectrophotometer ND-1000 (Fisher Scientific, Waltham, MA, USA). All samples were of high quality with an RNA Integrity Number (RIN-value) between 7.6 and 8.7 and 260/280 ratios greater than 2.00. 500-ng total RNA per sample was used as input for amplification and labeling with the Quick Amp Labeling kit (Agilent Technologies, Palo Alto, CA, USA), and according to the manufacturer's guidelines including control spikes. Labeled RNA was purified using the RNeasy Mini Kit (QIAGEN Ltd., Venlo, the Netherlands) yielding 7.5 μg or more of labeled cRNA and specific activities greater than 15.3 pg Cye3 dye/μg cRNA and 18.9 pg Cy5 dye/μg cRNA. Labeled samples were hybridized onto whole human genome GE 4×44K microarrays according to the manufacturers protocol (Agilent Technologies, Palo Alto, CA, USA). Scanning was performed using a microarray scanner G2505B (Agilent Technologies) and Feature Extraction v9.5 using the manufacturers protocols (Agilent Technologies). All samples were analyzed in duplicate. The microarray data have been submitted to the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus database (GEO, database number: GSE30658) (http://www.ncbi.nlm.nih.gov/geo).
Statistical analysis
All analyses of the gene expression microarray data were done within the R statistical software (http://www.r-project.org) developed by Bioconductor [14], using the Limma-package. Preprocessing of the gene expression data comprised of RNA background correction, loess within-array normalization and quantile between-array normalization. The moderated t-test implemented in Limma, employing empirical Bayes estimation of the variance, is used to evaluate the difference in gene expression between the two groups. The multiplicity problem (many genes are tested) is address through application of the Benjamini-Hochberg procedure to the raw p-values to control for the False Discovery Rate (FDR). Differences between groups were considered significantly different at p<0.01 and a FDR<0.1.
Functional classification of genes
Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Redwood City, CA, USA) was used to detect the biological functions and molecular networks of the differentially expressed (DE) genes. Additional functional annotation was performed using DAVID Bioinformatics Resources 6.7 (NIAID/NIH). Some of the functional categories were combined and some categorization was done manually (eg., the functional categories ‘inflammatory response’ and ‘inflammation’ were grouped) in order to facilitate the interpretation of the data.
Results
Patient characteristics
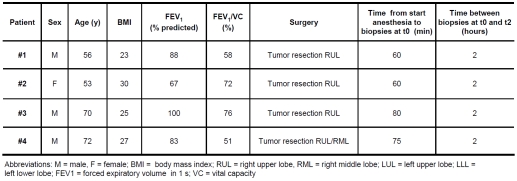
Patient charateristics are shown in Table 1. All patients underwent a thoracotomy for resection of a lung tumor (T1-3N0Mx). After induction of general anesthesia (propofol 3 mg/kg, sufentanil 0.20 mg/kg) a double-lumen tube was placed and anesthesia was maintained with propofol and sufentanil. In addition, patients received a thoracic epidural for postoperative pain therapy. During single lung ventilation one lung was allowed to deflate while the other was ventilated. From the patients, biopsies (each 50-75 mg) were taken as soon as the diaphragm had been exposed (t0) and again after two hours (t2). The diaphragm biopsies were obtained from the ‘non-ventilated’ side of the diaphragm. The duration of mechanical ventilation before the first biopsy varied between 60 and 80 minutes. Time between the first and second biopsies was exactly 2 hours for all four patients. Two of the four patients (#1, and 4) had mild airway obstruction (Table 1) and none of the four patients had a history of chronic heart failure or neuromuscular disease. All subjects tolerated surgery and biopsies well and no study-related postoperative complications were noted.
Table 1.
