Abstract
It has been shown that female mice with pneumonia have a survival advantage over males, but this is reversed if ozone exposure precedes infection. The purpose of this study was to investigate factors that underlie these observations, by studying histopathologic changes in lung and extrapulmonary (spleen and liver) tissues after ozone or filtered air (FA) exposure followed by pulmonary bacterial infection. Male and female wild type C57BL/6J mice were exposed to ozone or FA, then anesthetized and infected intratracheally with Klebsiella pneumoniae bacteria. Tissues (lung, spleen, and liver) were subjected to histopathologic analysis at 48 h post-infection. We found that after infection, 1) the severity of inflammation was higher, the affected area of the lung was larger, and spleen red pulp myelopoiesis was lower in ozone-exposed mice compared to FA-exposed animals in both sexes; 2) more pronounced extrapulmonary lesions (in liver and spleen) were observed in FA-exposed males compared to FA-exposed females; and 3) excessive lung inflammatory response was detected in ozone-exposed females compared to ozone-exposed males. We concluded that different risk factors contribute to the differential outcome of pneumonia between sexes in the presence or absence of ozone-induced oxidative stress. In specific, the excessive lung inflammation and higher risk for extrapulmonary lesions in ozone-exposed infected females and in FA-exposed infected males appear to play, respectively, a dominant role in the previously observed respective survival outcomes.
Keywords: Sex, infection, lung inflammation, spleen function, pathology
Introduction
According to American Lung Association (ALA) lung disease data from 2008 (www.lungusa.org), almost 400,000 Americans die from lung disease in the United States every year, and lung disease death rates are on the increase, while death rates due to other major causes of death, such as heart disease, cancer and stroke, are declining. More than 35 million Americans have chronic lung diseases. Moreover, pneumonia is one of the major causes of childhood mortality [1]. The high and increasing mortality and morbidity due to lung diseases necessitates the identification and study of factors that influence the incidence, susceptibility, and severity of lung diseases. Among these contributing factors are sex and air pollution.
Males, in general, are more susceptible than females to lung disease, as is the case with neonatal respiratory distress syndrome [2, 3], idiopathic pulmonary fibrosis and COPD [4, 5], and different types of pneumonia [5-8]. The incidence of asthma is often higher in young males than in prepubertal females [4]. Several animal models have also demonstrated that males exhibit a higher level of susceptibility to lung disease [9-12].
Air pollution is a worldwide problem. Ground level ozone is one of the major air pollutants. Ozone is a powerful oxidant and exposure to ozone can affect breathing, induce coughing, reduce lung function [13], and trigger asthma [14, 15]. Ozone exposure has been associated with impairment of the entire respiratory epithelium [16]. It can increase neutrophil recruitment into airways [17] that may contribute to the observed acute lung injury [18] and changes in pulmonary innate immunity [19]. However, millions of people in the USA still live under unfavorable environmental conditions, including living in areas with levels of ozone higher than the National Ambient Air Quality Standard limit (0.075 ppm for 8 h) set by the Environmental Protection Agency (www.epa.gov).
A number of epidemiological studies mention sex differences in the effect of air pollution, with females being more susceptible than males [20-25]. However, in other studies differences between the sexes in response to air pollution were not reported [26-30]. Furthermore, most studies have not considered sex as a factor. In our experimental model where we investigated the effect of ozone exposure on the survival of mice, as a function of sex, after pneumonia, we observed that although male mice were more susceptible to experimental pneumonia than females, ozone exposure reversed the trend and made females more susceptible than males [31].
The outcome of pneumonia is largely influenced by pathological changes in the lung (the primary infected organ) and by dissemination of the infection into other internal organs. Thus, in this report, we investigated the histopathologic changes in the lung, spleen, and liver in male and female mice after ozone exposure followed by pulmonary bacterial infection, to determine whether these changes contribute to the observed differences in the outcome of pneumonia between the sexes in the absence or presence of ozone-induced oxidative stress.
Materials and methods
Animals
Male and female C57BL/6J mice (stock number 000664, The Jackson Laboratory, Bar Harbor, ME) were used at 8-12 weeks of age. Animals were maintained under approved housing conditions and fed rodent chow and autoclaved water ad libitum. The Penn State Hershey Medical Center Institutional Animal Care and Use Committee (IACUC) approved all procedures involving animals.
Preparation of bacteria
Klebsiella pneumoniae bacteria (ATCC 43816) were obtained from the American Tissue Culture Collection (Rockville, MD) and prepared as described previously [31]. Briefly, bacteria were grown for 18 h in TSB media at 37°C to reach stationary phase. The overnight bacterial suspension was next diluted until the OD660 was equal to 0.4, and 200 μl were used to inoculate 50 ml of fresh TSB for sub-cultivation for 3 h to reach mid-log phase of growth. The sub-culture was then placed on ice to stop growth and serially diluted in PBS to obtain ∼ 9 × 103 CFU/ml, and 50 μl of this bacterial suspension (containing ∼ 450 CFU) were used immediately to infect mice. CFU per ml values were estimated based on the standard curve obtained at OD660 of the bacterial suspension, and an aliquot was also spread on TSA plates to confirm CFU estimates.
Exposure of mice to ozone
Mice were exposed to ozone (2 ppm for 3 h) or to FA (control) at the same time in separate chambers as described [32]. Mice were infected immediately after exposure. Each experiment involved 10 animals (5 mice exposed to ozone and 5 exposed to FA).
Infection of mice with K. pneumoniae
Infection was performed as described previously [31]. Briefly, the animals were anesthetized, the trachea was surgically exposed and ∼450 CFU/ mouse were inoculated intratracheally in 50 μl of PBS. Death during the first 12 h post-infection was considered to be due to the surgical procedure rather than infection and those mice were excluded from the study. In cases where infected mice were moribund with no chance of recovery, they were euthanized to prevent unnecessary suffering according to Penn State Hershey Medical Center IACUC recommendations. After exposure to ozone and subsequent infection, mice were studied by histopathologic analyses (n = 53), as described below.
Histopathologic analyses of tissues
Twenty-eight male and twenty-five female mice were exposed to filtered air (FA) or ozone and inoculated with ∼ 450 CFU of K. pneumoniae bacteria in 50 μl of PBS. As an additional control for the histopathologic changes induced by ozone only (without infection), male and female mice (10 mice of each sex) received sham infections with 50 μl of PBS rather than bacterial suspension after ozone or FA exposure. Mice were sacrificed by asphyxiation using carbon dioxide at 48 h post-inoculation. Lungs were infused through the trachea with 10% neutral buffered formalin (NBF), and tissues were immersion-fixed in NBF. The right (cranial, middle and caudal) and left lung lobes were bisected in a parasagittal plane, while the accessory lung lobe was sampled without sectioning. Longitudinal and cross sections of spleen were taken, and representative sections of all liver lobes were taken. Tissues were processed in an automated Tissue-Tek VIP processor (Sakura Finetek USA, Torrance, CA, USA) and paraffin-embedded using a Tissue-Tek TEC embedding station. Sections were cut at 6 μm for routine hematoxylin and eosin (H&E) staining.
