Abstract
Reduced-calorie diets (RCD) are difficult to follow, because they often require elimination of certain foods, leading to poor compliance and limited success. However, a low-calorie nutrient-dense diet has the potential to accommodate a daily snack without exceeding energy requirements, even during weight loss. This pilot study evaluated the effects of a RCD including either a daily dark chocolate snack (DCS) or a non-chocolate snack (NCS) on anthropometric and body composition measurements. In a randomized clinical trial, 26 overweight and obese (body mass index ≥25 to ≤43 kg/m2) premenopausal women were assigned to a RCD that included either a daily DCS or NCS (n=13/group) for 18 weeks. At baseline and end of study, body weight (BW, kg) and waist and hip circumferences (cm) were measured, along with fat mass (FM, kg), lean mass (LM, kg), and body fat percentage (BF%) by dual-energy X-ray absorptiometry. Energy and macronutrient intakes were estimated from four-day food records. Within and between group changes from baseline were analyzed using paired t-tests and independent t-tests, respectively. Women in both snack groups reduced estimated daily energy intake (p<0.001). Women in both the DCS and NCS groups, respectively, experienced decreases (p<0.001) in BW (−5.1 vs. −5.1 kg), hip (−5.8 vs. −5.4 cm) and waist circumferences (−5.7 vs. −3.5 cm), FM (−3.9 vs. −3.6 kg) and BF% (−3.4 vs. −3.1 %), with no change in LM. Improvements in anthropometric and body composition measurements among overweight and obese premenopausal women can be achieved with a RCD including either a daily DCS or NCS. A reduced-calorie dietary pattern including a daily sweet snack promotes body weight reduction and body composition improvements in premenopausal women who are overweight and obese: a pilot study
Keywords: obesity, overweight, premenopausal women, randomized clinical trial, snack, weight loss
INTRODUCTION
Based on cross-sectional observations of adults in the United States, it estimated that one in three individuals is trying to lose weight (1). In spite of this, sixty-eight percent of American adults are overweight or obese (2) suggesting that current weight-loss interventions are only modestly effective at helping individuals achieve and sustain a healthy weight status (3). The most commonly cited cause of unsuccessful weight loss during active intervention and long-term maintenance of weight loss is poor dietary compliance (4,5). Specifically, diets that promote rapid weight loss are challenging, because they typically require dramatic alterations in usual dietary intake leading to confusion and/or an inability or unwillingness to fully comply in the short-term (6,7). Long-term maintenance of restrictive diets is tenuous, and many individuals regain weight over time (8,9) and/or enter into patterns of weight-cycling (10,11) which present additional pathways for adverse health outcomes (12,13). Nutrition interventions that emphasize healthy lifestyle choices, include flexible dietary patterns, and encourage moderate weight reduction may be more compatible with short-term weight loss, successful long-term weight loss maintenance and reduction in risk of chronic disease (5).
Individuals who consume a nutrient-dense dietary pattern (rich in high-fiber whole grains, vegetables and fruits, and non-fat fluid milk, moderate in lean proteins) can meet their daily nutrient intake needs with additional energy remaining within their daily caloric recommendations (14). Using these “extra” kilocalories to incorporate a favorite food or snack into an energy-controlled dietary pattern could improve diet satisfaction, thereby lowering the perception of restriction, and increasing the likelihood of long-term adherence (15). Dietary compliance over a longer period of time can lead to greater weight loss (4,16) and weight stability (17) contributing to sustained improvements in health status (5,17,18).
Incorporating snacks between meals can be appropriate for both appetite and weight control when the energy content of the snack is moderated (19). When asked about their favorite snack, approximately 40% of women in the United States responded that chocolate is their sweet treat of choice (20). Chocolate is favored because of its pleasant sensory properties and ability to temporarily elevate mood (21). The overconsumption of any calorically-dense sweet treat can potentially lead to weight gain; therefore, women often abstain from chocolate and other sweet snacks when trying to lose weight. Unfortunately, restriction of a particular food can induce cravings and may lead to an undesirable rebound effect of increased or uncontrollable consumption when the particular food is reintroduced (22,23). Because chocolate is a highly desired snack, examining whether women can include a moderate amount of chocolate into a daily reduced-calorie diet (RCD) is important. Further, understanding whether chocolate elicits unique effects when incorporated into a RCD requires comparison to a sweet non-chocolate snack.
