Abstract
Lymphodepletion augments adoptive cell transfer during antitumor immunotherapy, producing dramatic clinical responses in patients with malignant melanoma. We report that the lymphopenia induced by the chemotherapeutic agent temozolomide (TMZ) enhances vaccine-driven immune responses and significantly reduces malignant growth in an established model of murine tumorigenesis. Unexpectedly, despite the improved antitumor efficacy engendered by TMZ-induced lymphopenia, there was a treatment related increase in the frequency of immunosuppressive regulatory T cells (TRegs; P = .0006). Monoclonal antibody (mAb)–mediated inhibition of the high-affinity IL-2 receptor α (IL-2Rα/CD25) during immunotherapy in normal mice depleted TRegs (73% reduction; P = .0154) but also abolished vaccine-induced immune responses. However, during lymphodepletion, IL-2Rα blockade decreased TRegs (93% reduction; P = .0001) without impairing effector T-cell responses, to augment therapeutic antitumor efficacy (66% reduction in tumor growth; P = .0024). Of clinical relevance, we also demonstrate that anti–IL-2Rα mAb administration during recovery from lymphodepletive TMZ in patients with glioblastoma reduced TReg frequency (48% reduction; P = .0061) while permitting vaccine-stimulated antitumor effector cell expansion. To our knowledge, this is the first report of systemic antibody-mediated TReg depletion during lymphopenia and the consequent synergistic enhancement of vaccine-driven cellular responses, as well as the first demonstration that anti–IL-2Rα mAbs function differentially in nonlymphopenic versus lymphopenic contexts.
Introduction
The alkylating chemotherapeutic agent temozolomide (TMZ) has been shown to prolong survival in patients with glioblastoma (GBM) and metastatic melanoma; however, patients with these diseases treated with TMZ possess a median survival of < 15 months.1,2 Novel approaches are required to treat these devastating malignancies, and the exquisite specificity inherent to immunotherapy makes it an appealing option.
Despite the potential of cancer immunotherapy, limited success has been achieved within this field due in large part to difficulties in generating appropriate numbers of high-avidity and persistent antitumor T cells.3–5 A recent and profound advance in immunotherapy is the use of lymphopenia to augment antitumor immunity through adoptive cellular therapy.6–8 Lymphodepletion induces homeostatic proliferation, enabling adoptively transferred activated T cells to become disproportionately overrepresented in the regenerating population and persist for months at high precursor frequencies.9–12 Recent studies have primarily examined the lymphodepletive properties of total body irradiation (TBI), and although informative, TBI is not routinely used therapeutically and has a limited clinical context. In contrast, lymphopenia resulting from various standard-of-care chemotherapies, although generally considered an undesirable but inevitable side effect of treatment, could provide a clinically significant means to augment immunotherapy.
TMZ is generally considered an immunosuppressive agent that induces lymphopenia in humans and patients receiving TMZ are routinely given prophylaxis to prevent the development of opportunistic infections.13–16 Low-dose TMZ has been shown to enhance “cross-priming” against tumor-derived antigens in experimental mice17; however, the direct effects of lymphodepletive doses of TMZ on vaccine-induced immunologic responses and regulatory T cells (TRegs) has not been examined.
Here, we report that lymphodepletive TMZ strongly augments vaccine-induced immune responses in a dose-dependent manner and that combinatorial vaccination and lymphopenia in mice bearing established B16/F10.9-OVA tumors significantly impaired malignant growth despite an increase in the frequency of CD4+CD25+Foxp3+ TRegs. This TMZ-induced enhancement of immunity is dramatically augmented when combined with anti–IL-2Rα monoclonal antibody (mAb)–mediated depletion of CD4+CD25+Foxp3+ TRegs, whereas identical treatment in normal mice impaired vaccine-induced effector responses. Anti–IL-2 receptor α (IL-2Rα) mAb treatment can suppress activated T cells in normal mice18 and can suppress vaccine-induced immune responses in patients with metastatic melanoma.19 However, to our knowledge, this is the first demonstration that the lymphopenic environment differentially impacts whether vaccination can be successfully combined with systemic antibody-mediated TReg depletion in the treatment of established tumors. To determine whether these findings could be translated in humans, TMZ-treated patients with GBM received combinatorial IL-2Rα blockade using daclizumab (Hoffman-La Roche) and DC vaccination targeting the human cytomegalovirus (CMV) antigen pp65 that we and others have shown to be expressed in a high proportion of GBM tumors.20–24 TReg frequencies in GBM patients were also significantly depleted and vaccine-induced antitumor immune responses were simultaneously enhanced. Cumulative preclinical and clinical results indicate that synergistic lymphopenia with concomitant IL-2Rα blockade selectively depletes TRegs and potentiates antitumor immunotherapy in both mice and humans.
Methods
Mice and tumor cell lines
C57BL/6J and OT-I transgenic mice were from The Jackson Laboratory and were bred under pathogen-free conditions at Duke University Medical Center. All animal experiments were performed according to Duke University Institutional Animal Care and Use Committee–approved protocols. B16F10.9-OVA25 was a gift from Dr Smita Nair (Duke University Medical Center).
Peripheral blood draws and complete blood counts
Fifty to 100 μL of blood was collected into heparinized tubes by retro-orbital bleeding for complete blood counts (CBCs) and flow cytometric analysis. CBCs were performed on a VetScan HM5 hematology analyzer (Abaxis).
Isolation of murine T cells and flow cytometry
Murine T cells were isolated and stained for FACS analysis using protocols established previously in our laboratory.26 For analysis of peripheral blood, whole blood was incubated with antibodies in the dark for 15 minutes at room temperature before lysing red blood cells (RBCs) with 1× ammonium chloride lysing solution (BD Biosciences), cells were washed and resuspended in 2% paraformaldehyde. All samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Antibodies and CFSE staining
Antibodies to CD3 (145-2C11), CD4 (L3T4), CD8 (53-6.7), CD16/32 (2.4G2), CD25 (7D4), and isotype controls were from BD Biosciences Pharmingen. Anti-Foxp3 (FJK-16s) was obtained from eBioscience. IL-2Rα mAb (PC61) ascites for in vivo administration were obtained from Accurate Chemical & Scientific. For CFSE labeling, harvested splenocytes were resuspended at 2 × 107 cells/mL and incubated in PBS with 2% FCS and 20nM CFSE (Invitrogen) for 6 minutes at 37°C. Cells were washed, resuspended in PBS and 2% FBS, incubated at 37°C for 10 minutes, and washed again before use.
Cytometric bead array
We collected 50 μL of peripheral blood from mice and transferred the blood to a 96-well plate. RBCs were lysed with 1× lysing buffer (BD Pharm Lyse; BD Biosciences), and cells were washed twice and stimulated with 100 μg/mL ovalbumin (OVA) peptide in AIM-5 media with 5% FBS. Cells were incubated at 37°C with 5% CO2, and supernatant was collected after 48 hours. We then mixed 50 μL of culture supernatant with 50 μL of capture beads and 50 μL of detection reagent (CBA mouse inflammation kit; BD Biosciences) and incubated the sample for 2 hours at room temperature. Next, the sample was washed and analyzed by flow cytometry per the manufacturer's instructions.