All tissues were examined by an American College of Veterinary Pathologists (ACVP) diplomat blinded to treatment/intervention. Percentages of the areas affected were visually estimated, and the lung volume affected by inflammation was semiquantitatively scored as 0 for <1%, 1 for 1-4%, 2 for 5-14%, 3 for 15-24%, and 4 for ≥25%. Severity and nature/type of inflammation were graded according to scoring system described in Table 1. Lungs were also evaluated for extension of infectious processes to pleural surfaces, and all tissues were evaluated for the presence of thromboemboli and bacteria. Spleen sections were independently evaluated semiquantitatively for depletion or hyperplasia of the white pulp lymphoid (both periarteriolar lymphoid sheaths (PALS) and follicles) and red pulp myeloid compartments, as well as congestion or contraction of the red pulp. Both liver and spleen were evaluated for the presence of bacteria, thromboemboli and inflammation, and areas of infarction were estimated in the liver. All images were captured with an Olympus BX51 microscope (Olympus America, Center Valley, PA, USA) and DP71 digital camera using Micro-Suite Basic 2.6 imaging software.
Table 1.
Scoring system for the nature and severity of inflammation in the lung of FA-exposed and ozone-exposed male and female mice after K. pneumoniae infection
| Score | Nature and severity of lung inflammation |
|---|---|
| 0 | Normal |
| 1 | Minimal and suppurative, consisting of occasional degenerate neutrophils |
| 2 | Minimal, necrosuppurative and hemorrhagic, consisting of occasional degenerate neutrophils with extravasated erythrocytes and fibrin and necrotic cellular debris |
| 3 | Mild and suppurative, consisting of low numbers of degenerate neutrophils |
| 4 | Mild, necrosuppurative and hemorrhagic, consisting of low numbers of degenerate neutrophils with extravasated erythrocytes and fibrin and necrotic cellular debris |
| 5 | Moderate and suppurative, consisting of moderate numbers of degenerate neutrophils |
| 6 | Moderate, necrosuppurative and hemorrhagic, consisting of moderate numbers of degenerate neutrophils with extravasated erythrocytes and fibrin and necrotic cellular debris |
| 7 | Severe and suppurative, consisting of large numbers of degenerate neutrophils |
| 8 | Severe, necrosuppurative and hemorrhagic, consisting of large numbers of degenerate neutrophils with abundant extravasated erythrocytes and fibrin and necrotic cellular debris |
Statistics
All data were analyzed with a simple t-test using SigmaPlot 10.0 software (Systat Software Inc. (SSI), San Jose, California). Results were considered statistically significant at p < 0.05.
Results
After FA exposure (control) or ozone exposure male and female mice were either inoculated with PBS (sham control) or infected with K. pneumoniae bacteria, and the histopathologic changes were analyzed at the 48 h time point as described in Methods.
After PBS inoculation
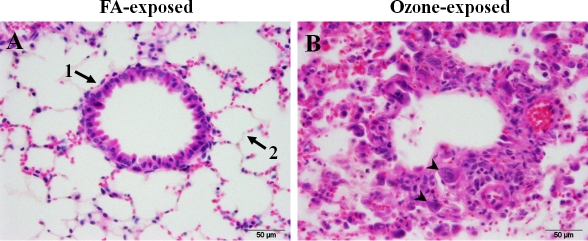
After FA exposure and PBS inoculation (no infection) male mice demonstrated mild respiratory epithelial changes. However, in response to ozone exposure followed by PBS inoculation there was evidence of bronchial and bronchiolar epithelial degeneration, necrosis, and regeneration, consistent with the known effects of ozone on the respiratory tract [33]. Inflammation was limited and mononuclear in nature, consistent with an essentially sterile resolution. FA-exposed or ozone-exposed PBS-inoculated female mice in general demonstrated changes in the lung that are similar to those in the respective male groups. However, while all males demonstrated from mild (FA-exposed) to moderate (ozone-exposed) epithelial changes, the pathology in females ranged from absent (FA-exposed) (Figure 1A) to mild to severe epithelial necrosis (ozone-exposed) (Figure 1B). No significant pathology was noted in the spleen or liver in either sex.
Figure 1.
Effect of ozone and FA exposures on the lung tissues of non - K. pneumoniae - infected mice. An example of severe histopathologic changes in the lungs of female mice in response to ozone exposure is shown. Mice were exposed to FA or to ozone, inoculated intratracheally with PBS (sham control), and their lungs were analyzed for histopathologic changes at 48 h post-infection, as described in Methods. Panels A and B depict the results for FA-exposed and ozone-exposed mice, respectively. FA-exposed animals (shown in panel A) demonstrated normal architecture of terminal bronchioles (arrow 1) and surrounding alveoli (arrow 2). In general, mice exposed to ozone both male and female (shown in Panel B) showed bronchiolar epithelial degeneration with exposed basement membranes (epithelial cell loss) or flattened attenuated epithelial cells stretched over exposed basement membranes. The remaining bronchiolar epithelium consists of short plump cuboidal cells with open chromatin, prominent nucleoli, increased nuclear to cytoplasmic (N:C) ratio and cytoplasmic basophilia (regenerative) with occasional binucleate cells (arrowheads). Cuboidal type II pneumocytes are prominent within alveoli with an interstitial and intra-alveolar infiltration of low to moderate numbers of macrophages. 600× magnification.
Thus, ozone exposure followed by PBS inoculation can result in lung pathology consistent with the known effects of ozone on the respiratory tract [33], whereas in the extrapulmonary tissues ozone exposure does not appear to have a significant impact at the time-point studied. After K. pneumoniae infection
1) effect of ozone exposure
The changes described below differed between ozone-exposed and FA-exposed animals in both sexes.