Therefore, this randomized clinical trial aimed to test the feasibility of purposefully including two different types of energy-controlled daily sweet snacks into a RCD on selected outcomes. The objective of this pilot study was to examine whether an energy-controlled dark chocolate snack (DCS) versus a non-chocolate snack (NCS) produced differential changes in anthropometric and body composition measurements when incorporated into a RCD over 18 weeks. It was hypothesized that premenopausal women who were overweight or obese and who followed a RCD with either a DCS or NCS incorporated into daily intake could maintain an energy deficit and experience significant positive changes in anthropometric and body composition measurements, despite the type of sweet snack consumed.
METHODS
Participants and Recruitment
The research was advertised as an 18-week weight loss study using posted flyers and electronic-mail notices. One hundred thirty-seven women responded to advertisements, of which 33 were eligible and completed baseline measurements (DCS, n=17; NCS, n=16). Remaining women did not meet eligibility criteria, return required forms or maintain interest in participating in the study. This was a pilot sample of women to test feasibility for a future, larger trial.
Enrolled participants were premenopausal women, aged 25 to 45 years, who were overweight or obese (body mass index ≥25 to ≤43 kg/m2). Women were eumenorrheic, engaged in <5 hours of physical activity per week and were weight stable during the 6 months before the study. Exclusion criteria included presence of metabolic disorders or chronic diseases, such as cardiovascular, renal, liver, and bone diseases. This study was approved by the Institutional Review Board for Research Involving Human Subjects at The Pennsylvania State University. Each participant provided written informed consent before entry into the study.
Data were collected before the dietary intervention (baseline) and after 18 weeks (February–July 2009) of the RCD (week 18). Women were compensated $80 dollars at the end of the study for their participation.
Dietary Intervention
After stratification by baseline age, body mass index and physical activity, participants were randomly assigned to either the weight loss with DCS or weight loss with NCS group. Participants in both groups followed a RCD designed to induce a 2-pound weight loss per week with a macronutrient composition of approximately 50% carbohydrate, 30% fat, and 20% protein. After baseline testing, energy intake levels were set at 1500, 1600, 1700, or 1800 kcal/day for each woman, using the Harris-Benedict equation (24).
Participants in the DCS group consumed one dark chocolate tasting square (Hershey’s® Extra Dark, 60% cacao, The Hershey Company, Hershey, PA) at two intervals each day (90 kcal/day) and one, 8-ounce sugar-free cocoa beverage (The Hershey Company) at the first meal of the day (65 kcal/day) as part of the RCD. Participants in the NCS group consumed a non-chocolate sweet snack of fruit-flavored licorice (The Hershey Company) at two intervals each day (90 kcal/day) along with one, 8-ounce sugar-free non-cocoa beverage (The Hershey Company) at the first meal of the day (65 kcal/day) as part of the RCD. Throughout the study, women refrained from consuming any cocoa or chocolate products unless part of the RCD. Snacks and beverages, as noted above, were provided to participants as part of the study protocol.
Participants were instructed on a food exchange system and portion sizes that represented exchanges from each of six exchange groups. Handouts with food choices, dietary patterns and menu plans were provided. For 18 weeks, women in both groups attended weekly nutrition education sessions that included lessons on basic nutrition knowledge, food purchasing and preparation, portion size moderation, recipe modification, and eating away from home. Compliance with assigned snack intervention was assessed using forms on which women self-recorded weekly intake of snacks and beverages and concurrent confirmation by an investigator via product counts. Compliance was defined as intake of ≥85% of weekly snacks and beverages.
Dietary Measurements
To assess change in food and nutrient intakes, participants completed 4-day food records at baseline and week 18. Participants recorded food and beverage intake on 3 weekdays and 1 weekend day in the week before data collection sessions. Food records were analyzed with the Food Processor® dietary analysis software (version 10.6.0, 2010, esha Research, Salem, OR) to estimate average daily intake of total energy (kcal), and carbohydrate, protein, and fat (% of total kcal).