MILLIPLEX cytokine panel
Peripheral blood was collected from mice 72 hours after the cessation of TMZ treatment. Plasma was derived and IL-2, IL-15, and IL-7 cytokine levels were determined (MILLIPLEX MAP mouse cytokine/chemokine kit protocol; Millipore) following the manufacturer's instructions.
T-cell suppression and proliferation assays
Cells were cultured and assayed as described previously.26
Generation of murine bone marrow-derived DCs, electroporation, and phenotyping
Bone marrow–derived DCs were generated from C57BL/6 mice as described previously using recombinant murine GM-CSF and IL-4. On day 8, nonadherent cells were harvested for RNA electroporation and suspended in Opti-MEM (Invitrogen) at 2.5 × 107 cells/mL. Aliquots (5 × 106) cell were mixed with 10 μg of OVA RNA, loaded into cold 2-mm cuvettes (Bio-Rad Laboratories), electroporated using a BTX model ECM 830 square wave electroporation system at 300 V for 500 μs, and replated in 60-mm culture dishes. On day 9, nonadherent cells were harvested for vaccination or in vitro stimulation.
Tumor implantation
B16/F10.9-OVA cells were grown in DMEM, 10% FCS, and 2mM l-glutamine. For tumor implantation, 1 × 105 cells in 100 μL of PBS were injected subcutaneously in the flank of C57BL/6 mice 3 days before treatment with TMZ. After 7 days, site of implantation was monitored daily for tumor growth, and tumor size was measured every 2-3 days. The volume of the tumor (cubic millimeters) was calculated by the formula (length × width2 × 0.52). Mice were killed when the tumor size reached 2 cm in any direction.
Preclinical temozolomide treatment, OT-I transfer, vaccination, and PC61 administration
C57BL/6 mice were injected intraperitoneally with TMZ (Temodar; Schering Plough) dissolved in a fresh solution of 85% saline and 15% DMSO; mice were weighed and injected intraperitoneally with a calculated single dose (milligrams per kilogram) or consecutive daily doses for 5 days (milligrams per kilogram per 5 days) as indicated. Standard-of-care TMZ (200 mg/m2) for a 65-kg, 60-inch human is equivalent to a murine dose of 66.31 mg/kg.27,28 Twenty-four to 48 hours after TMZ treatment, lymphodepleted mice received splenocytes from CD8+ OVA-specific TCR transgenic mice (OT-I mice) and normal C57BL/6 donor mice mixed at a 1:1 ratio (2 × 107 cells in total). Concomitant with lymphocyte infusion, mice were vaccinated intradermally with 5 × 105 OVA-loaded DCs or intradermally with 100 μg of OVA protein (Sigma-Aldrich) and 100μg of OVA class I peptide (sequence Ser-Ile-Ile-sn-Phe-Glu-Lys-Leu [SIINFEKL]; American Peptide) in 10% DMSO with an equal volume of complete Freund adjuvant (100 μL/mouse; Difco); for repeat peptide vaccinations incomplete Freund adjuvant was used. For mAb-mediated blockade of IL-2Rα, mice received 300 μL (0.62 mg/mL) PC61 intraperitoneally simultaneous with vaccination.
Patient selection and clinical protocol
Adults with a newly diagnosed, single lesion WHO grade 4 GBM who had gross total resection, Karnofsky Performance Scale score ≥ 80, and a Curran Group status of I-IV at the time of enrollment were eligible for enrollment. Postresection, patients received 6-7 weeks of conformal external beam radiotherapy with concurrent TMZ at 75 mg/m2. Patients with radiographic progression after external beam radiotherapy (> 20% increase in enhancement in comparison to postsurgical magnetic resonance imaging) did not receive vaccine. The trial design and informed consent were approved by the US Food and Drug Administration and the Duke University Medical Center Institutional Review Board.
Patients received adjuvant TMZ at 200 mg/m2 for 5 days of a 28-day cycle and 3 biweekly DC vaccines (2 × 107 DCs administered intradermally within 10 cm of the inguinal ligament) starting on day 21 of the first TMZ cycle. At vaccine 1, daclizumab was administered intravenously at a dose of 1 mg/kg. After daclizumab administration, patients received an autologous lymphocyte transfer intravenously at a dose of 3 × 107 cells/kg. The fourth and final vaccine was given on day 21 of the second TMZ cycle. Patients without tumor progression continued to receive monthly TMZ cycles for target of 6 to 12 cycles as tolerated.
Patients were monitored bimonthly by magnetic resonance imaging. Progressive disease was defined radiographically according to the Macdonald criteria29 or by the development of a new contrast-enhancing lesion more than 1 cm in diameter. Adverse events were defined according to the National Cancer Institute's Common Toxicity Criteria Version 2.0.
Production of pp65-LAMP/A64 mRNA
The 1.932-kb pp65 full-length cDNA insert was obtained from Dr Bill Britt (University of Alabama–Birmingham). The lysosome-associated membrane protein (LAMP)–targeting sequence ligated to pSP73/gp96ss/A64/Not was obtained from Dr Eli Gilboa (Miller School of Medicine, University of Miami). The PCR-generated pp65 full-length cDNA with SfiI ends was cloned into the vector pSP73/gp96ssLAMP/A64/Not Template DNA for in vitro transcription was linearized with restriction enzyme SpeI and purified using QIAquick PCR purification kit (QIAGEN). The gp96ss/pp65-LAMP/A64/mRNA was synthesized using the T7 mMessage mMachine RNA transcription kit (Ambion) according to the manufacturer's instructions. RNA was purified using RNeasy purification kit (Promega) according to the manufacturer's instructions.
In vitro generation of DCs
DCs were generated using the method of Romani et al30 and frozen in 80% human AB serum (HABS; Valley Biomedical) 10% DMSO (Bioniche Pharma), and 10% dextrose (Hospira). The cells were test thawed and checked for endotoxin, Mycoplasma, and bacterial and fungal contamination. DCs were assayed by FACS for presence of the DC markers CD11c and HLA-DR and the absence of the lineage markers CD3, CD14, CD19, and CD56. All fluorochrome antibodies were from BD Biosciences.
Analysis of human PBMCs
Human samples were obtained from individuals who had given written and informed consent, and peripheral blood mononuclear cells (PBMCs) were obtained and stained for FACS analysis as described previously.31,32 To determine the absolute number of TRegs and CD4+ T cells, the frequency of these populations as established by FACS analysis was multiplied by the absolute number lymphocytes as determined by CBC, white blood cell frequencies, and lymphocyte frequencies from the Duke University Clinical Laboratory.
Statistical analysis
Unless otherwise stated, an unpaired 2-tailed Student t test was used to determine statistical significance. Two-way and 3-way ANOVA with interaction was used to assess the effects of temozolomide, anti–IL-2Rα mAb, and vaccine on various immune measures and tumor volume. Parsimonious models were created by backward elimination. Pearson correlation was used to assess the association between TMZ dose and the levels of OVA-specific T cells. P values < .05 were considered significant. All figures shown are of representative experiments.