Lung
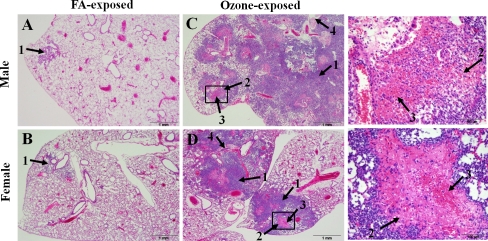
Ozone-exposed animals were found to have a significantly higher level of lung inflammation (severe in ozone-exposed vs. mild in FA-exposed) that encompassed a larger area (∼10-30 % in ozone-exposed males, and ∼10-15 % in ozone-exposed females versus less than 1-5 % in FA-exposed male and female mice) (Figure 2; Figure 3A). Moreover, different types of inflammation, i.e. mainly necrosuppurative and hemorrhagic were observed in ozone-exposed versus a typically purely suppurative in FA-exposed animals (Figure 2; Figure 3A). Furthermore, bacteria were detectable by routine H&E staining in the lungs of ozone-exposed mice only, except in one of the eleven FA-exposed female mice, in which bacteria were detected in the lung as well (Table 2). In the ozone-exposed mice, bacteria often formed large discrete colonies, suggesting uncontrolled local bacterial replication.
Figure 2.
Comparison of the pattern of inflammation in the lungs of ozone-exposed and FA-exposed male and female mice after K. pneumoniae infection. Male and female mice were exposed to ozone or to FA (control) as described in Methods, and then intratracheally infected with ∼ 450 CFU of K. pneumoniae bacteria. Lungs were sampled and analyzed for histopathologic changes at 48 h post-infection. The pattern of the lung inflammation was compared between ozone-exposed and FA-exposed male and female mice in this Figure. Both male and female mice exposed to FA showed small single foci of inflammation consisting of low to moderate numbers of predominantly degenerating neu-trophils in peri-bronchiolar airspaces (arrow 1, panels A and B). In male and female mice exposed to ozone normal lung architecture was extensively effaced by large numbers of degenerate neutrophils (arrow 1, panels C and D) and necrosis centered on and radiating from the bronchioles with abundant fibrin (arrow 2, panels C and D), hemorrhage (arrow 3, panels C and D), and necrotic cellular debris (arrow 4, panels C and D). Enlarged areas of Figures 2C and 2D (squares) are shown on the right side from the respective Figure panels. Hematoxylin-eosin staining, 40× magnification.
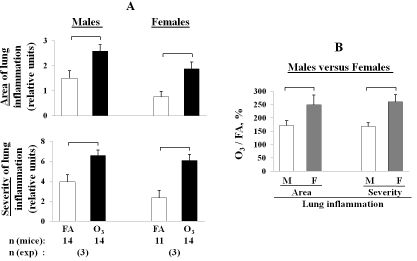
Figure 3.
Area and severity of inflammation in the lung of ozone-exposed and FA-exposed male and female mice after K. pneumoniae infection. Experimental design was as described in the legend for Figure 2. Male and female mice were exposed to ozone (solid bars) or to FA (control; open bars). The lungs were sampled and analyzed for histopa-thologic changes. A: Area of lung inflammation is the area (% of the total) affected by inflammation. These area values were converted to a 0-4 numerical scale: 0 for < 1 %, 1 for 1-4 %, 2 for 5-14 %, 3 for 15-24 %, and 4 for ≥ 25 % affected. Severity of lung inflammation was quantified based on a 0-8 numerical scale shown in Table 1. B: Comparison of lung inflammation in males versus females in response to ozone-exposure and pneumonia. The data are presented as the percent of the control (FA) for each sex, in accordance with the following formula: ozone-exposed (each mouse) / FA-exposed (average in the group) × 100 %. The values of area or severity of lung inflammation used to determine these ratios are shown in the panel A. The number of independent experiments was 3 for each sex, and the total number of mice is shown in the bottom of the Figure. Statistical analysis of data was performed using a t-test. Differences were considered significant if p < 0.05 and are indicated with the brackets.
Table 2.
Histopathologic changes in the lung, liver, and spleen in FA-exposed and ozone-exposed male and female mice after K. pneumoniae infection
| Lung* | Liver infarction* | Splenic thrombi* | ||||
|---|---|---|---|---|---|---|
| Sex | Treatment | Lobar type of pneumonia | Detection of bacteria in the lung with H&E | Pleuritis | ||
| Males | FA | 1/14 | 0/14 | 5/14 | 9/14 | 8/14 |
| Ozone | 3/14 | 7/14 | 1/14 | 3/14 | 4/14 | |
| Females | FA | 0/11 | 1/11 | 4/11 | 4/11 | 5/11 |
| Ozone | 0/14 | 4/14 | 3/14 | 4/14 | 1/14 | |
After ozone- or FA-exposure, mice were infected with K. pneumoniae, and the lung, spleen, and liver were sampled and analyzed for histopathologic changes at 48 h post-infection as described in Methods. The numerator indicates the number of mice that demonstrate the particular change, and the denominator indicates the total number of mice in the group. Number of independent experiments was 3 for each sex.
Spleen
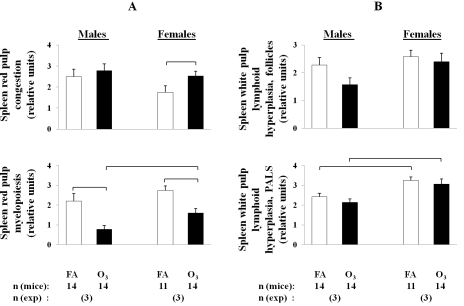
A higher frequency of thromboembolus formation was observed in the spleens of FA-exposed males and females compared to ozone -exposed animals (Figure 4A, Table 2). On the other hand, spleen immune function appeared to be compromised in ozone-exposed animals compared to FA-exposed; spleen red pulp myelopoiesis was significantly reduced in ozone-exposed male and female mice compared to FA-exposed animals, indicative of an inappropriate acute immune response to bacterial infection (Figure 5, Figure 6A).
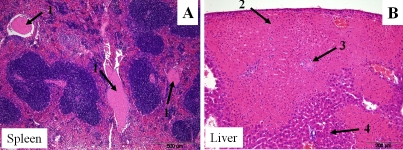
Figure 4.
Extra-pulmonary lesions (in spleen and in liver) in ozone-exposed male mice after K. pneumoniae infection. An example of extra-pulmonary lesions (in spleen and in liver) in ozone-exposed wild type male mice is shown. After ozone exposure, mice were infected with K. pneumoniae as described in the legend of Figure 2, and the spleen and liver were sampled and analyzed for histopathologic changes at 48 h post-infection. Recent fibrin thromboemboli (arrows 1, panel A) distend multiple blood vessels within the splenic red pulp; 100× magnification. In the liver there was focally extensive coagulative necrosis in which ghost cells retain their normal architecture (infarct) (arrow 2, panel B). A fibrin thromboembolus is shown in the center (arrow 3, panel B). Adjacent normal liver is shown (arrow 4, panel B); 200× magnification.