Physical Activity Record
At baseline and week 18, participants completed the Stanford 7-day Physical Activity Recall Scale (25). For each day of the week before data collection sessions, participants reported the approximate number of hours they slept, worked on the computer, watched television and spent in moderate, hard, and very hard activity from which kcals/day expended were estimated. Women were asked to maintain baseline level of physical activity throughout the 18-week intervention.
Anthropometric Measurements
At baseline and week 18, standing height was measured with a stadiometer (Seca 700, Seca North America East, Hanover, MD) to the nearest 0.1 cm, and fasting (overnight) body weight (BW) was measured to the nearest 0.1 kg using an electronic scale (TBF-410GS, Tanita Corporation, Arlington Heights, IL). Two measurements of the waist and hip were taken to the nearest 0.1 cm using an adjustable tape measure and then averaged. Waist circumference was measured at the narrowest point of the waist approximately 1 inch above the belly button and the hips were measured at the widest part of the buttocks. Participants wore lightweight clothing and were shoeless during measurements.
Soft Tissue Mass Measurements
Dual-energy X-ray absorptiometry (QDR 4500A, Hologic, Inc., Bedford, MA) was used to measure body composition at baseline and week 18. Total body scans were analyzed to determine fat mass (kg), lean mass (LM; kg) and body fat percentage. Participants were scanned in a supine position using standard protocols. All scans were completed by one technician to eliminate inter-tester variation. Quality control scans were completed daily, and the coefficients of variation for LM and fat mass were 1.02%, and 1.87%, respectively.
Statistical Analyses
Study participants were characterized using descriptive statistics (means ± SD). One-way analysis of variance was used to assess differences in all characteristics between snack groups at baseline. Statistical analyses were conducted with only those women who completed both baseline and week 18 measurements.
Changes within and between snack groups from baseline to week 18 were analyzed using paired t-tests, and independent t-tests, respectively, for anthropometric and body composition measurements, estimated dietary intake, snack and beverage compliance, and physical activity. Statistical tests were two-sided with significance set at p<0.05. Data analyses were conducted using the Statistical Package for the Social Sciences (version 17.0, 2008, SPSS Inc., Chicago, IL).
RESULTS AND DISCUSSION
Subject Characteristics
Of the 33 women who began the study, a total of 26 women (DCS, n= 13; NCS, n=13), with a mean age of 36.5 ± 4.9 years, completed the 18-week intervention. There were no differences in age, body mass index, or activity level between the study-completers and dropouts or between diet groups at baseline (Table).
Table.
Baseline and week 18 characteristics of study participants by snack group
| Dark Chocolate Snack (DCS) |
Non-Chocolate Snack (NCS) |
|||||
|---|---|---|---|---|---|---|
| Baseline n=13 |
Week 18 n=13 |
Baseline n=13 |
Week 18 n=13 |
|||
| Characteristic | Mean ± SD | Mean ± SD | Mean Change |
Mean ± SD | Mean ± SD | Mean Change |
| Age (years) | 36.3 ± 4.9 | 37.3 ± 4.8 | ||||
| Height (cm) | 163.3 ± 2.8 | 164.7 ± 6.9 | ||||
| Weight (kg) | 84.3 ± 16.2 | 79.2 ± 17.9 | −5.1*** | 83.1 ± 13.4 | 78.0 ± 4.3 | −5.1*** |
|
Body mass index (kg/m2) |
31.5 ± 5.4 | 29.6 ± 6.0 | −1.9*** | 30.6 ± 4.0 | 28.7 ± 4.9 | −1.9** |
|
Estimated daily energy intake (kcal/d) |
1939 ± 334 | 1496 ± 339 | −444* | 2092 ± 473 | 1462 ± 216 | −631*** |
|
Carbohydrate (% of total energy) |
48.5 ± 8.3 | 49.2 ± 3.6 | +0.7 | 52.0 ± 7.7 | 52.1 ± 8.5 | +0.1 |
|
Fat (% of total energy) |
34.3 ± 7.6 | 34.0 ± 6.4 | −0.3 | 31.1 ± 6.4 | 30.4 ± 9.0 | −0.6 |
|
Protein (% of total energy) |
15.8 ± 2.2 | 15.5 ± 2.4 | −0.3 | 14.9 ± 2.6 | 17.2 ± 3.3 | +2.3* |
p<0.05,
p<0.01,
p<0.001
p-value analyzed using paired t-tests for within snack group changes from baseline. There were no significant differences between snack group in changes in anthropometric measurements or estimated dietary intakes.