Results
Temozolomide as a lymphodepletive regimen
To determine a lymphodepletive dose (< 2000 cells/μL of peripheral blood) of TMZ in our murine model, mice were injected with increasing single doses (50-400 mg/kg) or multiday doses (20-80 mg/kg/5 days) of TMZ along with a separate cohort receiving lymphodepletive TBI (5 Gy; supplemental Table 1). Both 200 mg/kg TMZ single dose and 60 mg/kg/day TMZ for 5 days induced lymphopenia similarly to TBI (Figure 1A), significantly reduced the absolute numbers of CD4+and CD8+ T cells (supplemental Figure 1), and were considered lymphodepletive regimens.
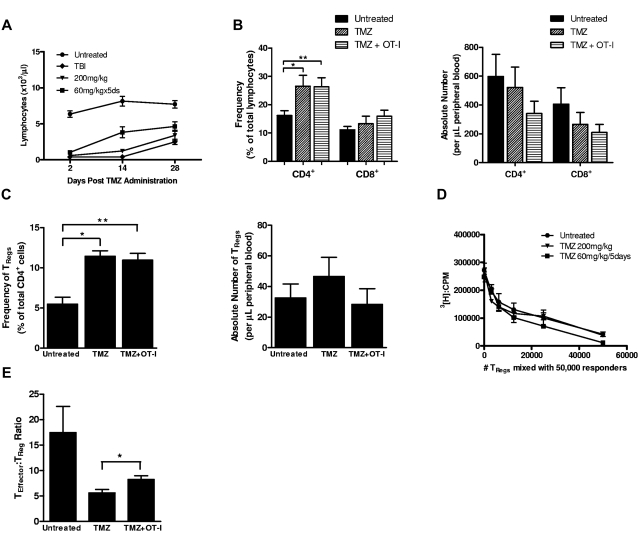
Figure 1.
Frequency of functional TRegs increases after lymphodepletive TMZ. (A) C57BL/6 mice were treated with 5 Gy of TBI (n = 3), single-dose TMZ (200 mg/kg; n = 5), or multiday TMZ (60 mg/kg/5 day; n = 5), and then CBCs were compared with untreated controls (n = 5). Total lymphocyte counts in peripheral blood were monitored over the course of 28 days; a representative experiment shown (n = 3). Postlymphodepletion values were evaluated for statistical significance (day 2): untreated vs 200 mg/kg TMZ, 60 mg/kg/5 day TMZ, and TBI, P < .0001 for all comparisons; and TBI vs 200 mg/kg TMZ and 60 mg/kg/5 day TMZ, P = .2042 and P = .0168, respectively. (B-C) The frequency and absolute number of CD4+ and CD8+ T cells and CD4+CD25+Foxp3+ TRegs in the peripheral blood of untreated (n = 5), lymphodepletive TMZ-treated (n = 5), and TMZ-lymphodepleted mice who also received OT-I transfer (n = 5) were monitored by FACS analysis 1 week after TMZ administration (frequency CD4+, *P = .0396 and **P = .0216; frequency TRegs, *P = .0006 and **P = .0019). For FACS analysis of all murine T-cell populations (supplemental Figure 11, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), total lymphocytes were first gated by side scatter and forward scatter. For CD4+ and CD8+ T-cell selection, total CD3+ T cells were selected from the lymphocyte gate by displaying side scatter by CD3+. CD4+ or CD8+ T cells were selected from the CD3+ T-cell population by displaying CD4+ by CD8+ and gating the desired population. For TRegs, lymphocytes were first gated by forward and side scatter, and from this gate CD4+ T cells were selected from side scatter. CD4+ T cells were displayed by CD25 versus Foxp3. For CD4+CD25+Foxp3+ TRegs, double-positive cells were selected and for CD4+Foxp3+ TRegs all Foxp3+ T cells were selected. Representative experiments are shown; all experiments performed in at least triplicate. (D) C57BL/6 mice were treated with 200 mg/kg single dose TMZ or 60 mg/kg/5day. Three days after the completion of TMZ treatment, CD4+CD25− responder T cells and CD4+CD25+ TRegs were isolated and cultured for 3 days in the presence of α-CD3e beads (all wells in triplicate). Proliferation was assessed after a 16-hour incubation with [3H]thymidine. (E) To determine the ratio of effector cells to TRegs, the absolute number of peripheral blood CD8+ cells was divided by the absolute number of peripheral blood TRegs (n = 5/cohort), ratios from representative experiments (B-C). *P = .0354.
CD4+CD25+Foxp3+ T-cell frequencies are elevated after lymphodepletive TMZ
To investigate the recovery from TMZ-induced lymphodepletion and to establish a model for evaluation of antigen-specific T cells, TMZ-treated mice were given an adoptive lymphocyte transfer of 2 × 107 naive splenocytes consisting of an equal proportion of OVA TCR transgenic splenocytes (OT-I) and wild-type C57BL/6 splenocytes. The absolute numbers of all assessed T-cell compartments in TMZ-treated mice were not altered by the addition of OT-I cells (Figure 1B-C), with mice exhibiting similar counts of CD4+, CD8+ (Figure 1B), CD4+Foxp3− (supplemental Figure 2), and CD4+CD25+Foxp3+ TRegs (Figure 1C). The frequencies of CD4+ T cells and TRegs were significantly increased by TMZ treatment, with the proportion of TRegs doubling after TMZ (P = .0006) or after TMZ + OT-I (P = .0019). This elevation in CD4+ T-cell frequency is attributable to changes in the TReg compartment, because analysis of CD4+Foxp3− T cells showed no significant increase in frequency after TMZ or TMZ + OT-I (supplemental Figure 2). The absolute numbers of CD4+ and CD8+ T cells remained depressed 1 week after TMZ (Figure 1B); however, the absolute numbers of TRegs were unchanged from untreated mice (Figure 1C). To determine whether TRegs were resistant to TMZ-mediated depletion or whether they simply recovered from depletion faster, the absolute numbers of CD4+ T cells and TRegs were examined early after TMZ treatment. Both T-cell subsets were significantly reduced (supplemental Figure 3A) with similar levels of depletion (supplemental Figure 3B), indicating that TRegs rebound faster than CD4+ T cells. Importantly, TRegs isolated after TMZ treatment were equivalently immunosuppressive in vitro, suggesting that lymphodepletive TMZ has no impact on the suppressive function of CD4+Foxp3+ TRegs (Figure 1D).
We also evaluated the impact of TMZ and TMZ + OT-I on the effector T cell to TReg (TEff:TReg) ratio, defined as the ratio of the absolute number of peripheral blood CD8+ T cells to TRegs, because decreased TEff:TReg ratios have been associated with reduced immune responses and diminished antitumor efficacy after immunotherapy.33 Although there was a small increase in the TEff:TReg ratio in the TMZ + OT-I cohort in comparison with TMZ alone, the TEff:TReg ratios in both the TMZ and TMZ + OT-I cohorts were reduced in comparison with untreated controls. Therefore, lymphodepletive TMZ could impair antitumor immunity due to both a decrease in circulating lymphocytes and because of an elevated proportion of TRegs (Figure 1E).