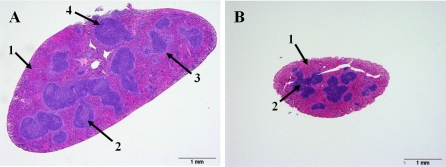
Figure 5.
Comparison of splenic architecture after K. pneumoniae infection. Representative examples of two extremes of splenic architecture are shown. Both images are from females. The quantitative data analysis is shown in Figure 6. After FA or ozone exposure, mice were infected with K. pneumoniae as described in the legend of Figure 2, and the spleens were sampled and analyzed for histopathologic changes at 48 h post-infection. Panel A depicts an example of a spleen which is more typical for FA-exposed males and females. This spleen is markedly enlarged with rounded edges and a hypercellular red pulp composed of numerous myeloid precursors (arrow 1) and minimal congestion. In the white pulp, germinal centers are pale (due to medium and large size lymphoblasts (arrow 2)), the marginal zone is prominent (pale color around white pulp can be identified) (arrow 3), and periarteriolar lymphoid sheaths (PALS) are expanded by mature and immature lymphocytes (arrow 4). Panel B depicts an example of a spleen, which is more typical for ozone-exposed males and females. The red pulp is mildly expanded by acute congestion with an absence of myelopoiesis (arrow 1). The white pulp is composed of mature quiescent (small blue) lymphocytes (arrow 2). Both images are taken at 40× magnification.
Figure 6.
Changes in leukocyte populations in the spleens of ozone-exposed and FA-exposed male and female mice after K. pneumoniae infection. After ozone or FA-exposure, mice were infected with K. pneumoniae as described in the legend of Figure 2, and the spleens were sampled and analyzed for histopathologic changes in both the red and white pulp. The myeloid compartment of the red pulp and the follicular and PALS compartments of the white pulp were each individually semiquantitatively scored for hyperplasia of the relevant leukocyte populations on a 0-4 numerical scale: 0 for normal, 1 for minimal hyperplasia, 2 for mild, 3 for moderate, and 4 for severe florid hyperplasia. Acute congestion of the red pulp was graded by a similar scale. A: Comparison of histopathologic changes in the red pulp in males versus females in response to ozone-exposure and pneumonia. B: Comparison of histopathologic changes in the white pulp in males versus females in response to ozone-exposure and pneumonia. The number of independent experiments was 3 for each sex, and the total number of mice is shown in the bottom of the Figure. Statistical analysis of data was performed using a t-test. Differences were considered significant if p < 0.05 and are indicated with the brackets.
Liver
As in the case of splenic thromboemboli, liver infarction (Figure 4B) was observed more frequently in FA-exposed compared to ozone-exposed animals in both sexes (9 of 14, and 3 of 14 FA-exposed and ozone-exposed males, respectively, were affected, and 4 of 11, and 4 of 14 FA-exposed and ozone-exposed females were affected, respectively) (Table 2).
Thus, after pulmonary infection the inflammation in the lung was higher and the spleen immune response was lower in ozone-exposed compared to FA-exposed animals of both sexes.
2) effect of sex
The changes described below differed in males and females.
Lung
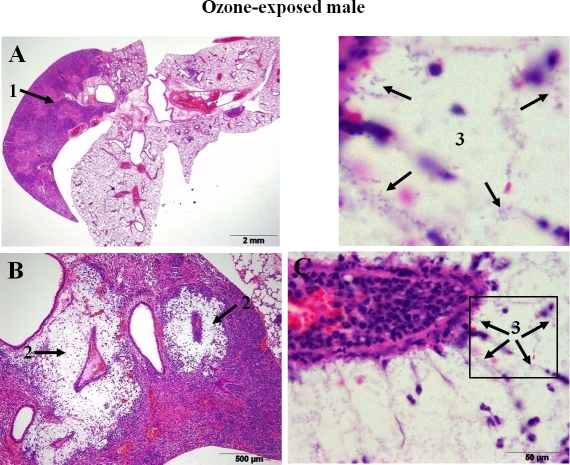
Only male mice (3 of 14 ozone-exposed and 1 of 14 FA-exposed) demonstrated the lobar type of pneumonia, which is considered to be a more severe form of pneumonia, and none of the 14 ozone- and 11 FA-exposed female mice developed this type of pneumonia (Figure 7, Table 2). Although no significant differences were found directly between males and females after ozone- or FA-exposure with regards to both the area and the severity of inflammation in the lung (Figure 3A), if the data from ozone-exposed mice were normalized to the data from FA-exposed mice (the baseline for each sex), the area of the lung inflammation was larger and the severity of pneumonia was higher in females than in males after ozone exposure (p < 0.05) (Figure 3B).
Figure 7.
Lobar pneumonia in the lung of ozone-exposed male mice after K. pneumoniae infection. An example of pneumonia development in ozone-exposed wild type male mice is shown. After ozone exposure, mice were infected with K. pneumoniae as described in the legend of Figure 2, and the lungs were sampled and analyzed for histopa-thologic changes at 48 h post-infection. Infection with K. pneumoniae in ozone-exposed mice resulted in severe inflammation involving the entire anatomical unit (right cranial lobe) (arrow 1, panel A). In these mice large colonies of encapsulated bacteria were readily visible as large punched out spaces within the inflammatory foci surrounding blood vessels or bronchioles (arrow 2, panel B) and large number of plump bacterial bacilli surrounded by clear halos (capsules) (arrow 3, panel C, higher magnification). Enlarged area of Figure 7C (square) is shown above Figure 7C. Hematoxylin-eosin staining; 20×, 100×, and 1,000× magnifications for panels A, B, and C, respectively.
Spleen
Splenic fibrin thromboembolus formation in general was more frequently observed in both FA and ozone-exposed males compared to female mice with most occurrences in FA-exposed males (8 of 14 and 4 of 14 FA-exposed and ozone-exposed males, and 5 of 11 and 1 of 14 FA-exposed and ozone-exposed females were affected, respectively) (Figure 4A; Table 2). Ozone-exposed females had a significantly higher acute congestion in the red pulp than FA-exposed females, whereas no comparable significant differences were detected for males (Figure 6A). Regarding spleen immune function, ozone-exposed female mice were found to have a significantly higher level of myelopoiesis in the red pulp of the spleen than their male counterparts (Figure 6A). In the white pulp of the spleen, FA- and ozone-exposed females showed significantly higher levels of lymphoid hyperplasia in the periarteriolar lymphoid sheaths (PALS) than their male counterparts (Figure 6B).