Patient characteristics
 |
Overview of gene expression analysis
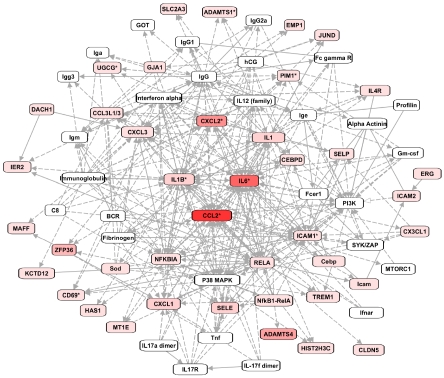
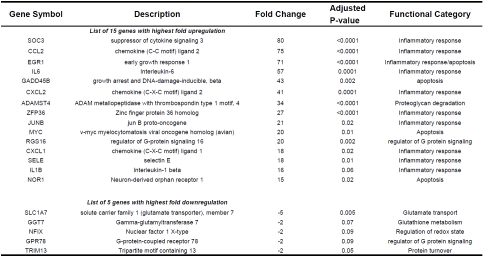
Gobal differences in gene expression in diaphragm biopsies were assessed by microarray analysis. Microarray analysis identified 346 differentially expressed (DE) gene transcripts in the diaphragm at t2 vs. t0. The vast majority of the DE genes at t2 was upregulated (326/346; 95%). There were no noticeable differences between patients in the gene expression response, although the limited number of patients did not allow for strict sub-grouping. Table 1 shows an overview of the 15 genes that were most highly upregulated as well as those 5 with the highest downregulation. IPA was used to visualize the interconnectivity of the DE genes. In Figure 1 the network with the most DE genes is shown. At the center of this network are the pro-inflammatory mediators IL6, IL1β, CXCL2, and CCL2, implying a central role for these molecules in the muscle's response to thoracic surgery. Pathway analysis using IPA software revealed that inflammation (83 genes; p<0.0001) and cell death (118 genes, p<0.0001) pathways were significantly overrepresented.
Figure 1.
The top network of differentially expressed (DE) genes, as constructed by Ingenuity Systems Pathway Analysis (IPA). Rectangles in red represent genes significantly upregulated after two hours of thoracic surgery (color intensity indicates the magnitude of upregulation). White rectangles are hub molecules, of which expression is not altered, but that generally have many connections with DE genes. Uninterrupted and interrupted lines indicate physical and indirect intreactions between molecules.
Next, DAVID software was used to retrieve Gene Ontology classifications of the differentially expressed genes. The most prominent functional categories were inflammatory response (45 genes) apoptosis (108 genes). Tables 3 and 4 show a selection, per category, of the 15 genes with the highest fold upregulation at t2. Genes within the inflammatory response functional category included interleukin-6 (IL6, 57 fold upregulated at t2), interleukin-1 beta (IL1-ß, 16 fold upregulated at t2), and chemokine (C-C motif) ligand 2 (CCL2, 75 fold upregulated at t2).
Table 3.
Expression of Inflammatory Response Genes in the Diaphragm, t2 vs t0
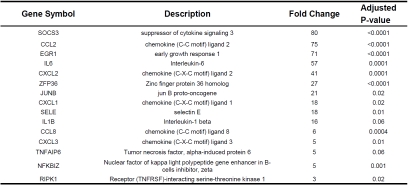 |
Table 4.
Expression of Apoptosis-related Genes in the Diaphragm, t2 vs t0
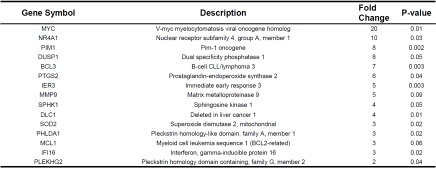 |
Genes upregulated within the apoptosis functional category included V-myc myelocytomatosis viral oncogene homolog (MYC, 20 fold upregulated at t2) and superoxide dismutase 2 mitochondrial (SOD2, 3 fold upregulated at t2). Note that several of the upregulated genes within the inflammatory response category were also grouped in the apoptosis category (eg., SOCS3, CCL2, IL6, CXCL2, ZFP36, CXCL1, IL1B, SELE; for details see Table 3). These genes are not listed in Table 4.
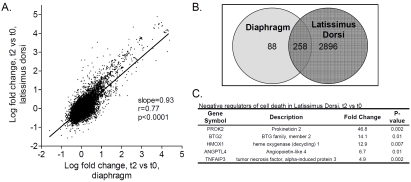
Gene expression analysis of m. latissimus dorsi from the same patients showed that of the 346 genes that were differentially expressed in the diaphragm, 258 were also differentially expressed in the latissimus dorsi (Figure 2A and B). In addition to these 258 genes, latissimus dorsi showed differential expression at t2 of 2896 other genes (Figure 2B). These 2896 genes were mainly grouped into inflammatory response and apoptosis categories, and in addition, into a skeletal muscle contraction category (33 DE genes; top 5 (gene symbol/fold change at t2): MYOM1/-2, MYH2/-2, MYH3/-2, RYR1/-2, MYLK2/-2). When these 2896 genes were separated into upregulated and downregulated genes, upregulated genes were overrepresented in the category negative regulation of programmed cell death (42 genes; p= value 5.4 × 10-7, FDR 3.6 × 10-5), while downregulated genes were clustered in muscle organ development category (29 genes; p=9.5 × 10-5, FDR 6.9 × 10-2). Top 5 from negative regulation of programmed cell death are shown in Figure 2C.