TMZ-mediated lymphodepletion enhances vaccine-induced immune responses despite increased frequencies of TRegs
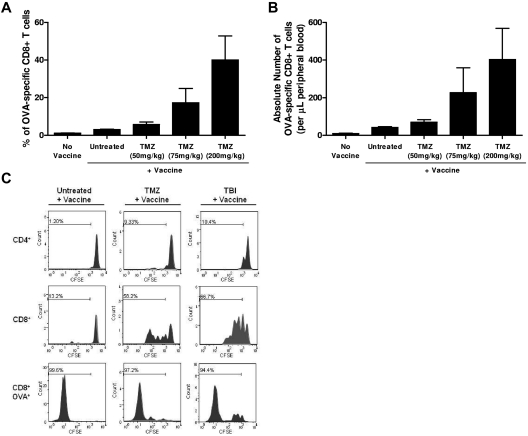
Because TMZ quickly increases the proportion of TRegs, and because TRegs have been shown to suppress lymphodepletion-induced homeostatic proliferation and vaccine-stimulated immunity,34,35 we next assessed the impact of TMZ treatment on vaccine-induced immunologic responses. C57BL/6 mice were given escalating doses of TMZ before OVA immunization. We found that the frequency and absolute number of vaccine-induced immunologic responses were increased in a dose-dependent manner in mice previously treated with TMZ (Figure 2A, Pearson correlation = 0.69, P = .0008; Figure 2B, Pearson correlation = 0.54, P = .014) and that this increase in T-cell expansion was observed in mice after receiving a single dose of TMZ or a multiday regimen of TMZ (supplemental Figure 4). These results indicate that TMZ-induced lymphopenia leads to increased vaccine-induced immunologic responses despite increased TReg proportions. To determine whether TMZ induces homeostatic proliferative responses in mice and to evaluate the relative kinetics of lymphopenia-induced homeostatic proliferation versus vaccine-induced proliferation, mice received CFSE-labeled OT-I T cells after lymphodepletive TMZ or 5 Gy of TBI and before vaccination. Proliferation was assessed by flow cytometric analysis of CFSE dilution 1 week after vaccination (Figure 2C). In both TMZ- and TBI-treated animals, proliferation was observed in the overall CD8+ and CD4+ compartments, with greater cell division (CFSE loss) seen in CD8+ T cells, demonstrating that the lymphodepletive environments induced by 5 Gy of TBI and TMZ induce similar levels of lymphopenia-induced homeostatic proliferation. To examine the proliferation of vaccine-induced T cells, CFSE dilution was examined within the OVA tetramer-positive CD8+ T-cell population. Vaccine-induced OVA-specific T cells, however, proliferated extensively, with > 94% of the cells exhibiting almost a complete loss of CFSE label (Figure 2C bottom panel). The vaccine-induced T cells proliferated to a similar extent in lymphopenic and normal hosts, demonstrating that the lymphopenic environment, while inducing homeostatic proliferation within the overall T-cell compartments, did not convey a proliferative advantage to vaccine-induced T cells. Cumulatively, the data demonstrate that despite increased TReg frequencies, TMZ-induced lymphopenia stimulates homeostatic proliferation and enhances vaccine-mediated immune responses.
Figure 2.
TMZ-mediated lymphodepletion induces homeostatic proliferation and enhances vaccine-induced immune responses. (A-B) Mice were given increasing doses of TMZ before vaccination as described in “Preclinical temozolomide treatment, OT-I transfer, vaccination and PC61 administration.” The frequency and absolute number of OVA-specific T cells were monitored in peripheral blood using an OVA-specific tetramer and anti-CD8 mAb. For all murine tetramer FACS analyses, cells were gated with the following strategy: total lymphocytes were gated from forward and side scatter, CD8+ T cells were selected from the lymphocyte gate, and the population of OVA+ T cells was selected from total CD8+ lymphocytes (supplemental Figure 11). No vaccine, n = 3; untreated, n = 5; 50 mg/kg TMZ, n = 5; 75 mg/kg TMZ, n = 5; and 200 mg/kg TMZ, n = 5. Increasing TMZ dose was associated with both increasing frequencies of OVA-specific T cells (Pearson correlation = 0.69; P = .0008) and increasing absolute numbers of OVA-specific T cells (Pearson correlation = 0.54; P = .014). This experiment was repeated twice with similar results. (C) Untreated (n = 3), TMZ-lymphodepleted (n = 3), or 5-Gy TBI-treated (n = 3) C57BL/6 mice received CFSE-labeled OT-I T cells and OVA vaccination. Proliferation of CD4+, CD8+, and CD8+OVA+ T cells was monitored by CFSE dilution. Representative FACS plots are shown.
Anti–IL-2Rα mAb administration synergizes with lymphopenia to deplete TRegs and potentiate immune responses
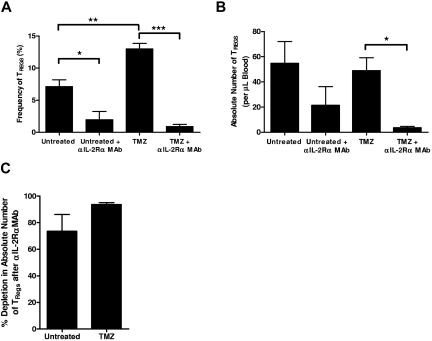
Although it has been proposed that lymphopenia augments immunotherapeutic protocols in part via depletion of inhibitory TRegs, our results demonstrate that immune responses are strongly potentiated after lymphodepletive TMZ despite an early increase in the frequency of functional CD4+CD25+Foxp3+ regulatory T cells and a decreased TEff:TReg ratio (Figure 1C-E). These data suggest that complementary TReg blockade strategies may synergize well with TMZ-induced lymphodepletion to further enhance immunotherapeutic antitumor immunity. To assess whether depletion or inhibition of TRegs in the context of TMZ treatment would further amplify vaccine-induced immunity, we examined the effect of anti–IL-2Rα mAb administration on TRegs in normal hosts and during TMZ-mediated lymphopenia. Untreated or lymphopenic mice were injected intraperitoneally with 10 mg/kg of the anti–IL-2Rα mAb PC61 concomitant with vaccination; peripheral blood TReg levels were assessed 1 week later. In both untreated (P = .0154) and TMZ-treated (P = .0001) mice, anti–IL-2Rα mAb administration caused a significant decrease in the frequency of remaining CD4+CD25+Foxp3+ TRegs (Figure 3A), and the absolute number was significantly depleted by anti–IL-2Rα blockade in TMZ-treated mice (P = .0025; Figure 3B). In addition, the percentage of depletion induced by anti–IL-2Rα mAb administration was not significantly different between TMZ-treated mice and controls (Figure 3C). Kohm et al36 have alternatively shown that in vivo injection of anti–IL-2Rα mAb causes down-regulation or shedding of IL-2Rα on TRegs rather than TReg depletion. To confirm that our anti–IL-2Rα mAb-induced reduction of CD4+CD25+Foxp3+ TRegs was not from IL-2Rα expression loss, we examined the impact of anti–IL-2Rα mAb administration on CD4+Foxp3+ TRegs. Similar to our data on CD4+CD25+Foxp3+ cells, the frequency and absolute number of CD4+Foxp3+ TRegs 1 week after PC61 and vaccination were reduced in both untreated and TMZ-treated cohorts, and the percentage of depletion induced by IL-2Rα blockade was not significantly different between untreated and TMZ-treated mice (supplemental Figure 5A-C). However, because work from our laboratory26 has shown that CD25− TRegs isolated after anti–IL-2Rα mAb administration are no longer functionally suppressive, we have continued to examine CD4+CD25+Foxp3+ TRegs as our primary population of interest.