Liver
Sex difference in the development of liver infarction was found between FA-exposed males and females (9 of 14 and 4 of 11 males and females were affected, respectively). Moreover, liver infarction, when present, involved a larger area in FA-exposed males compared to that of females. However, in ozone-exposed animals the difference between sexes was not evident (3 of 14 and 4 of 14 males and females were affected, respectively) (Figure 4B; Table 2).
Thus, after pneumonia infection, FA-exposed males had a higher risk for extrapulmonary organ lesions (in spleen and liver) than FA-exposed females, whereas ozone-exposed females had higher degree of inflammation in the lung compared to ozone-exposed males.
Discussion
Pulmonary infection can cause a number of pathological changes in the lung, as well as in the extrapulmonary organs as in the case of bacterial dissemination, and these changes can impact the outcome of disease. Moreover, the clinical course of pneumonia is influenced by sex and environmental conditions, such as ozone exposure. We have shown previously that ozone-exposed animals had lower survival rates than FA-exposed animals, and although female mice had higher survival rates than males after K. pneumoniae infection, ozone exposure reversed the trend and caused females to survive less than males [31]. The purpose of this study was to assess risk factors that may contribute to the observed differences in survival. For this, we analyzed, via histopathology, tissues (lung, spleen, liver) from mice after pneumonia in the presence or absence of ozone-induced oxidative stress. We found: 1) severe inflammation in the lung and reduction of spleen immune function to be among the contributing factors for lower survival of ozone-exposed compared to FA-exposed animals for both sexes; 2) a higher risk for extrapulmonary organ lesions (in spleen and liver) in FA-exposed males appears to contribute to the previously observed lower survival of males compared to their FA-exposed female counterparts; and 3) a severe inflammation in the lung of ozone-exposed females appears to contribute to the previously observed lower survival of ozone-exposed females compared to ozone-exposed males. Thus, a histopathologic analysis of tissues revealed that different risk factors contribute to and thus can/may explain the previously observed [31] differential survival of mice after pneumonia between sexes in the presence or absence of ozone-induced oxidative stress.
Analysis of histopathologic changes in the lungs of non-infected mice (PBS inoculated control group) in response to ozone revealed that ozone exposure induced epithelial changes in the lungs of mice from both sexes compared to FA-exposure conditions, which is consistent with published data regarding the impact of ozone exposure on the lung [33]. Because no significant pathology was observed in the extrapulmonary tissues (spleen, liver) from either sex, ozone exposure does not appear to have a significant impact on the extrapulmonary tissues at the time-point studied.
However, after pulmonary infection, the inflammation in the lung was found to be more severe in ozone-exposed than the FA-exposed infected animals. Although an appropriately lung host defense-mediated inflammatory response is necessary to combat infection, excessive lung inflammation may potentially cause detrimental effects. In the case of the severe lung inflammation seen in the ozone-exposed male and female mice, reactive oxygen species and proteases, localized in the vacuoles of phagocytes that are normally intended to clear microorganisms from the tissues, may be produced in excess and released into the extracellular environment, causing tissue damage that, in turn, may lead to further loss of function of the organ rather than clearance of the infection [34, 35]. Therefore, the observed excessive inflammatory response may be pathological, and potentially result in death [36]. In the present study the observed increased severity of the lung inflammatory response in ozone-exposed animals may make a significant contribution to the increased mortality from pneumonia shown previously [31].
A higher frequency of splenic thromboemboli in FA-exposed infected males and females compared to their ozone-exposed counterparts observed in this study, could be the result of excessive bacterial proliferation in the spleen of FA-exposed animals. This bacterial proliferation may, in turn, produce large amounts of lipopolysaccharide (LPS) that can contribute to the vasodilatation of the blood vessels with increased activation of the clotting cascade [37], thereby interfering with splenic function. On the other hand, the immune function of spleen (red pulp myelopoiesis) was found to be significantly reduced in ozone-exposed animals compared to FA-exposed mice for both sexes. This reduction indicates that an inappropriate acute immune response to bacterial infection in ozone-exposed mice may be among the contributing factors to their lower survival rates [31]. It is also possible that certain environmental factors (such as ozone) may compromise the development of the adaptive immune response, especially when this is earlier on in its developmental process. Together these findings indicate that differences between FA- and ozone-exposed pneumonia-infected mice are not only observed in the lung, the primary site of pneumonia infection, but also in extrapulmonary organs, such as spleen.
It is well known that the spleen has two major functions: 1) removal of old or defective red blood cells (i.e. serving as a blood filter) and acting as a blood reservoir; and 2) generation of an appropriate immune response to blood-borne foreign substances. Moreover, it was recently reported that the red pulp of spleen is an important site for the storage of half of the body's monocytes [38]. In mice, the spleen is also an important site of hematopoieses, including myelopoieses. Swirski and co-authors [38] suggested that following infection or tissue injury, monocytes can rapidly migrate from the red pulp of the spleen to infected tissues through the bloodstream to differentiate into mature macrophages at the sites of infection in order to facilitate the clearance of infecting agents and assist in wound healing [38, 39]. Myeloid cells localized in the spleen red pulp, such as neutrophils and monocytes, are involved in the first stages of the immune response. Thus, a significantly lower myeloid response in spleen red pulp of ozone-exposed mice when compared to FA-exposed animals shows an inability to maintain myeloid leukocyte production in the face of an overwhelming bacterial infection and in the presence of oxidative stress. Furthermore, the importance of splenic function in the development of pneumonia was demonstrated in the study of Robinette and Fraumeni [40]. Their study involved 740 American servicemen splenectomized because of trauma during World War II. They showed that splenectomized individuals demonstrated significant excess of mortality from pneumonia and ischemic heart-disease. Moreover, sepsis can occur more readily in asplenic or hyposplenic patients and the most severe disease is associated with infections due to encapsulated organisms (such as K. pneumoniae used in this study) [41, 42]. From animal studies it has been reported that asplenic rats are more susceptible to experimentally induced H. influenzae bacteremia [43]. Thus, splenic function is one of the important factors that may impact pneumonia outcome. Based on our observations, we speculate that bacterial dissemination in ozone-exposed animals may not generate an adequate immune response in the mouse spleen to maintain an active host defense. This is consistent with the lower survival in ozone-exposed and infected mice compared to their FA-exposed counterparts [31].
Thus, the excessive inflammatory response in the lung (which can be associated with excessive lung tissue damage), coupled with the inability of the spleen to adequately respond to dissemination of the lung infection appear to explain in part the lower survival of ozone-exposed and pneumonia-infected mice compared to their FA-exposed counterparts [31] (Figure 8A).