Snack Compliance and Educational Session Attendance
Compliance with snack and beverage intakes, respectively, was 93.2% and 93.0% for the DCS group and 94.7% and 94.9% for the NCS group. Class attendance for the DCS group was 78.0% and for the NCS group was 73.1%. Compliance and class attendance did not significantly differ between groups.
Estimated Dietary Intake Data
Mean estimated energy intake at baseline was 1939 kcals and 2092 kcals for the DCS and NCS group, respectively (Table). Macronutrient composition of intakes in the DCS and NCS groups, respectively, were 48.5% carbohydrate, 34.3% fat, and 15.8% protein and 52.0% carbohydrate, 31.1% fat, and 14.9% protein (not significantly different). At week 18, the DCS group significantly reduced estimated daily energy intake by 444 kcals (p<0.01). The NCS group significantly reduced estimated daily energy intake by 631 kcals (p<0.01) and significantly increased estimated dietary protein intake by 2.3% (p<0.05). The assigned snack and beverage mix accounted for 6.1% and 3.0% of estimated daily kcals, respectively, for a total of 9.1%, within the 5–15% range of kcals reported as available for non-essential or “extra” kcals by the 2010 Dietary Guidelines Advisory Committee (14). Estimated energy and macronutrient intake did not differ significantly between groups at baseline or week 18.
Physical Activity
Estimated physical activity did not significantly differ between DCS or NCS groups at baseline or week 18. Estimated energy expended during daily tasks and physical activity significantly decreased (p<0.01) from baseline to week 18 for all women and within each group. Change in physical activity may largely be explained by the seasonal change from the beginning of the study (summer) to week 18 (winter). This change also may partially explain the discrepancy between the estimated reduction in energy intake (~445 to ~630 kcals/day) and the magnitude of weight loss over the 18-week intervention (~5.1 kg). While the decrease in energy intake should have precipitated greater weight loss, women did not maintain energy expenditure from physical activity, potentially resulting in less weight loss than expected. This further illustrates the importance of energy restriction and exercise for weight loss and maintenance (26).
Body Weight and Composition Changes
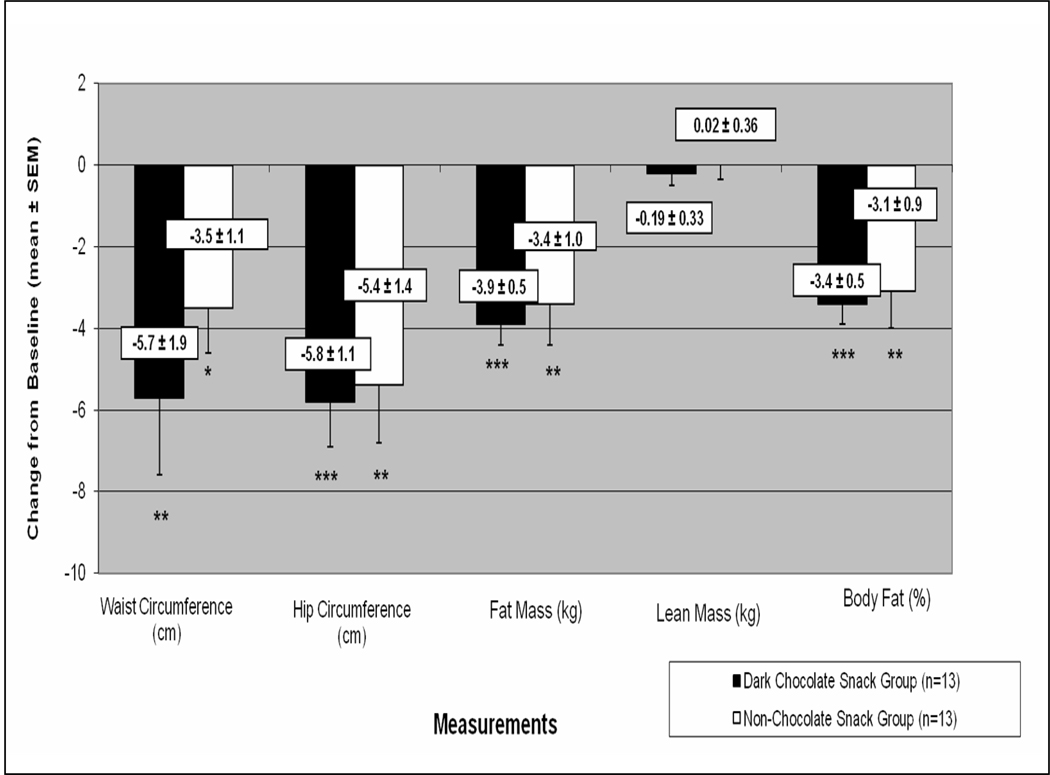
After 18 weeks, mean weight loss for each snack group was 5.1 kg (p<0.001) (Table). For the DCS group, waist and hip circumferences decreased by 5.7 and 5.8 cm, respectively (Figure). Fat mass decreased by 3.9 kg (p<0.001) and body fat percentage decreased by 3.4% (p<0.001). For the NCS group, waist and hip circumference decreased by 3.5 and 5.4 cm, respectively. Fat mass decreased by 3.6 kg (p<0.01) and body fat percentage decreased by 3.1% (p<0.01). Neither the DCS nor NCS group experienced a change in LM (Figure). Significant differences in BW and composition measurements between groups were not found at baseline or week 18.