Figure 3.
Inhibition of high-affinity IL-2Rα during lymphopenia depletes TRegs. (A-C) Untreated or TMZ-lymphodepleted C57BL/6 mice received OVA vaccination with or without concomitant αIL-2Rα mAb treatment (n = 5/group). One week after mAb administration and vaccination, mice were bled and CD4+CD25+Foxp3+ TReg levels in the peripheral blood were assessed by FACS analysis. Experiments performed in at least triplicate with similar results. (A-B) For frequency TRegs: *P = .0154, **P = .0027, and ***P = .0001. By 2-way ANOVA with interaction, the magnitude of effect of anti–IL-2Rα mAb was greater among TMZ-treated mice as opposed to mice not treated with TMZ (P = .0024). For absolute number of TRegs, *P = .0025. (C) In the untreated and TMZ-treated cohorts, the percentage depletion of CD4+CD25+Foxp3+ TRegs after αIL-2Rα mAb administration was determined from the absolute number of TRegs after αIL-2Rα mAb treatment in comparison with the absolute number of TRegs in the cohort that did not receive IL-2Rα blockade.
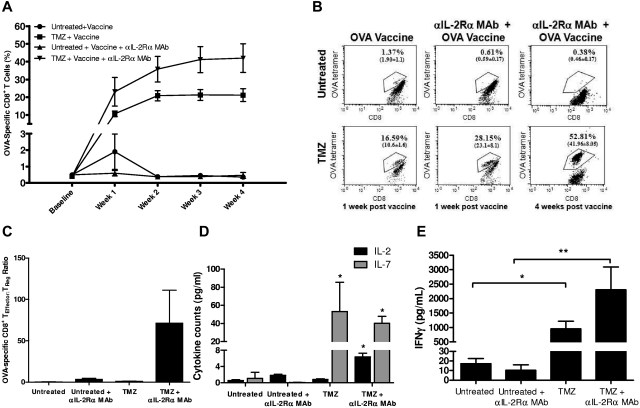
Although TRegs have been shown to be particularly dependent on the expression of IL-2Rα and consequent IL-2 signaling, it is important to note that activated T cells also transiently express the high-affinity IL-2R. Therefore, it is possible that PC61 administration may impair vaccine-induced antigen-driven effector responses in addition to depleting TRegs. To investigate this possibility, we analyzed the kinetics of OT-I expansion in untreated and TMZ-lymphodepleted hosts after simultaneous OVA vaccination and PC61 administration (Figure 4A-B). The vaccine-driven expansion of OT-I cells in untreated animals was effectively abrogated in the presence of IL-2Rα blockade. This corroborates the work of others showing the dependence of activated T cells on IL-2 signaling for fully realized immunologic responses and the impairment of these responses by anti–IL-2Rα blockade.18,19 In sharp contrast, the dramatic expansion of OT-I cells engendered by the lymphopenic environment was not abolished during anti–IL-2Rα mAb administration but was increased in both magnitude and duration over the observed period of time. Four weeks after immunization, the percentage of OT-I cells increased from 0.3% among untreated animals to 21% among TMZ only–treated animals (P = .0004). TMZ-treated animals receiving PC61 and vaccine had a 2-fold increase in the percentage of OT-I cells in comparison with vaccinated mice receiving TMZ only (P = .036) and greater than a 100-fold increase over untreated and vaccinated animals (P = .0009). Importantly, this led to increases in the TEff:TReg ratio for both CD4+CD25+Foxp3+ and CD4+Foxp3+ TReg populations in TMZ-treated animals (Figure 4C; supplemental Figure 6). These animals did not exhibit any signs of autoimmune toxicity during the observation period of 60 days (data not shown). Therefore, in the context of a lymphopenic setting, but not in a normal environment, administration of anti–IL-2Rα antibodies allows for the selective depletion of TRegs and enhancement of vaccine-stimulated effector T cells.
Figure 4.
Anti–IL-2Rα mAb blockade synergizes with TMZ-induced lymphodepletion to enhance antigen-specific immunity. (A) Forty-eight hours after TMZ treatment, lymphodepleted or untreated C57BL/6 mice received OVA vaccination with or without simultaneous anti–IL-2Rα mAb treatment (n = 5/group). Immune responses were monitored for 1 month by FACS analysis of peripheral blood OT-I T-cell levels. This experiment was repeated twice with similar results, and unpaired t tests were generated against the untreated + vaccine cohort; TMZ + vaccine and TMZ + vaccine + αIL-2Rα mAb cohorts were significantly different from untreated + vaccine for weeks 1 to 4. Baseline values were determined in untreated and TMZ-treated mice that did not receive vaccination. At week 4, the percentage of CD8+ T cells observed with TMZ significantly increased with the addition of anti–IL-2-Rα mAb (P = .036). (B) Representative FACS plots shown at 1 and 4 weeks after vaccination. (C) To determine the ratio of effector cells to TRegs, the absolute number of CD8+OVA+ effectors in peripheral blood 1 week after vaccination was assessed by FACS analysis with OVA+ tetramers and divided by the absolute number of CD4+CD25+Foxp3+ TRegs. (D) Peripheral blood was collected from untreated and TMZ-lymphodepleted (60 mg/kg/5 days) C57BL/6 mice treated with or without αIL-2Rα mAb 72 hours after the termination of TMZ administration. Cytokine levels in plasma were measured by Luminex according to manufacturer's instructions. For cytokine measurements, analysis of variance with interaction was conducted with TMZ and αIL-2Rα mAb as the main effects and their statistical interaction. TMZ + αIL-2Rα had a significant effect on IL-2 production (P = .0005), and TMZ alone had a significant effect on IL-7 production (P = .0001). Asterisks denote significance (P < .05) versus untreated. (E) Memory recall responses were evaluated 5 weeks after vaccination in treated mice by measurement of IFNγ secretion in peripheral blood lymphocytes using CBA. *P = .0082 and **P = .0198 by unpaired t test.
To investigate the impact of TMZ treatment on lymphocyte homeostatic cytokines in our model of lymphodepletive TMZ, we examined the levels of IL-2, IL-7, and IL-15 in the plasma of untreated or TMZ-lymphodepleted mice treated with or without anti–IL-2Rα mAb (Figure 4D). Plasma levels of IL-15 were unchanged; however, IL-7 levels were increased after TMZ treatment (analysis of variance, P = .0001) and IL-2 levels were increased after the combination of TMZ and anti–IL-2Rα mAb administration (analysis of variance with interaction, P = .0005). In vitro anti–IL-2Rα mAb administration has been shown previously to liberate IL-2, which may account for the up-regulation of IL-2 after lymphodepletion and IL-2Rα blockade.37 Therefore, the data indicate that in the context of TMZ-induced lymphopenia, the selective depletion of TRegs via IL-2Rα blockade simultaneous with vaccine-induced effector T-cell expansion may be mediated by an early surge in IL-7.