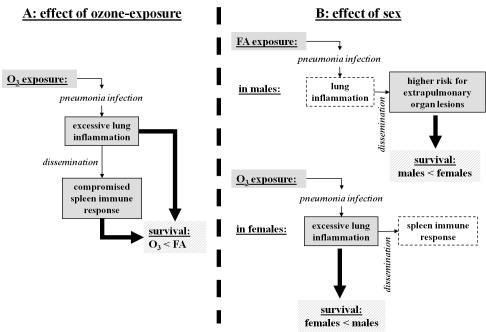
Figure 8.
Impact of ozone exposure and sex on the outcome of pneumonia infection: overview and potential underlying mechanisms. Summary of our findings from the histopathologic examinations of tissues is presented in the diagram. Potential risk factors for survival rates [31] of male and female mice after pneumonia under different exposure conditions [31] are noted. Panel A depicts pathological effects of ozone exposure that may contribute to mouse survival after pneumonia. Ozone-exposed male and female mice survived less than FA-exposed mice after pneumonia. Both the excessive lung inflammatory response (p < 0.05) and the functional inability of the spleen to adequately respond to the dissemination of pneumonia infection (red pulp myelopoiesis is reduced, p < 0.05) in ozone-exposed animals (compared to FA-exposed animals) may account for this difference. Panel B depicts potential factors that may explain the observed sex differences. It has been previously shown [31] that FA-exposed males survived less than FA-exposed females, and ozone-exposed females survived less than ozone-exposed males after pneumonia, indicating that different mechanisms may be involved. The higher risk of extrapulmonary organ lesions (liver infarction, spleen thrombosis) in FA-exposed males may potentially cause a higher mortality after pneumonia compared to FA-exposed females. Lung inflammation does not appear to play a major role in this difference, because although lung inflammation in the FA-exposed male lungs was slightly increased compared to females, this difference did not reach a significant level (p > 0.05). However, in spite of a significantly higher spleen immune response (red pulp myelopoiesis) in ozone-exposed females compared to males, the excessive lung inflammatory response observed in ozone-exposed female mice (p < 0.05) may be the likely or major factor responsible for their lower survival rate [31] compared to ozone-exposed males.
A lobar type of pneumonia, which is considered to be a more severe form of pneumonia, was observed only in male mice, and none of the female mice developed this type of pneumonia. Although this lobar type of inflammation is suggestive of a failure to contain the infection, such that the spread is limited only by anatomical boundaries, the ozone to FA lung inflammation ratio (ozone/FA) was significantly higher in females compared to males. This indicates that the previously observed lower survival of females from pneumonia after ozone exposure [31] may be due to excessive lung inflammation compared to males.
Splenic fibrin thromboembolus formation was observed more frequently in both FA and ozone-exposed males compared to female mice, with most occurrences in FA-exposed males. Based on our unpublished preliminary findings, where more pronounced bacteremia in FA-exposed males was observed compared to FA-exposed females, we speculate, that the splenic fibrin thromboembolus formation (as well as the liver infarction, described below) is due to the large number of bacteria colonizing the spleen. This, in turn, may compromise the functions of the spleen and thus negatively influence the clinical course of pneumonia and survival in males, as previously shown both in animal and epidemiological studies [5-8, 31].
The FA- and ozone-exposed infected females showed a significantly higher level of lymphoid hyperplasia in PALS of the white pulp of spleen than did the males, indicating higher T-lymphocyte production in the PALS, and consequently, a more appropriate cell-mediated immune response. However, even though T-cells play an important role in the immune response to infection, these results do not fully explain the sex differences previously observed with survival after pneumonia [31]. Although a better host defense, as assessed by survival, may have occurred in FA-exposed females, this was not the case for the ozone-exposed females, who showed the highest level of mortality [31]. It is interesting to note that most of the mortality occurred during the first 4 days post-infection when the adaptive immune response had not yet been well developed. We also found that ozone-exposed female mice had significantly higher red pulp myelopoiesis than their male counterparts. However, in this case the excess production of myeloid cells may have negative consequences for the ozone-exposed animals, resulting in an even higher degree of inflammation and damage in their lungs [35] that may increase morbidity and mortality [31]. These observations indicate that in ozone-exposed females, in spite of an increase in spleen immune response in both white and red pulp, the higher lung inflammatory response may play a dominant role in the poor outcome. Moreover, ozone-exposed females had significantly more acute congestion in the red pulp than FA-exposed females, whereas no significant differences were detected for males. The splenic congestion could be symptomatic of decreased vasomotor tone consistent with the effect of endotoxic shock [44, 45]. Based on this, we speculate that ozone-exposed females in contrast to their male counterparts develop a higher level of bacterial dissemination from the lung that, in turn, will increase the splenic red pulp congestion. Because spleen congestion is associated with storage of mature erythrocytes in red pulp, sequestration of red blood cells from the blood flow can decrease the red blood cell concentration in the circulation [46] resulting in anemia. Furthermore, the splenic red pulp congestion may also affect the filtering capacity of the spleen and subsequently, host defense against bacterial infection [47]. Thus, the function of the spleen may be compromised in ozone-exposed animals when compared to FA-exposed, with this reduction being more pronounced in females than in males. The latter, in part, explains our previous observations of survival, where the gap between survival curves of ozone- and FA-exposed females was larger than that in males [31].
As in the case of splenic thromboemboli, sex differences in liver infarction were found between FA-exposed infected males and females with males being more affected. However, in ozone-exposed animals the difference between sexes was not evident. As with splenic thromboembolus formation, this could be associated with higher bacteremia in FA-exposed male animals that could reduce their survival compared to their female counterparts [31]. The liver is an essential organ for survival. A number of studies have demonstrated an association of liver transplantation with bacterial infection [48, 49]. Sepsis during bacterial infection can cause hypotension, shock, and thrombosis, which, in turn, may lead to hepatic infarction [50-53]. Pneumonia was found to be among the factors that can cause morbidity and mortality in liver transplant recipients [51, 54-56]. Moreover, liver transplantation with simultaneous splenectomy increases the risk for opportunistic pneumonia [57]. These results point to the importance of both the integrity and functional ability of the spleen and liver for a better pneumonia outcome. Based on our observations in the present study, these (integrity and function of the liver and spleen) appear to be more compromised in FA-exposed infected males than in females, and may negatively influence the pneumonia outcome. Thus, extrapulmonary organ lesions (in spleen and liver) are among the factors that may explain the lower survival observed in FA-exposed males compared to FA-exposed females [31] (Figure 8B).