Figure.
Changes in anthropometric and body composition measurements from baseline to week 18 by snack group
*p<0.05, **p<0.01, *** p<0.001; p-value analyzed using paired t-tests for within snack group changes from baseline. There were no significant differences between snack group in changes in anthropometric or body composition measurements.
Results from this randomized clinical trial demonstrated that premenopausal women who were overweight or obese and who included either daily sweet snack while following a RCD were able to maintain an energy deficit, lose a significant amount of BW, and improve specific anthropometric and body composition measurements over an 18-week period. In addition, women in both intervention groups reduced BW and fat mass without negatively impacting LM which was likely the result of slow, steady BW loss (27).
The most salient finding from this study is that while women were required to consume a daily sweet snack, they did not exceed the energy requirements of the RCD. The anticipated consumption of the daily sweet snack may have alleviated some of the cravings commonly experienced during food restriction (22,23). Further, either sweet snack when included as a part of an overall RCD did not inhibit positive changes in BW and body composition. This research finding suggests that any energy-controlled sweet snack of choice could be incorporated into a RCD as long as overall energy deficit remains.
Other dietary intervention studies have explored the effectiveness of “popular” diets (4,17) on weight loss. Diets such as the Atkin’s (28), Zone (29), and Ornish diet (30) have the ability to precipitate weight loss if followed strictly. However, such interventions differ from the current study in that the aforementioned diets have rigid macronutrient composition prescriptions which can be difficult to follow for longer periods of time particularly for women who prefer more unstructured eating patterns (31). As the length and intensity of these more complex interventions increase, a steady decline in dietary compliance is commonly observed (4,16,17,32). As demonstrated in previous studies, poor compliance prohibits additional weight loss (4,16,17,32).
Women in the current study were able to reduce energy intake by over 400 kcal per day and consume snacks without substantially altering the macronutrient composition of their pre-intervention intake, unlike other, more restrictive weight loss approaches (28–30). Drastic changes in usual intake of macronutrients were not required, potentially facilitating dietary compliance. Dietary compliance was demonstrated by the clinically significant weight loss and change in body composition experienced by the women in the current study. Therefore, this intervention technique may provide an effective weight-loss strategy for women who struggle with other more restrictive diet plans.