Previous studies also have demonstrated that although IL-2 signaling is dispensable during primary responses, it is essential for normal immunologic memory responses,38 thus prompting the possibility that immunologic memory responses may be significantly impaired in mice receiving PC61 treatment during vaccination. To investigate this possibility, the cytokine recall responses (IFNγ) of OVA-specific T cells were evaluated 5 weeks after vaccination in untreated and TMZ-treated mice. IFNγ responses were diminished in normal mice receiving IL-2Rα blockade but enhanced in mice receiving IL-2Rα mAb treatment during recovery from TMZ (Figure 4E), suggesting that the selective depletion of TRegs in TMZ-treated mice led to enhanced primary and memory T-cell responses.
IL-2Rα mAb blockade during lymphopenia effectively enhances antitumor efficacy in an established model of tumorigenesis
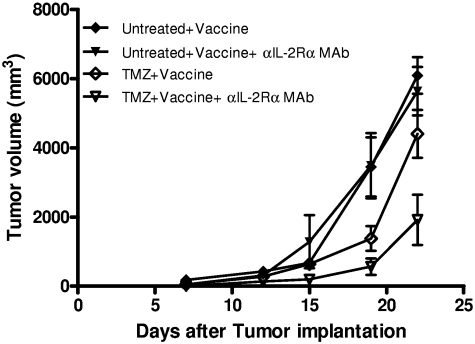
We and others have observed that TReg depletion via antibody-mediated blockade of IL-2Rα enhances antitumor efficacy most effectively in prophylactic rather than therapeutic settings,26,33,39 potentially because of the simultaneous inhibition of physiologic or vaccine-induced antitumor immune responses by anti–IL-2Rα mAbs when used against established tumors.18 To determine whether PC61 administration in the context of recovery from TMZ-induced lymphopenia could mediate significant improvements in therapeutic antitumor immunity, we assessed the efficacy of OVA vaccination on established B16/F10.9-OVA tumors (Figure 5; supplemental Figure 7). Mice were subcutaneously inoculated with tumor cells 3 days before lymphodepletive TMZ treatment and 1 week before initial immunization and PC61 administration, with additional vaccinations occurring on days 12 and 17 after tumor implantation. Mice were assessed for tumor burden throughout the course of the experiment. On day 22 after implantation, tumors reached maximal size in untreated mice, and the experiment was terminated for end point evaluation. TMZ in combination with vaccine significantly reduced the tumor volume (P = .0015), and the addition of PC61 only in the context of TMZ and vaccine provided a further significant reduction in tumor volume (P = .0035; Figure 5; supplemental Figure 7). TMZ treatment alone or vaccine alone did not impair tumor growth in comparison with untreated tumor-bearing mice (P = .1922 and P = .3343, respectively; supplemental Figure 7). Administration of PC61 alone in untreated or TMZ-treated mice had no effect on tumorigenesis (P = .3343 and P = .5515, respectively; supplemental Figure 7) in comparison with controls, indicating that the systemic depletion of TRegs in normal or lymphopenic mice with established tumor did not impair tumor growth. These results offer a striking parallel to our immune response data and indicate that IL-2Rα blockade during lymphopenia uniquely enables the selective depletion of TRegs to augment vaccine-induced effector T-cell function and potentiate antitumor immunotherapy in an established model of tumorigenesis.
Figure 5.
Administration of anti–IL-2Rα mAb during lymphopenia augments vaccine-induced antitumor efficacy. B16/F10.9-OVA tumors were implanted subcutaneously in C57BL/6 mice, and TMZ was administered 3 days later. One week after implantation, cohorts of mice received vaccination with or without αIL-2Rα mAb administration. Mice were additionally vaccinated on days 12 and 17 after implantation (n = 8 per group). Statistical significance by unpaired t test on day 22 (maximal tumor burden in untreated mice): untreated + vaccine versus TMZ + vaccine, P = .0729; untreated + vaccine + αIL-2Rα mAb versus TMZ + vaccine + αIL-2Rα mAb, P = .0024; and TMZ + vaccine versus TMZ + vaccine + αIL-2Rα mAb, P = .0268).
Impact of anti–IL-2Rα mAb administration in TMZ-treated patients with GBM receiving targeted immunotherapy
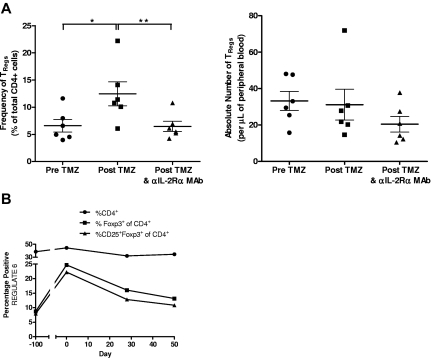
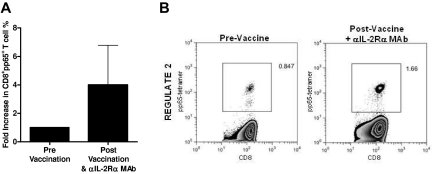
Because of these preclinical findings, we assessed the potential of a single intravenous dose of a humanized anti–IL-2Rα mAb (daclizumab) to reduce or eliminate TRegs and augment vaccine-induced antitumor immunity in a pilot study of patients with newly diagnosed GBM receiving adjuvant TMZ therapy (“REGULATE” Protocol, Food and Drug Administration IND-BB-12839; Duke Institutional Review Board Protocol 0581). Six patients were treated in this pilot study (demographic data shown in Table 1). On day 21 ± 2 of the first TMZ cycle (during lymphocyte nadirs), patients received 1 mg/kg daclizumab, adoptive transfer of 3 × 107/kg naive lymphocytes harvested during leukapheresis to serve as a responder population to vaccination, and a vaccine consisting of 2 × 107 DCs electroporated with CMV pp65 RNA. Patients also received 2 biweekly RNA-pulsed DC vaccinations and a fourth vaccine on day 21 ± 2 after their second TMZ cycle. In parallel to our findings in mice, patients displayed a significantly increased frequency of CD4+CD25+Foxp3+ TRegs after TMZ treatment (Figure 6A; P = .0236). After daclizumab administration, the frequency of TRegs was significantly reduced (P = .0061) in treated patients, with absolute numbers decreasing by ∼ 34% (P = .1494). Examination of CD4+Foxp3+ TRegs revealed the same trends, with TReg frequency increased after TMZ (P = .0239) and decreased after daclizumab (P = .0160) and with the absolute number reduced after daclizumab by 25% (P = .3461; supplemental Figure 8). The kinetics of TReg depletion revealed that TReg levels spiked after the first cycle of TMZ but dropped rapidly after anti–IL-2Rα treatment (day 0) without returning to baseline over a 50-day period of observation (Figure 6B; supplemental Figure 9). Importantly, overall CD4+ and CD4+Foxp3− T cells experienced no reduction in absolute number or frequency after daclizumab administration in comparison with post-TMZ levels, indicating that anti–IL-2Rα mAb administration during TMZ-induced lymphopenia selectively eliminates TRegs, as was observed in our murine studies (supplemental Figure 10). IL-2Rα mAb blockade did not prevent our capacity to enhance immunologic responses, because we found that 4 of 6 patients displayed an increase in pp65-specific T cells after vaccination with a mean 4-fold increase in tetramer-positive CD8+ T cells (Figure 7). The administration of daclizumab in combination with DC vaccination was well tolerated without any adverse events associated with immunotherapy, and although clearly not powered for a clinical end point, 4 of 6 patients treated have experienced progression-free survival times exceeding 24 months, warranting further exploration of the safety and efficacy of this treatment strategy in larger clinical trials (Table 1). The cumulative clinical data parallel our preclinical work and strongly suggests that anti–IL-2Rα mAb treatment in TMZ-treated patients with GBM may enhance vaccine-driven antitumor immunity through a selective reduction in immunosuppressive regulatory T cells.