Based on the results of the present study, we conclude that different risk factors contribute to the differential survival of mice from pneumonia under different exposure conditions (in the presence or absence of oxidative stress) between sexes [31]. Specifically, the higher degree of the lung inflammatory response and the reduction in the ability of the spleen to generate an appropriate immune response are among the contributing factors for the previously observed [31] negative effect of ozone on the survival from pneumonia for both sexes. However, the higher degree of the lung inflammatory response observed in ozone-exposed females, and the greater risk of extrapulmonary lesions in spleen and liver, due perhaps to a higher dissemination of bacteria from the lung as it may be in the case of FA-exposed males, provide support and may explain the previously observed sex differences seen in survival from pneumonia [31]. Further studies are needed to understand the underlying molecular mechanisms.
Acknowledgments
We gratefully thank Dr. Dani Zander from The Pennsylvania State University College of Medicine for her critical review of this manuscript and helpful suggestions. This work was supported by National Institute of Environmental Health Sciences Grant 1RO1-ES09882.
References
- 1.Nohynek H, Madhi S, Grijalva CG. Childhood bacterial respiratory diseases: past, present, and future. Pediatr Infect Dis J. 2009;28:S127–132. doi: 10.1097/INF.0b013e3181b6d800. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen HC. Testosterone regulation of sex differences in fetal lung development. ProcSo-cExpBiolMed. 1992;199:446–452. doi: 10.3181/00379727-199-43379. [DOI] [PubMed] [Google Scholar]
- 3.Perelman RH, Palta M, Kirby R, Farrell PM. Discordance between male and female deaths due to the respiratory distress syndrome. Pediatrics. 1986;78:238–244. [PubMed] [Google Scholar]
- 4.Caracta CF. Gender differences in pulmonary disease. MtSinai JMed. 2003;70:215–224. [PubMed] [Google Scholar]
- 5.Gordon HS, Rosenthal GE. The relationship of gender and in-hospital death: increased risk of death in men. Med Care. 1999;37:318–324. doi: 10.1097/00005650-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez F, Masia M, Mirete C, Soldan B, Rodriguez JC, Padilla S, Hernandez I, Royo G, Martin-Hidalgo A. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. JInfect. 2006;53:166–174. doi: 10.1016/j.jinf.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Gannon CJ, Pasquale M, Tracy JK, McCarter RJ, Napolitano LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21:410–414. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999;159:2058–2064. doi: 10.1001/archinte.159.17.2058. [DOI] [PubMed] [Google Scholar]
- 9.Yancey AL, Watson HL, Cartner SC, Simecka JW. Gender is a major factor in determining the severity of mycoplasma respiratory disease in mice. InfectImmun. 2001;69:2865–2871. doi: 10.1128/IAI.69.5.2865-2871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Saito H, Setogawa T, Tomioka H. Sex differences in host resistance to Mycobac-terium marinum infection in mice. Infect Immun. 1991;59:4089–4096. doi: 10.1128/iai.59.11.4089-4096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashman RB, Kay PH, Lynch DM, Papadimitriou JM. Murine candidiasis: sex differences in the severity of tissue lesions are not associated with levels of serum C3 and C5. Immunol Cell Biol. 1991;69(Pt1):7–10. doi: 10.1038/icb.1991.2. [DOI] [PubMed] [Google Scholar]
- 12.Huber SA, Job LP, Auld KR. Influence of sex hormones on Coxsackie B-3 virus infection in Balb/c mice. Cell Immunol. 1982;67:173–179. doi: 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB. Health effects of air pollution. J Allergy Clin Immunol. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 14.McBride DE, Koenig JQ, Luchtel DL, Williams PV, Henderson WR., Jr Inflammatory effects of ozone in the upper airways of subjects with asthma. Am J Respir Crit Care Med. 1994;149:1192–1197. doi: 10.1164/ajrccm.149.5.8173759. [DOI] [PubMed] [Google Scholar]
- 15.Currie WD, van Schaik S, Vargas I, Enhorning G. Breathing and pulmonary surfactant function in mice 24 h after ozone exposure. Eur Respir J. 1998;12:288–293. doi: 10.1183/09031936.98.12020288. [DOI] [PubMed] [Google Scholar]
- 16.Kehrl HR, Vincent LM, Kowalsky RJ, Horstman DH, O'Neil JJ, McCartney WH, Bromberg PA. Ozone exposure increases respiratory epithelial permeability in humans. Am Rev Respir Dis. 1987;135:1124–1128. doi: 10.1164/arrd.1987.135.5.1124. [DOI] [PubMed] [Google Scholar]
- 17.Bassett D, Elbon-Copp C, Otterbein S, Barraclough-Mitchell H, Delorme M, Yang H. Inflammatory cell availability affects ozone-induced lung damage. J Toxicol Environ Health A. 2001;64:547–565. doi: 10.1080/15287390152627237. [DOI] [PubMed] [Google Scholar]
- 18.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 19.Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proc Am Thorac Soc. 2007;4:240–246. doi: 10.1513/pats.200701-023AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy SM, Chambers R, Du W, Dimich-Ward H. Environmental and occupational exposures: do they affect chronic obstructive pulmonary disease differently in women and men? Proc Am Thorac Soc. 2007;4:692–694. doi: 10.1513/pats.200707-094SD. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis D, Chinn S, Sterne J, Luczynska C, Burney P. The association of respiratory symptoms and lung function with the use of gas for cooking. European Community Respiratory Health Survey. Eur Respir J. 1998;11:651–658. [PubMed] [Google Scholar]
- 22.Schwela D. Air pollution and health in urban areas. Rev Environ Health. 2000;15:13–42. doi: 10.1515/reveh.2000.15.1-2.13. [DOI] [PubMed] [Google Scholar]
- 23.Dow L, Fowler L, Phelps L, Waters K, Coggon D, Kinmonth AL, Holgate ST. Prevalence of untreated asthma in a population sample of 6000 older adults in Bristol, UK. Thorax. 2001;56:472–476. doi: 10.1136/thorax.56.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, Margolis H, Rappaport E, Vora H, Gong H, Jr, Thomas DC. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159:768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 25.Li YF, Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Rappaport EB, Peters JM. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med. 2000;162:2097–2104. doi: 10.1164/ajrccm.162.6.2004178. [DOI] [PubMed] [Google Scholar]