Figure 2.
A. Correlation between the gene expression changes in the diaphragm and those in latissimus dorsi at t2. Note the strong and significant correlation. B. Venn-diagram showing the number of genes that were differential in both diaphragm and latissimus dorsi and the number of genes that were differential exclusively in diaphragm or in latissimus dorsi. C. Table listing the top 5 genes involved in negative regulation of programmed cell death that were differential exclusively in the latissiums dorsi at t2.
Discussion
In the present study genome-wide gene expression profiling was used to disentangle the complex molecular processes that might underlie the development of diaphragm muscle fiber weakness during thoracic surgery. It was found that two hours of thoracic surgery elicit a profound inflammatory and pro-apoptotic response in the diaphragm muscle. A similar gene expression response was observed in the non-respiratory latissimus dorsi muscle. Interestingly, in addition to the upregulation of pro-apoptotic genes, latissiums dorsi also showed strong upregulation of anti-apoptotic genes. This was not observed in diaphragm muscle. Although future studies should validate our microarray results, these findings suggest that the development of selective diaphragm muscle fiber weakness in patients during thoracic surgery [4] might be related to an exaggerated apoptotic response.
Inflammatory response in the diaphragm during thoracic surgery
The majority of the genes that were most upregulated after two hours of surgery included many inflammatory response genes (Tables 2 and 3). For instance, CXCL2, IL6, SOCS3, EGR1, CCL2, IL1-ß, and ADAMTS4 showed a 16-80 fold upregulation at t2. Network analysis revealed that the upregulated inflammatory genes, IL6, IL1-ß, CXCL2, and CCL2 are key mediators that influence expression of many of the other DE genes (Figure 1). Although inflammation has been associated with impaired muscle fiber function [10,15], little data is available on the effects of specific inflammatory mediators on muscle fiber function. One of the mediators that gained the most attention over the past years is IL6, a proinflammatory cytokine that has been shown to impair cardiac function and to a lesser extent diaphragm function [16]. IL1-ß, another proinflammatory cytokine, has been shown to depress muscle contractility, presumably through effects on sarcoplasmic reticulum calcium handling [17]. CCL2, also known as MCP-1 (monocyte chemoattractant protein), is member of the CC subfamily of chemokines and has been shown to play an important role in the pathogenesis of diaphragm weakness during sepsis [18].
Table 2.
Genes with highest fold up/down regulation in the diaphragm, t2 vs t0
 |
Upregulation of inflammatory response genes has also been reported recently in human diaphragm muscle after five hours of cardiothoracic surgery [19]. In line with our findings, Huang et al. [19] reported upregulation of CCL2, IL6 , SOCS1, SOCS3, and of other inflammatory mediators that were also upregulated in the present study. Thus, upregulation of inflammatory mediators appears to be a general response of the diaphragm to thoracic surgery. Interestingly, the magnitude of upregulation of inflammatory response genes was more pronounced in the present study than was reported by Huang et al [19]. For instance, whereas IL6 was upregulated 16 fold during cardiothoracic surgery, it was upregulated nearly 60 fold in the present study. Similarly, CCL2 was upregulated 8 fold in the study by Huang et al, but 75 fold in the present study. The mechanisms underlying the more pronounced inflammatory response in the present study might involve the time-point of observations. In the present study, time between biopsies was only two hours, whereas in the study by Huang et al. [19], time between biopsies was five hours. It could be speculated that the diaphragm undergoes a strong inflammatory response during the first hours of surgery followed by a blunted response as time proceeds. However, previous work on mechanically ventilated rats suggested that the gene expression response within the diaphragm is more pronounced when the duration of mechanical ventilaton increases [20]. Thus, these findings suggest that the more pronounced response in the present study is likely not due to different time-points of biopsy collection, but is rather the result of the different conditions associated with the two types of surgeries. The cardiothoracic surgery, decribed in Huang et al., was performed under hypothermic conditions. Previous studies reported a reduced inflammatory response within skeletal muscle following cold therapy [21,22]. We hypothesize that hypothermia, which is not present during the lung surgery in the present study, blunted the inflammatory response within the diaphragm during cardiothoracic surgery.