While this study has novel findings, limitations exist. This was a pilot study; hence, the sample size was small. Only premenopausal women were included, thus limiting generalizability to women in other stages of the lifespan and to men. The DCS was compared to only a NCS, and snacks were included as part of the RCD. Further comparison of sweet snacks as part of a daily RCD to other non-sweet snacks and/or to a RCD without snacks is warranted. Participants were not able to choose their sweet snack (i.e., randomized to group)—a choice that if given, may have led to overconsumption. Conversely, chocolate is considered the most highly craved snack in North America (33) and is recognized as the favorite food of 40% of women (20) making it an appropriate choice for this study.
CONCLUSIONS
This study tested the feasibility of purposefully incorporating a highly desirable sweet snack such as chocolate compared to a non-chocolate snack into a RCD. All women who followed this RCD with either a DCS or NCS were able to maintain an energy deficit, lose a significant amount of BW and improve body composition regardless of the type of snack consumed. When prescribing a dietary plan for weight loss, an individual’s food preferences and usual intake pattern, which also includes snacks, should be considered. Education about the importance of controlling sweet snack portion size and its contribution to overall daily energy intake is critical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathryn E. Piehowski, 110 Chandlee Laboratory, Department of Nutritional Sciences, The Pennsylvania State University, University Park, Pennsylvania, 16802-6504, USA, Phone: 814-865-5926, Fax: 814-863-6103, kep158@psu.edu.
Amy G. Preston, The Hershey Center for Health and Nutrition, The Hershey Company, 1025 Reese Avenue, Hershey, PA 17033 USA, Phone: 717-534-5252, Fax: 717-534-5076, apreston@hersheys.com.
Debra L. Miller, The Hershey Center for Health and Nutrition, The Hershey Company, 1025 Reese Avenue, Hershey, PA 17033 USA, Phone: 717-534-5686, Fax: 717-534-5076, debramiller@hersheys.com.
Sharon M. Nickols-Richardson, 323 Chandlee Laboratory, Department of Nutritional Sciences, The Pennsylvania State University, University Park, Pennsylvania, 16802, USA, Phone: 814-863-2920, Fax: 814-863-6103, smn13@psu.edu.
REFERENCES
- 1.Bish CL, Blanck HM, Serdula MK, Marcus M, Kohl HW, III, Khan LK. Diet and physical activity behaviors among Americans trying to lose weight: 2000 Behavioral Risk Factor Surveillance System. Obes Res. 2005;13(3):596–607. doi: 10.1038/oby.2005.64. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 4.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, Champagne CM, Dalcin A, Erlinger TP, Funk K, Laferriere D, Lin PH, Loria CM, Samuel-Hodge C, Vollmer WM, Svetkey LP. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35(2):118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin PH, Appel LJ, Funk K, Craddick S, Chen C, Elmer P, McBurnie MA, Champagne C. The PREMIER intervention helps participants follow the Dietary Approaches to Stop Hypertension dietary pattern and the current Dietary Reference Intakes recommendations. J Am Diet Assoc. 2007;107(9):1541–1551. doi: 10.1016/j.jada.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Winham DM, Collins CB, Hutchins AM. Dietary intakes, attitudes toward carbohydrates of postmenopausal women following low carbohydrate diets. Can J Diet Pract Res. 2009;70(1):44–47. doi: 10.3148/70.1.2009.44. [DOI] [PubMed] [Google Scholar]
- 8.Latner JD, Stunkard AJ, Wilson GT, Jackson ML, Zelitch DS, Labouvie E. Effective long-term treatment of obesity: a continuing care model. Int J Obes Relat Metab Disord. 2000;24(7):893–898. doi: 10.1038/sj.ijo.0801249. [DOI] [PubMed] [Google Scholar]
- 9.Strohacker K, McFarlin BK. Influence of obesity, physical inactivity, and weight cycling on chronic inflammation. Front Biosci (Elite Ed) 2010;2:98–104. doi: 10.2741/e70. [DOI] [PubMed] [Google Scholar]