- 26.Bates DV. The effects of air pollution on children. Environ Health Perspect. 1995;103(Suppl 6):49–53. doi: 10.1289/ehp.95103s649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Lubin JH, Zhang SR, Metayer C, Xia Y, Brenner A, Shang B, Wang Z, Kleinerman RA. Lung cancer and environmental tobacco smoke in a non-industrial area of China. Int J Cancer. 2000;88:139–145. doi: 10.1002/1097-0215(20001001)88:1<139::aid-ijc22>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Oosterlee A, Drijver M, Lebret E, Brunekreef B. Chronic respiratory symptoms in children and adults living along streets with high traffic density. Occup Environ Med. 1996;53:241–247. doi: 10.1136/oem.53.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbey DE, Nishino N, McDonnell WF, Burchette RJ, Knutsen SF, Lawrence Beeson W, Yang JX. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159:373–382. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- 30.Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 31.Mikerov AN, Gan X, Umstead TM, Miller L, Chinchilli VM, Phelps DS, Floros J. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res. 2008;9:24. doi: 10.1186/1465-9921-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, Floros J. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. ToxicolApplPharmacol. 2007;220:72–82. doi: 10.1016/j.taap.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez A. Respiratory system. In: McGavin MD, Zachary JF, editors. Pathological Basis of Veterinary Disease. 4th. St. Louis, MO: Mosby Elsevier; 2007. pp. 463–558. [Google Scholar]
- 34.Si-Tahar M, Touqui L, Chignard M. Innate immunity and inflammation–two facets of the same anti-infectious reaction. Clin Exp Immunol. 2009;156:194–198. doi: 10.1111/j.1365-2249.2009.03893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin TR. Neutrophils and lung injury: getting it right. J Clin Invest. 2002;110:1603–1605. doi: 10.1172/JCI17302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cazzola M, Matera MG, Pezzuto G. Inflammation–a new therapeutic target in pneumonia. Respiration. 2005;72:117–126. doi: 10.1159/000084039. [DOI] [PubMed] [Google Scholar]
- 37.Wang X. Lipopolysaccharide augments venous and arterial thrombosis in the mouse. Thromb Res. 2008;123:355–360. doi: 10.1016/j.thromres.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 40.Robinette CD, Fraumeni JF., Jr Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet. 1977;2:127–129. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- 41.Hansen K, Singer DB. Asplenic-hyposplenic overwhelming sepsis: postsplenectomy sepsis revisited. Pediatr Dev Pathol. 2001;4:105–121. doi: 10.1007/s100240010145. [DOI] [PubMed] [Google Scholar]
- 42.de Porto AP, Lammers AJ, Bennink RJ, ten Berge IJ, Speelman P, Hoekstra JB. Assessment of splenic function. Eur J Clin Microbiol Infect Dis. 2010;29:1465–1473. doi: 10.1007/s10096-010-1049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moxon ER, Goldthorn JF, Schwartz AD. Haemophilus influenzae b infection in rats: effect of splenectomy on bloodstream and meningeal invasion after intravenous and intranasal inoculations. Infect Immun. 1980;27:872–875. doi: 10.1128/iai.27.3.872-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abel FL, Beck RR. Portal venous compliance in canine endotoxin shock. Circ Shock. 1991;35:96–101. [PubMed] [Google Scholar]
- 45.Sayk F, Vietheer A, Schaaf B, Wellhoener P, Weitz G, Lehnert H, Dodt C. Endotoxemia causes central downregulation of sympathetic vasomotor tone in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R891–898. doi: 10.1152/ajpregu.90444.2008. [DOI] [PubMed] [Google Scholar]
- 46.Frangioni G, Borgioli G. Effect of spleen congestion and decongestion on newt blood. J Zool, Lond. 1991;223:15–25. [Google Scholar]
- 47.Chen LT, Moxon ER. Effect of splenic congestion associated with hemolytic anemia on mortality of rats challenged with Haemophilus influenzae b. Am J Hematol. 1983;15:117–121. doi: 10.1002/ajh.2830150203. [DOI] [PubMed] [Google Scholar]
- 48.Blair JE, Kusne S. Bacterial, mycobacterial, and protozoal infections after liver transplantation–part I. Liver Transpl. 2005;11:1452–1459. doi: 10.1002/lt.20624. [DOI] [PubMed] [Google Scholar]
- 49.George DL, Arnow PM, Fox AS, Baker AL, Thistlethwaite JR, Emond JC, Whitington PF, Broelsch CE. Bacterial infection as a complication of liver transplantation: epidemiology and risk factors. Rev Infect Dis. 1991;13:387–396. doi: 10.1093/clinids/13.3.387. [DOI] [PubMed] [Google Scholar]
- 50.Haque M, Schumacher PA, Harris A, Scudamore CH, Steinbrecher UP, Chung SW, Buczkowski AK, Erb SR, Yoshida EM. Late acute celiac and hepatic artery thrombosis with portal vein thrombosis resulting in hepatic infarction 12 years post orthotopic liver transplantation. Ann Hepatol. 2009;8:396–399. [PubMed] [Google Scholar]
- 51.Bert F, Larroque B, Paugam-Burtz C, Janny S, Durand F, Dondero F, Valla DC, Belghiti J, Moreau R, Nicolas-Chanoine MH. Microbial epidemiology and outcome of bloodstream infections in liver transplant recipients: an analysis of 259 episodes. Liver Transpl. 2010;16:393–401. doi: 10.1002/lt.21991. [DOI] [PubMed] [Google Scholar]
- 52.Brown RK, Memsic LD, Busuttil RW, Pusey E, Ray RA, Kangarloo H, Hawkins RA. Accurate demonstration of hepatic infarction in liver transplant recipients. J Nucl Med. 1986;27:1428–1431. [PubMed] [Google Scholar]
- 53.Carroll R. Infarction of the human liver. J Clin Pathol. 1963;16:133–136. doi: 10.1136/jcp.16.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golfieri R, Giampalma E, Morselli Labate AM, d'Arienzo P, Jovine E, Grazi GL, Mazziotti A, Maffei M, Muzzi C, Tancioni S, et al. Pulmonary complications of liver transplantation: radiological appearance and statistical evaluation of risk factors in 300 cases. Eur Radiol. 2000;10:1169–1183. doi: 10.1007/s003309900268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh N, Gayowski T, Wagener MM, Marino IR. Pulmonary infiltrates in liver transplant recipients in the intensive care unit. Transplantation. 1999;67:1138–1144. doi: 10.1097/00007890-199904270-00009. [DOI] [PubMed] [Google Scholar]
- 56.Singh N, Gayowski T, Wagener M, Marino IR, Yu VL. Pulmonary infections in liver transplant recipients receiving tacrolimus. Changing pattern of microbial etiologies. Transplantation. 1996;61:396–401. doi: 10.1097/00007890-199602150-00013. [DOI] [PubMed] [Google Scholar]
- 57.Neumann UP, Langrehr JM, Kaisers U, Lang M, Schmitz V, Neuhaus P. Simultaneous splenectomy increases risk for opportunistic pneumonia in patients after liver transplantation. Transpl Int. 2002;15:226–232. doi: 10.1007/s00147-002-0399-8. [DOI] [PubMed] [Google Scholar]