Table 1.
Characteristics, progression-free survival, and overall survival of patients in the REGULATE Trial
| Patient | Age (y) | Karnofsky performance score | Recursive partitioning analysis class | Progression-free survival, mo (surgery) | Overall survival, mo (surgery) | Progression-free survival, mo (vaccine 1) | Overall survival, mo (vaccine 1) |
|---|---|---|---|---|---|---|---|
| 1 | 43 | 100 | III | > 42.0 | > 42.0 | > 38.0 | > 38.0 |
| 2 | 45 | 100 | III | 7.2 | 22.1 | 2.8 | 17.7 |
| 3 | 55 | 100 | IV | 5.4 | 15.5 | 1.8 | 11.8 |
| 4 | 49 | 90 | III | 27.2 | > 35.1 | 23.5 | > 31.5 |
| 5 | 20 | 80 | IV | > 27.5 | > 31.1 | > 24.3 | > 27.9 |
| 6 | 62 | 90 | IV | > 26.4 | > 26.4 | > 22.8 | > 22.8 |
| Median | 47 | 95 | 27.2 | Not reached | 23.5 | Not reached |
Figure 6.
Patients with GBM possess an increased frequency of TRegs after TMZ treatment that can be reduced by a single administration of an anti–IL-2Rα mAb. (A-B) Percentages and absolute numbers of TRegs (CD4+CD25+Foxp3+) from leukapheresis (pre-TMZ and post-TMZ and daclizumab) and peripheral blood (post-TMZ) samples were determined by CBC counts and FACS analysis. TReg levels from preoperative samples (pre-TMZ, ∼ day − 100), before initial vaccination and daclizumab (post-TMZ, day 0) and ∼ 7 weeks after vaccination and daclizumab (post-TMZ and daclizumab, day ∼ 50) were assessed. (A) Frequency of TRegs by paired t test, *P = .0236 and **P = .0061. (B) Total CD4+, CD4+Foxp3+, and CD4+CD25+Foxp3+ T-cell frequencies from leukapheresis and peripheral blood samples from a representative patient were determined before and after daclizumab administration (day 0). Gating strategies for flow cytometric analyses were as follows and as shown in supplemental Figure 12: (1) For CD4+ T cells, all cells were displayed using forward and side scatter, and the lymphocyte population was selected. Lymphocytes were then displayed by side scatter and CD4 on a dot plot. The CD4+ population was then selected out of this lymphocyte population. (2) For Foxp3+ of CD4+, an identical gating strategy as described in 1 was used to select CD4+ T cells. The CD4+ T cells were then displayed by dot plot against Foxp3, and all Foxp3+ cells were selected. (3) For CD25+Foxp3+ of CD4+, an identical gating strategy as described in (1) was used to select the CD4+ population. The selected CD4+ population was then displayed by dot plot as CD25 versus Foxp3. CD25 and Foxp3 double-positive cells were then selected out of the CD4+ population.
Figure 7.
Anti–IL-2Rα mAb blockade does not prevent the vaccine-induced expansion of antigen-specific CD8+ T cells. CMV-pp65–specific CD8+ T cells were determined by flow cytometric analysis of PBMCs both before (pre) and 43 to 55 days after (post) administration of CMV-pp65 DC vaccine and daclizumab. (A) Data are shown as the average fold increase (post/pre) in the frequency of pp65+CD8+ T cells from 4 REGULATE patients (individual patient average of tetramer+CD8+ T cells across tested HLA alleles). (B) Representative FACS plot of pp65-tetramer+CD8+ T cells. Gating strategy: total lymphocytes selected from forward scatter and side scatter display, CD3+CD8+ T cells selected from total lymphocytes, and pp65+ T cells selected from total CD3+CD8+ population (supplemental Figure 12).
Discussion
We report that TMZ has lymphopenic side effects that can be potently leveraged to improve immunotherapy as determined by both immune responses and therapeutic antitumor efficacy. We also demonstrate that improved antitumor immunity occurs despite an increased proportion of immunosuppressive TRegs and that the dramatic potentiation of vaccination by TMZ-induced lymphopenia can itself be significantly heightened through the concomitant depletion of TRegs by anti–IL-2Rα mAb treatment. High-affinity IL-2R antibodies administered during lymphodepletion selectively deplete TRegs to promote vaccine-stimulated immune responses and inhibit malignant growth. Although these results need confirmation in larger patient studies, we demonstrate that anti–IL-2Rα mAb administration in TMZ-treated patients also selectively depletes regulatory T cells while permitting the expansion of vaccine-induced effector T cells.
The significance of these observations are substantial, suggesting that the treatment of GBM and metastatic melanoma with TMZ provides a clinically relevant therapeutic window that can be manipulated to promote antitumor immunity. Of additional import is that TMZ-induced lymphopenia actually increases regulatory T cells in mice and humans, prompting us to examine the efficacy of tumor vaccination during combinatorial lymphopenia and systemic TReg depletion. In normal animals, our work and the work of others demonstrates that IL-2Rα blockade will impair immune activation, probably because of the transitory expression of IL-2Rα on activated T cells during priming.18,19,40 However, our data demonstrate that although TRegs are depleted or functionally inhibited by anti–IL-2Rα mAbs during both normal and lymphopenic conditions, it is only during lymphodepletion that anti–IL-2Rα mAb treatment does not impair immune responses but in fact strongly accentuates them while simultaneously inhibiting suppressive TRegs.
Several mechanisms may account for the selective depletion of TRegs by anti–IL-2Rα mAbs during lymphopenia. In our model of lymphodepletive TMZ, early elevation of IL-7 cytokine levels (Figure 4D) may permit effector T cells to expand in the absence of IL-2–mediated signaling, whereas TRegs will remain dependent on IL-2 for survival and proliferation. Alternatively, an indirect mechanism of daclizumab suppression mediated through CD56bright regulatory natural killer cells may be bypassed through the depletion of these cells during lymphodepletive regimens.41 Importantly, the above-mentioned hypotheses are not dependent on any unique properties of TMZ, and theoretically, the differential effects of anti–IL-2Rα mAbs during lymphopenia should hold true for other methods of lymphodepletion as well.