- 10.Field AE, Manson JE, Taylor CB, Willett WC, Colditz GA. Association of weight change, weight control practices, and weight cycling among women in the Nurses' Health Study II. Int J Obes Relat Metab Disord. 2004;28(9):1134–1142. doi: 10.1038/sj.ijo.0802728. [DOI] [PubMed] [Google Scholar]
- 11.Yoo HJ, Kim BT, Park YW, Park KH, Kim CW, Joo NS. Difference of body compositional changes according to the presence of weight cycling in a community-based weight control program. J Korean Med Sci. 2010;25(1):49–53. doi: 10.3346/jkms.2010.25.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lissner L, Odell PM, D'Agostino RB, Atokes J, III, Kreger BE, Belanger AJ, Brownell KD. Variability of body weight and health outcomes in the Framingham population. New Engl J Med. 1991;324(26):1839–1844. doi: 10.1056/NEJM199106273242602. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128(2):81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 14.United States Department of Agriculture and United States Department of Health and Human Services. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. Washington, DC: U.S. Government Printing Office; 2010. Jul, [Google Scholar]
- 15.Jiménez-Cruz A, Jiménez AB, Pichardo-Osuna A, Chaudry T, Bacardi-Gascon M. Long term effect of Mediterranean diet on weight loss. Nutr Hosp. 2009;24(6):753–754. [PubMed] [Google Scholar]
- 16.Acharya SD, Elci OU, Sereika SM, Music E, Styn MA, Turk MW, Burke LE. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence. 2009;3:151–160. doi: 10.2147/ppa.s5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, Armiento M, D’Andrea F, Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278(17):1407–1411. [PubMed] [Google Scholar]
- 19.Keast DR, Nicklas TA, O'Neil CE. Snacking is associated with reduced risk of overweight and reduced abdominal obesity in adolescents: National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Clin Nutr. 2010;92(2):428–435. doi: 10.3945/ajcn.2009.28421. [DOI] [PubMed] [Google Scholar]
- 20.Rozin P, Levine E, Stoess C. Chocolate craving and liking. Appetite. 1991;17(3):199–212. doi: 10.1016/0195-6663(91)90022-k. [DOI] [PubMed] [Google Scholar]
- 21.Yanovski S. Sugar and fat: cravings and aversions. J Nutr. 2003;133 suppl:835S–837S. doi: 10.1093/jn/133.3.835S. [DOI] [PubMed] [Google Scholar]
- 22.Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40(1):15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 23.Barnes RD, Tantleff-Dunn S. Food for thought: examining the relationship between food thought suppression and weight-related outcomes. Eat Behav. 2010;11(3):175–179. doi: 10.1016/j.eatbeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington: Carnegie Institute of Washington publication; 1919. p. 279. [Google Scholar]
- 25.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS., Jr Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 26.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zalesin KC, Franklin BA, Lillystone MA, Shamoun T, Krause KR, Chengelis DL, Mucci SJ, Shaheen KW, McCullough PA. Differential loss of fat and lean mass in the morbidly obese after bariatric surgery. Metab Syndr Relat Disord. 2010;8(1):15–20. doi: 10.1089/met.2009.0012. [DOI] [PubMed] [Google Scholar]
- 28.Atkins R. Dr. Atkins’ New Diet Revolution. New York, NY: Harper Collins: New York; 2002. [Google Scholar]
- 29.Sears B, Lawren W. Enter The Zone: A Dietary Road Map to Lose Weight Permanently: Reset Your Genetic Code: Prevent Disease: Achieve Maximum Physical Performance. New York, NY: Harper Collins: New York; 1995. [Google Scholar]
- 30.Ornish D. Eat More Weigh Less. New York, NY: Harper Collins: New York; 2001. [Google Scholar]
- 31.Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nutr. 2006;84(5):1215–1223. doi: 10.1093/ajcn/84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhassan S, Kim S, Bersamin A, King AC, Gardner CD. Dietary adherence and weight loss success among overweight women: results from the A TO Z weight loss study. Int J Obes (Lond) 2008;32(6):985–991. doi: 10.1038/ijo.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruinsma K, Taren DL. Chocolate: food or drug? J Am Diet Assoc. 1999;99(10):1249–1256. doi: 10.1016/S0002-8223(99)00307-7. [DOI] [PubMed] [Google Scholar]