Apoptotic response in the diaphragm during thoracic surgery
In the present study we found activation of early apoptotic pathways after only two hours of contractile inactivity of the diaphragm. For instance, the proapoptotic gene MYC was upregulated 20 fold in the diaphragm after two hours of thoracic surgery. Induction of apoptosis, including upregulation of MYC, has been described before in inactive muscle [23]. Furthermore, our findings are in line with previous work showing significant upregulation of apoptosis regulating BCL2 in the diaphragm after five hours of cardiothoracic surgery [19]. A recent study revealed activation of downstream proapoptotic genes in mechanically ventilated brain dead patients [24]. They identified induction of a Fos/ FoxO1/Stat3-Bim intrinsic apoptotic pathway that lead to activation of caspases following 18-75 hours of diaphragm inactivity. Together, these results suggest that acute mediators of apoptotic pathways are rapidly activated during thoracic surgery, and that longer durations of mechanical ventilation are likely needed to significantly activate downstream apoptotic pathways.
Diaphragm versus latissimus dorsi gene expression during thoracic surgery
An important finding of the present study was that the gene expression response in the latissimus dorsi (a non-respiratory muscle) to two hours of thoracic surgery was similar to that observed in the diaphragm (Figure 2A). Of the 346 differential genes in the diaphragm at t2, 258 were also differential in the latissimus dorsi muscle (Figure 2B), with the direction of change being similar for all differential genes. Analysis of the genes that were differential exclusively within the latissimus dorsi revealed that these genes are mainly involved in inflammatory response and apoptotic pathways. This suggests that the gene expression response to thoracic surgery was similar in the latissimus dorsi compared to the diaphragm. Interestingly, latissimus dorsi showed, in addition to upregulation of positive regulators of apoptosis, also significant upregulation of negative regulators of apoptosis. These negative regulators of apoptosis were not upregulated in diaphragm muscle at t2. Previous work from our lab showed that two hours of thoracic surgery induces marked diaphragm muscle fiber weakness [4]. This diaphragm muscle fiber weakness was associated with reduced contractile protein content and activation of proteolytic pathways. Strikingly, weakness was not observed in latissimus dorsi muscle fibers from the same patients. The present findings might suggest that the development of selective diaphragm weakness in these patients might be related to a stronger apoptotic response to two hours of surgery in the diaphragm muscle than in the latissiums dorsi muscle.
Conclusions
This study has demonstrated that thoracic surgery results in rapid changes in the expression of genes involved in inflammation and apoptosis in the diaphragm. The present study design can not rule out that other surgery related conditions, in addition to diaphragm inactivity, contributed to the changes in gene expression. General anesthesia and neuromuscular blockade have been shown to affect diaphragm physiology [25] and might have impacted gene expression in the present study. Compared to the non-respiratory latissimus dorsi muscle, the diaphragm showed a stronger apoptotic response to two hours of thoracic surgery. Therefore, we speculate that the development of selective diaphragm muscle fiber weakness in these patients might be related to a more profound apoptotic response.
Acknowledgments
C.A.C.O. (VENI 016.096.043) and A.V.N. (VIDI 917.96.306) are supported by the Netherlands Organisation for Scientific Research (NWO).
References
- 1.Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, Weiss K, Owens DK, Aronson M, Barry P, Casey DE, Jr, Cross JT, Jr, Fitterman N, Sherif KD, Weiss KB. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144(8):575–580. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 2.Carrel TP, Eisinger E, Vogt M, Turina MI. Pneumonia after cardiac surgery is predictable by tracheal aspirates but cannot be prevented by prolonged antibiotic prophylaxis. Ann Thorac Surg. 2001;72(1):143–148. doi: 10.1016/s0003-4975(01)02669-8. [DOI] [PubMed] [Google Scholar]
- 3.Pasquina P, Tramer MR, Granier JM, Walder B. Respiratory physiotherapy to prevent pulmonary complications after abdominal surgery: a systematic review. Chest. 2006;130(6):1887–1899. doi: 10.1378/chest.130.6.1887. [DOI] [PubMed] [Google Scholar]
- 4.Welvaart WN, Paul MA, Stienen GJ, van Hees HW, Loer SA, Bouwman RA, Niessen HW, de Man FS, Witt CC, Granzier H, Vonk-Noordegraaf A, Ottenheijm CAC. Selective diaphragm muscle weakness following contractile inactivity during thoracic surgery. Ann Surg. 2011 doi: 10.1097/SLA.0b013e318232e75b. In Press. [DOI] [PubMed] [Google Scholar]