The anti–IL-2Rα mAbs basiliximab and daclizumab are already used clinically as immunosuppressants. Although the adoptive transfer of T cells depleted of TRegs possess enhanced antitumor efficacy in lymphopenic animals,42 TRegs may quickly regenerate,43,44 and a method to systemically deplete TRegs during successive rounds of chemotherapy, such as IL-2Rα–specific mAbs, would have high clinical applicability.45 Thus, our cumulative preclinical and clinical data demonstrate that the administration of anti–IL-2Rα mAbs during lymphopenia is not only a novel theoretical concept but also a broadly applicable and clinically relevant therapeutic modality for the potent enhancement of antitumor immunotherapy.
Supplementary Material
Acknowledgments
The authors thank the staff who supported this study, including Denise Lally-Goss, Sharon McGehee-Norman, and Beth Perry.
This work was supported by grants from the National Institutes of Health National Institute of Neurological Disorders and Stroke Specialized Program of Research Excellence in brain cancer (P50CA108786; D.D.B. and J.H.S.), the National Brain Tumor Society (D.A.M. and J.H.S.), the American Brain Tumor Association (D.A.M.), the Accelerate Brain Cancer Cure Foundation Young Investigator's Award (D.A.M.), and in part by Duke University's Clinical & Translational Science Awards grant 1UL2 RR024128-01 from the National Institutes of Health National Center for Research Resources.
Footnotes
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.A.M., L.S.-P., X.C., and R.J.S. conceived and designed experiments; D.A.M., X.C., L.S.-P., R.J.S., D.J.S., G.E.A., A.D., A.H.F., H.S.F., J.E.H., R.E.M., D.A.R., and J.J.V. performed experiments; J.H.S., D.A.M., L.S.-P., X.C., R.J.S., G.E.A., J.E.H., and K.L.C. performed data analysis and interpretation; J.H.S., D.D.B., D.A.M. contributed reagents and tools; and D.A.M. and K.L.C. wrote the paper.
Conflict-of-interest disclosure: D.A.M. and J.H.S. have patents pending related to technology disclosed in this manuscript. D.A.M. has served as a paid member of the Schering Plough North American Investigators Advisory Board. D.A.R. has served as paid speaker for Schering/Merck and Genentech/Roche. The remaining authors declare no competing financial interests.
Correspondence: John H. Sampson, Duke Brain Tumor Immunotherapy Program, Division of Neurosurgery, Department of Surgery, The Preston Robert Tisch Brain Tumor Center at Duke, Box 3050, 220 Sands Bldg, Research Dr, Duke University Medical Center, Durham, NC 27710; e-mail: john.sampson@duke.edu.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 3.Berger C, Turtle CJ, Jensen MC, Riddell SR. Adoptive transfer of virus-specific and tumor-specific T cell immunity. Curr Opin Immunol. 2009;21(2):224–232. doi: 10.1016/j.coi.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehrotra S, Mougiakakos D, Johansson CC, Voelkel-Johnson C, Kiessling R. Oxidative stress and lymphocyte persistence: implications in immunotherapy. Adv Cancer Res. 2009;102:197–227. doi: 10.1016/S0065-230X(09)02006-5. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat Clin Pract Oncol. 2006;3(12):668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui Y, Zhang H, Meadors J, Poon R, Guimond M, Mackall CL. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood. 2009;114(18):3831–3840. doi: 10.1182/blood-2009-03-212134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asavaroengchai W, Kotera Y, Mule JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A. 2002;99(2):931–936. doi: 10.1073/pnas.022634999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dummer W, Niethammer AG, Baccala R, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110(2):185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11(11):1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarzberg AB, Stover EH, Sengupta T, et al. Selective lymphopenia and opportunistic infections in neuroendocrine tumor patients receiving temozolomide. Cancer Invest. 2007;25(4):249–255. doi: 10.1080/07357900701206380. [DOI] [PubMed] [Google Scholar]
- 14.Wick W, Weller M. How lymphotoxic is dose-intensified temozolomide? The glioblastoma experience. J Clin Oncol. 2005;23(18):4235–4236. doi: 10.1200/JCO.2004.00.8417. author reply 4236. [DOI] [PubMed] [Google Scholar]
- 15.Gajewski TF. Temozolomide for melanoma: new toxicities and new opportunities. J Clin Oncol. 2004;22(4):580–581. doi: 10.1200/JCO.2004.11.957. [DOI] [PubMed] [Google Scholar]
- 16.Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22(4):610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 17.Kim TG, Kim CH, Park JS, et al. Immunological factors relating to the antitumor effect of temozolomide chemoimmunotherapy in a murine glioma model. Clin Vaccine Immunol. 2010;17(1):143–153. doi: 10.1128/CVI.00292-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtin JF, Candolfi M, Fakhouri TM, et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS One. 2008;3(4):e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs JF, Punt CJ, Lesterhuis WJ, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res. 2010;16(20):5067–5078. doi: 10.1158/1078-0432.CCR-10-1757. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell DA, Xie W, Schmittling R, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10(1):10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cobbs CS, Harkins L, Samanta M, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62(12):3347–3350. [PubMed] [Google Scholar]
- 22.Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008;116(1):79–86. doi: 10.1007/s00401-008-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prins RM, Cloughesy TF, Liau LM. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med. 2008;359(5):539–541. doi: 10.1056/NEJMc0804818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Söderberg-Nauclér C. HCMV microinfections in inflammatory diseases and cancer. J Clin Virol. 2008;41(3):218–223. doi: 10.1016/j.jcv.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Porgador A, Feldman M, Eisenbach L. H-2Kb transfection of B16 melanoma cells results in reduced tumourigenicity and metastatic competence. J Immunogenet. 1989;16(4–5):291–303. doi: 10.1111/j.1744-313x.1989.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 26.Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12(14 Pt 1):4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. Oncology tools: dose calculator. [Accessed April 12, 2011]. http://www.accessdata.fda.gov/scripts/cder/onctools/animalquery.cfm.
- 28.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50(4):219–244. [PubMed] [Google Scholar]
- 29.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 30.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180(1):83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 32.Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205(9):2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winstead CJ, Fraser JM, Khoruts A. Regulatory CD4+CD25+Foxp3+ T cells selectively inhibit the spontaneous form of lymphopenia-induced proliferation of naive T cells. J Immunol. 2008;180(11):7305–7317. doi: 10.4049/jimmunol.180.11.7305. [DOI] [PubMed] [Google Scholar]
- 35.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohm AP, McMahon JS, Podojil JR, et al. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176(6):3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 37.McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci U S A. 2011;108(18):7529–7534. doi: 10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441(7095):890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, et al. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32(2):266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Waldmann TA. The IL-2/IL-15 receptor systems: targets for immunotherapy. J Clin Immunol. 2002;22(2):51–56. doi: 10.1023/a:1014416616687. [DOI] [PubMed] [Google Scholar]
- 41.Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl Acad Sci U S A. 2004;101(23):8705–8708. doi: 10.1073/pnas.0402653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kline J, Brown IE, Zha YY, et al. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14(10):3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 43.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201(5):723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neujahr DC, Chen C, Huang X, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176(8):4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 45.Morse MA, Hobeika AC, Osada T, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112(3):610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.