- 5.Hulzebos EH, Helders PJ, Favie NJ, de Bie RA, Brutel dlR, van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006;296(15):1851–1857. doi: 10.1001/jama.296.15.1851. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence VA, Cornell JE, Smetana GW. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 7.Weiner P, Man A, Weiner M, Rabner M, Waizman J, Magadle R, Zamir D, Greiff Y. The effect of incentive spirometry and inspiratory muscle training on pulmonary function after lung resection. J Thorac Cardiovasc Surg. 1997;113(3):552–557. doi: 10.1016/S0022-5223(97)70370-2. [DOI] [PubMed] [Google Scholar]
- 8.Weiner P, Zeidan F, Zamir D, Pelled B, Waizman J, Beckerman M, Weiner M. Prophylactic inspiratory muscle training in patients undergoing coronary artery bypass graft. World J Surg. 1998;22(5):427–431. doi: 10.1007/s002689900410. [DOI] [PubMed] [Google Scholar]
- 9.Maeda H, Nakahara K, Ohno K, Kido T, Ikeda M, Kawashima Y. Diaphragm function after pulmonary resection. Relationship to postoperative respiratory failure. Am Rev Respir Dis. 1988;137(3):678–681. doi: 10.1164/ajrccm/137.3.678. [DOI] [PubMed] [Google Scholar]
- 10.Ottenheijm CA, Heunks LM, Dekhuijzen PN. Diaphragm muscle fiber dysfunction in chronic obstructive pulmonary disease: toward a pathophysiological concept. Am J Respir Crit Care Med. 2007;175(12):1233–1240. doi: 10.1164/rccm.200701-020PP. [DOI] [PubMed] [Google Scholar]
- 11.Bouhemad B, Langeron O, Orliaguet G, Coriat P, Riou B. Effects of halothane and isoflurane on the contraction, relaxation and energetics of rat diaphragmatic muscle. Br J Anaesth. 2002;89(3):479–485. [PubMed] [Google Scholar]
- 12.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 13.Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P. Mechanical Ventilation-induced Diaphragm Disuse in Humans Triggers Autophagy. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- 14.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottenheijm CA, Heunks LM, Dekhuijzen RP. Diaphragm adaptations in patients with COPD. Respir Res. 2008;9:12–19. doi: 10.1186/1465-9921-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen SP, Gayan-Ramirez G, Van Den BA, Herijgers P, Maes K, Verbeken E, Decramer M. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111(8):996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 17.Duncan DJ, Yang Z, Hopkins PM, Steele DS, Harrison SM. TNF-alpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium. 2010;47(4):378–386. doi: 10.1016/j.ceca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labbe K, Danialou G, Gvozdic D, Demoule A, Divangahi M, Boyd JH, Petrof BJ. Inhibition of monocyte chemoattractant protein-1 prevents diaphragmatic inflammation and maintains contractile function during endotoxemia. Crit Care. 2010;14(5):R187. doi: 10.1186/cc9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang TT, Deoghare HV, Smith BK, Beaver TM, Baker HV, Mehinto AC, Martin AD. Gene expression changes in the human diaphragm after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2011 doi: 10.1016/j.jtcvs.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Deruisseau KC, Shanely RA, Akunuri N, Hamilton MT, Van Gammeren D, Zergeroglu AM, McKenzie M, Powers SK. Diaphragm unloading via controlled mechanical ventilation alters the gene expression profile. Am J Respir Crit Care Med. 2005;172(10):1267–1275. doi: 10.1164/rccm.200503-403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puntel GO, Carvalho NR, Amaral GP, Lobato LD, Silveira SO, Daubermann MF, Barbosa NV, Rocha JB, Soares FA. Therapeutic cold: An effective kind to modulate the oxidative damage resulting of a skeletal muscle contusion. Free Radic Res. 2011;45(2):125–138. doi: 10.3109/10715762.2010.517252. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho N, Puntel G, Correa P, Gubert P, Amaral G, Morais J, Royes L, da Rocha J, Soares F. Protective effects of therapeutic cold and heat against the oxidative damage induced by a muscle strain injury in rats. J Sports Sci. 2010;28(9):923–935. doi: 10.1080/02640414.2010.481722. [DOI] [PubMed] [Google Scholar]
- 23.Siu PM, Alway SE. Id2 and p53 participate in apoptosis during unloading-induced muscle atrophy. Am J Physiol Cell Physiol. 2005;288(5):C1058–C1073. doi: 10.1152/ajpcell.00495.2004. [DOI] [PubMed] [Google Scholar]
- 24.Tang H, Lee M, Budak MT, Pietras N, Hittinger S, Vu M, Khuong A, Hoang CD, Hussain SN, Levine S, Shrager JB. Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3-Bim axis. FASEB J. 2011 doi: 10.1096/fj.11-183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ermilov LG, Pulido JN, Atchison FW, Zhan WZ, Ereth MH, Sieck GC, Mantilla CB. Impairment of diaphragm muscle force and neuromuscular transmission after normothermic cardiopulmonary bypass: effect of low-dose inhaled CO. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R784–R789. doi: 10.1152/ajpregu.00737.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]